INTRODUCTION
Systemic lupus erythematosus (SLE) is a complex disease that's characterized by an autoantibody response to nuclear and cytoplasmic antigens. The autoantibody response is associated with the inflammatory cascades and end-organ damage in the kidney, skin, brain and other organs. Especially in the kidney, immune-complex deposits of autoantibodies have been implicated as major pathogenic mediators [1].
The diagnosis of SLE can be made by fulfilling the revised criteria of the American College of Rheumatology [2]. One of the laboratory hallmarks of SLE is the presence of antinuclear antibodies (ANA) in the serum [3]. On the other hand, a small subset of SLE patients with the typical clinical findings of SLE was reported to have persistently negative ANA tests. These patients were designated as ANA-negative SLE and their clinical picture was notable for the high incidence of photosensitive dermatitis and the low incidence of nephritis and neuropsychiatric manifestations [4].
We report here on a case of an ANA-negative SLE patient who presented with lupus nephritis. The other serum autoantibodies, including anti-double stranded DNA antibody (anti-dsDNA), anti-extractable nuclear antigens antibodies and antiphosopholipid antibodies, and the repeated ANA testing were all negative.
CASE REPORT
A 16-yr-old female was admitted due to pain in multiple joints she had experienced for a week and also for a skin rash on her face and legs she had experienced for 2 days. Her blood pressure was 119/74 mmHg, the pulse rate 115/min, the respiratory rate 20/min and the body temperature 37.0Ōäā. She had a malar rash, photosensitivity and a erythematous maculopapular rash on both legs. There was active synovitis that involved her proximal interphalangeal joints, metacarpophalangeal joints, wrists, elbows, metatarsophalangeal joints, ankles and knees. Her initial laboratory investigations showed a white blood cell count of 9,830/mm3 (lymphocyte 777/mm3), a hemoglobin level of 12.3 g/dL, a platelet count of 182,000/mm3, the erythrocyte sedimentation rate was 24 mm/h and the C-reactive protein level was 1.63 mg/dL. The levels of C3 and C4 were normal. She had no proteinuria on urinalysis. Antinuclear antibody, anti-dsDNA, anti-cardiolipin antibody, lupus anticoagulant, anti-RNP antibody, anti-Sm antibody, anti-Ro antibody, anti-La antibody and rheumatoid factor were all negative. A skin biopsy was taken from an erythematous lesion on her leg. The histopathology of the biopsy specimen showed leukocytoclastic vasculitis without immune deposits. She complained of chest pain on day 3 of admission. The chest X-ray and electrocardiogram were normal, but an echocardiogram showed a minimal pericardial effusion.
She was diagnosed as having ANA-negative SLE on the basis of the malar rash, photosensitivity, arthritis, lymphopenia and pericarditis. She was treated with nonsteroidal antiinflammatory drugs, hydroxychloroquine, methotrexate and low dose corticosteroids. Most of her symptoms were controlled and she was discharged on the 11th day of admission.
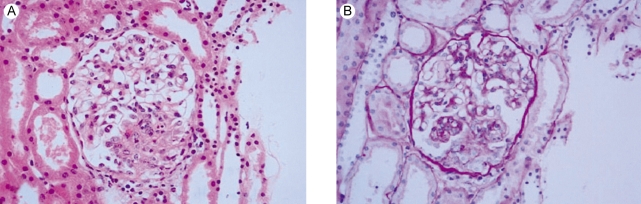
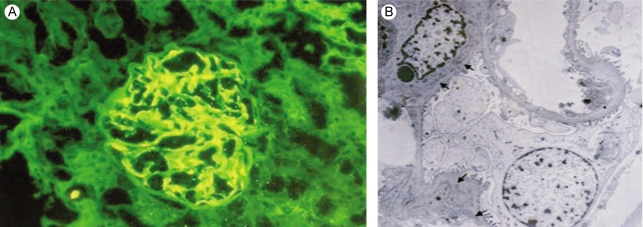
One month later, she was readmitted with diffuse abdominal pain she had experienced for 2 weeks. Abdominal computed tomography demonstrated diffuse bowel wall thickening in the cecum and terminal ileum (Fig. 1). There was mild leukocytosis, but the blood urea nitrogen and serum creatinine levels were normal. The retests for ANA and anti-dsDNA were negative. A urine examination showed 2+ proteinuria by a dipstick test and the protein/creatinine ratio of a random urine test was 2.84 (800/282). Renal biopsy was performed and that contained 15 glomeruli. Most of the glomeruli showed slight expansion of the mesangial matrix with focal and segmental mesangial cell proliferation (Fig. 2). Segmental collapse and adhesion to Bowmann's capsule was noted in 5 glomeruli. Immunofluorescent staining revealed 6 glomeruli that showed strong IgG deposits, mild IgA, C3 and fibrinogen deposits and minimal C1q deposits that were mainly seen at the mesangium (Fig. 3A). The electron microscopic findings showed mesangial electron dense deposits (Fig. 3B). The subendothelial electron dense deposits were focal and segmental. No tubuloreticular structure was present. Three of them showed a small active cellular crescent.
ANA-negative lupus nephritis was considered and she was treated by monthly pulse cyclophosphamide and high-dose corticosteroids, and she showed persistently improving proteinuria. During the 2-year follow-up period, repeated tests for ANA and anti-dsDNA were all negative. Currently, she has 1+ proteinuria by the dipstick test and the protein/creatinine ratio of a random urine test was 0.42 (58/137).
DISCUSSION
Systemic lupus erythematosus is a chronic inflammatory autoimmune disease that involves many different organ systems, and this illness exhibits a wide spectrum of clinical manifestations. The diagnosis of SLE depends on the patient's clinical and laboratory abnormalities [2]. Various kinds of autoantibodies are present in the sera of SLE patients, and ANA is a diagnostic hallmark for SLE, having a frequency of 95% or greater in SLE patients [5].
However, several investigators have reported that small groups of patients with the clinical features of SLE have negative tests for ANA. These patients appear to represent 1-5% of the SLE population. The age of onset and the female predominance are the same for ANA-negative SLE as for ANA-positive SLE [6]. ANA-negative SLE patients are known to have a higher prevalence of anti-Ro antibody and cutaneous manifestations, as well as a lower prevalence of both central nervous system and renal involvement [4,7]. Maddison et al. described 66 SLE patients with negative ANA, as was determined by indirect immuno-fluorescence. They found serum antibodies to cytoplasmic components; 62% of patients had anti-Ro antibody and 27% had anti-La antibody [4].
One explanation for the ANA-negative finding is technical inaccuracy. McHardy et al. identified 38 adults who had a high DNA-binding capacity, but negative fluorescent ANA testing (with a rat liver substrate), and the clinical diagnosis of SLE was established for these patients [8]. In another study, the previously ANA-negative finding, with using mouse liver substrate, in the sera of patients with SLE or subacute cutaneous lupus erythematosus was found to be anti-Ro antibody positive by performing enzyme-linked immunosorbent assays [9]. Actually, the increasing use of human epithelial (HEp-2) substrate has increased the sensitivity of ANA assays and as a result, the perceived incidence of ANA-negative SLE has decreased [8,10].
Another cause of ANA-negative findings is that ANA is present, but its bound in the form of immune complexes. This has been described in five patients with lupus nephritis whose ANAs, which were primarily reactive with DNA, were not detected in the serum by indirect immunofluorescence until the ANAs were dissociated from circulating immune complexes [11]. Loss of ANA through the kidney in a patient with profuse proteinuria has been reported as another possibility. In that case, the tests for ANA became positive upon clinical recovery [12]. However, most ANA-negative patients have persistently negative tests for ANA after a long follow-up period. Technical factors or prozone effects have been described as the possible reasons for this [13].
Lupus nephritis is believed to be a consequence of the deposition of immunoglobulins in the kidney, and the predominant immunoglobulin isotype is IgG. Also, lupus nephritis is frequently associated with ANA and anti-dsDNA. On rare occassions, the serological markers may be initially absent, but they become positive after some time in most patients [14]. Gianviti et al. reported on three patients aged 9, 8 and 10 years old, respectively, who presented with a glomerulopathy suggestive of lupus nephritis, but they were without clinical or serological evidence of SLE [15]. All developed ANA and anti-dsDNA at 5, 3 and 10 years, respectively, after their original presentation, but only one patient developed clinical symptoms of SLE in 6 years. Development of ANA and anti-dsDNA secondary to the release of nuclear antigens into the blood stream during hemodialysis treatment has been reported in the literature. Ozdemir et al. described a 28-yr-old female who was admitted with acute renal failure following her fourth delivery [16]. The serum immuno-logical markers were negative and renal biopsy showed histopathological changes consistent with SLE as the etiology of her nephritic syndrome. During the follow-up period with administering immunosuppressive treatment, the ANA became positive as the proteinuria declined. Yet in our patient, the autoantibodies including ANA were negative during the follow-up period, even though there was strong IgG deposit in the glomeruli. There are several possible explanations for this. First, the level of ANA in the sera may be too low to detect by conventional clinical assays.Second, our follow-up period may not have been long enough for her to develop ANA. Finally, the renal damage in our patient may have been mediated by autoantibodies that do not react with ANA.
In conclusion, our patient fulfilled the ACR criteria, including having a renal disorder without serologic evidence of SLE at presentation. During the follow-up period, the repeated ANA and anti-dsDNA tests were all negative, irrespective of her improving proteinuria. Our patient may be a very rare case of ANA-negative lupus nephritis without seroconversion and this suggests that ANA may not be required in the pathogenesis of lupus nephritis.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





