 |
 |
| Korean J Intern Med > Volume 21(4); 2006 > Article |
|
Abstract
Background
The radiographic characteristics of tuberculous pneumonia in adults are similar to primary tuberculosis that occurs in childhood, and upper lobe cavitary tuberculosis is the hallmark of postprimary tuberculosis. The purpose of this study was to investigate the factors associated with tuberculous pneumonia by making comparison with cavitary tuberculosis.
Methods
The medical records and radiographic findings of patients with cavitary tuberculosis and tuberculous pneumonia, and who were diagnosed between March 2003 and February 2006, were analyzed retrospectively.
Results
Forty patients had cavitary tuberculosis and sixteen patients had tuberculous pneumonia. Fever was more frequent for tuberculous pneumonia, whereas hemoptysis was more frequent for cavitary tuberculosis. The duration of symptoms before visiting the hospital was shorter, but the diagnosis after admission was more delayed for tuberculous pneumonia patients than for cavitary tuberculosis patients. The prevalence of underlying comorbidities such cancer, diabetes, alcoholism and long-term steroid use was not different between the two groups. The patients with tuberculous pneumonia were older and they had lower levels of serum albumin and hemoglobin than those with cavitary tuberculosis. The patients with tuberculous pneumonia showed a tendency to have more frequent endobronchial lesion. Tuberculous pneumonia occurred in any lobe, whereas the majority of cavitary tuberculosis patients had upper lung lesion, but the prevalence of lymphadenopathy, pleural effusion and previous tuberculosis scar was not different between the two groups.
Conclusions
Older age, a lower level of serum albumin and hemoglobin and a random distribution of lesion were associated with tuberculosis pneumonia as compared with cavitary tuberculosis. These findings suggest that the pathogenesis of tuberculous pneumonia might be different from that of cavitary tuberculosis.
Active tuberculosis disease has been classified as either primary or postprimary tuberculosis (TB)1, 2). Primary TB is common in the pediatric age group, and it is caused by initial Mycobacterium tuberculosis (M. tuberculosis) infection. The radiological characteristics are focal lung infiltration or homogeneous consolidation that usually affects the middle and lower lobes and lymphadenopathy is also present1). Postprimary TB is common in adults; this is mainly located in the apical and posterior segments of the upper lobes and it is characterized by cavitary lung lesion2).
While the radiographic features of childhood TB have apparently not changed, there has been an increased number of reports of atypical TB in adults3-10). One of these atypical radiological findings in adult is tuberculous pneumonia that resembles childhood primary TB. The radiographic characteristics of tuberculous pneumonia are homogeneous, segmental or lobar consolidation6, 7) and it occurs in any site of the lung6, 10).
There have been many reports concerning the pathogenesis of postprimary TB in adults. Stead et al. proposed that postprimary TB was usually caused by reactivation of dormant M. tuberculosis rather than by new exogenous reinfection11). Yet the recent reports that have employed mycobacterial genotyping techniques have suggested that exogenous reinfection was also a significant cause of postprimary TB in adults, and especially in an area with a high incidence of tuberculosis12-15). But it is still unknown if tuberculosis pneumonia in adults is caused by primary infection, endogenous reactivation or exogenous reinfection. Comparing the characteristics of tuberculous pneumonia with that of typical postprimary tuberculosis might be useful for understanding the pathogenesis of tuberculous pneumonia. Despite conducting a search of the related articles, we could not find any literature that compared tuberculous pneumonia with cavitary TB.
In this study we analyzed the clinical and radiological characteristics of tuberculous pneumonia and cavitary TB, and we tried to determine the factors associated with the two characteristic radiological patterns.
We first reviewed the electronic hospital records of the patients diagnosed with active TB at Chungbuk National University Hospital from March 2003 to February 2006. Through reviewing the medical charts and radiographs, we enrolled the patients who met all of following criteria: 1) they had active pulmonary TB, 2) they were older than 15 years, 3) there was no history of prior active TB and 4) they had cavitary pulmonary TB or tuberculous pneumonia. We divided them into two groups: the cavitary TB group (Group I) and the tuberculous pneumonia group (Group II). Cavitary TB was defined as the presence of a gas-filled space surrounded by a discrete cavity wall in the lung parenchyma on a chest X-rays or a chest computed tomography (CT) scan (Figure 1). Tuberculous pneumonia was defined as the presence of homogeneous parenchymal consolidation on chest X-ray that was interpreted as bacterial pneumonia by a chest radiology specialist, and if chest CT scan was performed, the findings were also homogeneous parenchymal consolidation (Figure 2).
The demographic data, underlying comorbidities and laboratory data were compared between the two groups. The location of the main lesion on chest X-rays or chest CT scans was classified as upper lung lesion (including the apical segment of the lower lobe), middle (including the lingular lobe) or lower lung lesion. We also assessed the presence of the following findings on radiographs 1) bronchogenic spread, which was defined as air space consolidation, a cavity or a tree with a bud pattern that was seen on chest X-rays or chest CT scans in another lobe other than the lobe with the main tuberculous lesion, 2) a tree with a bud pattern on CT scan was defined as centro-lobular branching linear structures, 3) hilar or mediastinal lymphadenopathy was defined as a lymph node larger than 1cm on the short axis on chest CT scan, 4) pleural effusion or 5) a previous TB scar (dense calcified pulmonary nodules, calcified lymph nodes or pleural thickening2, 16).
Forty patients with cavitary TB and sixteen patients with tuberculous pneumonia were enrolled in this study. Among the 56 cases, the final diagnosis of active pulmonary tuberculosis was made by the following methods (Table 1). The diagnosis in 31 patients was confirmed by positive culture for M. tuberculosis in the specimens (sputum, bronchial washing or pleural fluid). Among the patients without positive culture for M. tuberculosis, seven cases were confirmed by the typical histology (endobronchial mucosal biopsy, percutaneous pleural biopsy or percutaneous transthoracic lung biopsy). Twelve cases among the patients without positive culture for M.tuberculosis or typical histology had positive AFB smears of the sputum or the bronchial washing specimens. The remaining six cases were diagnosed as active pulmonary TB by the clinical and radiological findings; all of them had cavitary lesion and they improved after receiving antituberculous medication.
Fever was more frequent in the patients with tuberculous pneumonia, while hemoptysis was the more frequent presentation for cavitary TB patients. The duration of symptoms before visiting the hospital was shorter and the diagnosis after admission was more delayed for the patients with tuberculous pneumonia compared with those patients with cavitary TB, but after antituberculous medication, fever mostly subsided within several days for both groups. The prevalence of underlying comorbidities such as cancer, diabetes, long-term steroid use and alcoholism showed no statistical difference between both groups (Table 2).
The patients with tuberculous pneumonia were older, they had lower levels of serum albumin and hemoglobin and they had a tendency to have a lower yield on the positive sputum AFB smear and culture for M. tuberculosis than those patients with cavitary TB (Table 3). Because of non-responsiveness to empirical antibacterial treatment and the diagnostic need for the cases with negative sputum AFB smear, bronchofibroscopy was more frequently performed for the tuberculous pneumonic patients (7 cases). We found stenotic endobronchial lesion at the proximal bronchus of the lung lobe with the main lesion in six cases, such as endobronchial TB (3 cases), anthracotic pigmentation (2 cases), and severe bronchial mucosal swelling (one case). Bronchofibroscopy was performed for diagnosis in two cases of cavitary TB and endobronchial lesion was not found in either case (p=0.083).
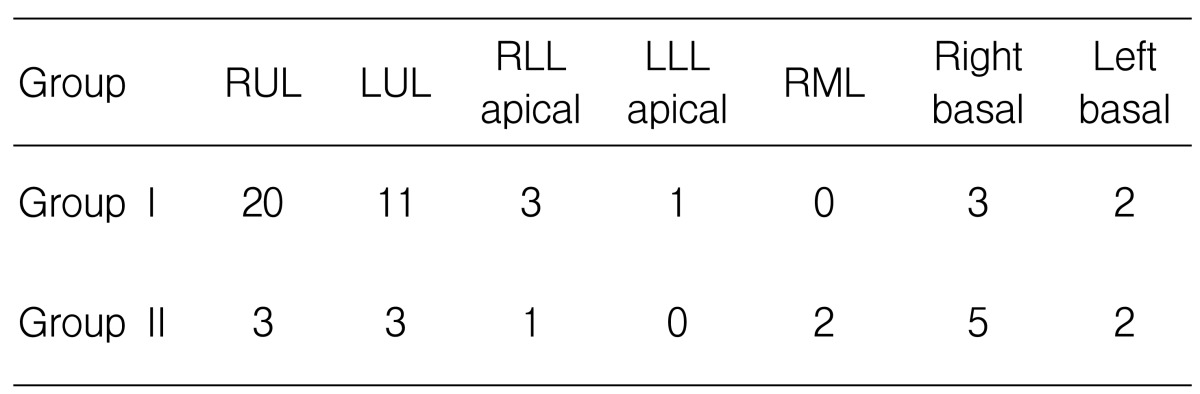
The distribution of the main parenchymal lesions is shown in Table 4.
Most of the patients with cavitary TB had a main cavity at both upper lobes and the apical segment of the lower lobe. The main lesion of tuberculous pneumonia occurred in all sites of the lungs. The classified location of the main lesion was significantly different in the two groups. Cavitary TB predominantly occurred at the upper lung compared with tubeculous pneumonia. The prevalence of lymphadenopathy, pleural effusion and previous tuberculosis scar lesion was not different between the two groups. Bronchogenic spreading was more common in cavitary TB patients (without statistical significance), but the finding of a tree with a bud pattern on chest CT was more frequent in cavitary TB patients than in tuberculous pneumonia patients (Table 5).
Primary TB is caused by an airborne infection of M. tuberculosis and its location reflects the pulmonary airflow. This can occur in any site of the lung, yet it is more frequently in the mid or lower lung field due to these areas greater ventilation. From the primary focus, tuberculous bacilli are spread via the lymphatics or blood stream. Dormant states mainly occur in such areas such as the apicoposterial segment of the upper lobe or the apical segment of the lower lobe, where lymph production and drainage are deficient and high oxygen tension is present2). Stead et al. proposed that postprimary TB could occur at these sites by reactivation of dormant disease states11). Lung cavitation is the hallmark of postprimary TB and this appears in about half of the patients17).
Tuberculous pneumonia, similar to childhood primary tuberculosis18), is the unusual radiographic finding in adults and this occurs in any site of the lung6, 10) Tuberculosis occurring in the lower lung fields in adult is a frequent characteristics of tuberculous pneumonia19), which is homogeneous segmental or lobar consolidation6, 7). Fever was more common and the duration of symptom until the hospital visit was shorter for tuberculous pneumonia than for cavitary TB. Because tuberculous pneumonia may frequently be indistinguishable from bacterial pneumonia, the diagnosis at a hospital is generally more delayed6, 10). The white blood cell count in the peripheral blood is frequently normal in both groups, and this is one of the clues to distinguish tuberculosis pneumonia from bacterial pneumonia19).
The patients with tuberculous pneumonia in our study had a tendency to have a lower yield for a positive sputum AFB smear and culture for M. tuberculosis than did the patients with cavitary TB, and bronchocopic examination is useful for these cases to make the diagnosis of tuberculosis20). In our study the positive culture rate for M. tuberculosis in a specimen was lower than that of other reports3, 6, 10). Three cases with positive AFB smears didnt have culture for M. tuberculosis
ordered. In our hospital, culture for M. tuberculosis is done at another laboratory, so the result of culture might be influenced by many factors, including storage, transport and processing.
Many authors have reported that lower lung field TB and tuberculous pneumonia more commonly occur in specific groups of patients such as those with diabetes mellitus (DM), cancer or infection with human immunodeficiency virus (HIV)3, 6, 19, 21). But in our study, the prevalence of comorbidity such as cancer, DM, alcoholism and long-term steroid use was not different between the two groups. The prevalence of DM, cancer and long-term steroid use has been variable in the reports on tuberculous pneumonia6, 10) and these studies did not compare tuberculous pneumonia with cavitary TB3, 6, 10, 19, 21).
In our study the patients with tuberculous pneumonia were older and had lower levels of serum albumin and hemoglobin than did those patients with cavitary TB. In many reports, lung cavitation was less frequent in the elderly patients22-24) and lower lung field TB and tuberculous pneumonia are more common in older patients than in the younger patients22, 25). Because elderly people have more impaired T-lymphocyte function than younger patients26), the decrease in their immunologic status associated with aging might be related to the development of tuberculous pneumonia. Also, the findings of lower serum albumin and hemoglobin levels in patients with tuberculous pneumonia might reflect malnutrition or more severe catabolic states in older patients than in those patients with cavitary TB.
Recent reports concerned with the use of mycobacterial genotyping techniques have suggested that exogenous reinfection appears to be a significant cause of postprimary TB in adults, and the incidence of exogenous reinfection is variable according to the patients age and the incidence of TB in a country12-15). The incidence of TB in our country was 64.5 cases per 100.000 persons in 2004 (http://tbnet.go.kr) and exogenous reinfection might be a substantial cause of postprimary TB in Korean adults. In our study, the main lesion in tuberculous pneumonia patients occurred in any site of the lung, which is similar to other studies6, 10). This finding suggests that tuberculous pneumonia might be caused by exogenous reinfection via airflow implantation of M tuberculosis rather than by reactivation of dormant bacilli. Further, the elderly might be more susceptible to exogenous reinfection due to impaired host immunity.
In addition to exogenous reinfection, endobronchial stenosis in adults might be involved in the pathogenesis of tuberculosis pneumonia, as compared to the enlarged lymph nodes in the pathogenesis of childhood TB. Lymphadenopathy in tuberculous pneumonia patients was uncommon in our study and also in other studies7, 10) and it was different from that of childhood primary TB, in which lymphadenopathy was observed in almost all such patients27). Goussard et al. speculated that the pathogenesis of tuberculous pneumonia in children was enlarged tuberculous lymphadenitis that ruptured into the bronchus; the caseous material was aspirated into the affected lobe and the subsequent exudative hypersensitivity reaction to the aspirated tuberculoprotein caused tuberculous pneumonia28). Tuberculous pneumonia, including that in the lower lung in adults, is frequently associated with endobronchial lesions7, 10) and it is more common in female patients25, 29). In our study, seven non-responders to antibiotic therapy and they had negative sputum AFB smears were examined by bronchofibroscopy, and endobronchial lesion was discovered in six of them.
Therefore we can speculate that tuberculous pneumonia might develop by reinfection with aging, and implantation of M. tuberculosis occurred via the airflow and this caused a random distribution of lesion. In addition to exogenous reinfection, endobronchial narrowing hinders the drainage of infected materials and so contributes to the development of consolidative hypersensitivity reaction.
Our retrospective study had the following limitations. Although there were some limitations in interpreting the tuberculin skin test in our country because of BCG vaccination30), the enrolled patients in our study were not examined by tuberculin skin test and the scar from the BCG vaccination was not recorded. However, the prevalence of a previous TB scar on radiographs, which is visible in one third of healed primary tuberculosis patients2), was not different between the two groups. Second, the exact prevalence of combined endobronchial lesion could not be estimated because bronchofibroscopy was not performed in all of the patients of both groups.
A large scale prospective study using DNA genotyping methods and bronchofibroscopic examination is needed to elucidate the pathogenesis of tuberculosis pneumonia and cavitary TB.
References
2. McAdams HP, Erasmus J, Winter JA. Radiological manifestations of pulmonary tuberculosis. Radiol Clin North Am 1995;33:655–678PMID : 7610237.

3. Wilcke JT, Askgaard DS, Nybo Jensen B, Dossing M. Radiographic spectrum of adult pulmonary tuberculosis in a developed country. Respir Med 1998;92:493–497PMID : 9692111.


4. Miller WT, MacGregor RR. Tuberculosis: frequency of unusual radiographic findings. AJR Am J Roentgenol 1978;130:867–875PMID : 417585.


5. Khan MA, Kovnat DM, Bachus B, Whitcomb ME, Brody JS, Snider GL. Clinical and roentgenographic spectrum of pulmonary tuberculosis in the adult. Am J Med 1977;62:31–38PMID : 835590.


6. Lee SH, Hur GY, Jung KW, Lee SY, Lee SY, Kim JH, Park SM, Shin C, Shim JJ, In KH, Kang KH, Ryu SH. Clinical investigation of tuberculous pneumonia. Tuberc Respir Dis 2004;57:19–24.

7. Park S, Hong YK, Joo SH, Choe KO, Cho SH. CT findings of pulmonary tuberculosis presenting as segmental consolidation. J Comput Assist Tomogr 1999;23:736–742PMID : 10524858.


8. Choi D, Lee KS, Suh GY, Kim TS, Kwon OJ, Rhee CH, Han J. Pulmonary tuberculosis presenting as acute respiratory failure: radiologic findings. J Comput Assist Tomogr 1999;23:107–113PMID : 10050819.


9. Wei CJ, Tiu CM, Chen JD, Chou YH, Chang CY, Yu C. Computed tomography features of acute pulmonary tuberculosis. Am J Emerg Med 2004;22:171–174PMID : 15138951.


10. Paeng MH, Kim YK, Shim SS, Chang JH, Lee JH, Kwag HJ. Pulmonary tuberculosis mimicking pneumonia on CT: retrospective analysis of clinical and CT features. Tuberc Respir Dis 2003;55:31–40.

11. Stead WW, Kerby GR, Schueleter DP, Jordahl CW. The clinical spectrum of primary tuberculosis in adults: confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann Intern Med 1968;68:731–745PMID : 5642961.


12. Chiang CY, Riley LW. Exogenous reinfection in tuberculosis. Lancet Infect Dis 2005;5:629–636PMID : 16183517.


13. Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, Enarson DA, Behr MA, Helden PD. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 2005;171:1430–1435PMID : 15831840.


14. van Rie A, Warren R, Richardson M, Victor TC, Gie RP, Enarson DA, Beyers N, van Helden PD. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med 1999;341:1174–1179PMID : 10519895.


15. du Plessis DG, Warren R, Richardson M, Joubert JJ, van Helden PD. Demonstration of reinfection and reactivation in HIV-negative autopsied cases of secondary tuberculosis: multilesional genotyping of Mycobacterium tuberculosis utilizing IS6110 and other repetitive element-based DNA fingerprinting. Tuberculosis 2001;81:211–220PMID : 11466033.


16. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O'Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA. American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med 2003;167:603–662PMID : 12588714.


17. Lee KS, Im JG. CT in adults with tuberculosis of the chest characterictics findings and role in management. AJR Am J Roentgenol 1995;164:1361–1367PMID : 7754873.


18. Lamont AC, Cremin BJ, Pelteret RM. Radiological patterns of pulmonary tuberculosis in the paediatric age group. Pediatr Radiol 1986;16:2–7PMID : 3945496.


20. Zhu G, Ji S. Analysis of lobar pneumonic tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi 1998;21:85–87PMID : 11263390.

21. Sunderam G, McDonald RJ, Maniatis T, Oleske J, Kapila R, Reichman LB. Tuberculosis as a manifestation of the acquired immunodeficiency syndrome (AIDS). JAMA 1986;256:362–366PMID : 3723722.


22. Lee JH, Hwangbo B, Yoo CG, Lee CT, Han SK, Shim YS, Chung HS. Clinical features of pulmonary tuberculosis in the elderly. Tuberc Respir Dis 2001;51:334–345.

23. Perez-Guzman C, Vargas MH, Torres-Cruz A, Villarreal-Velarde H. Does aging modify pulmonary tuberculosis?: a meta-analytical review. Chest 1999;116:961–967PMID : 10531160.


24. Wang JY, Lee LN, Hsueh PR. Factors changing the manifestation of pulmonary tuberculosis. Int J Tuberc Lung Dis 2005;9:777–783PMID : 16013774.

25. Umeki S. Comparison of younger and elderly patients with pulmonary tuberculosis. Respiration 1989;55:75–83PMID : 2788909.


26. Ben-Yehuda A, Weksler ME. Host resistance and the immune system. Clin Geriatr Med 1992;8:701–711PMID : 1423129.


27. Leung AN, Mller NL, Pineda PR, FitzGerald JM. Primary tuberculosis in children: radiographic manifestations. Radiology 1992;182:87–91PMID : 1727316.


Figure 1
Chest X-ray of a 24-year-old woman reveals a cavitary lung lesion at the right upper lung (A). The chest CT scan demonstrates the thick walled cavity at the posterior segment of right upper lung (white arrowed) with a tree in bud pattern (black arrows) (B).

Figure 2
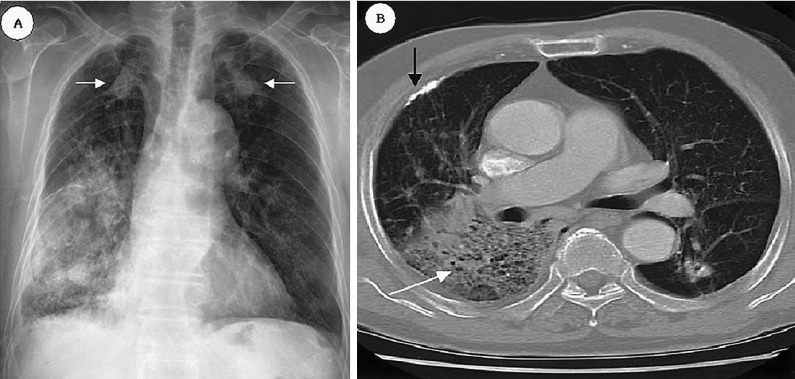
Chest X-ray of an 82-year-old man reveals homogenous consolidation at the right lower lung and the dense nodular lesions in both upper lungs (white arrowed) have not changed during 10 years (A). Chest CT scan demonstrates homogeneous consolidation with an air bronchogram (white arrow) and an old calcified pleural lesion (black arrow) (B).
-
METRICS

- Related articles
-
Paradoxical reaction of tuberculosis masquerading as a gastric subepithelial tumor2022 March;37(2)







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


