 |
 |
| Korean J Intern Med > Volume 21(2); 2006 > Article |
|
Abstract
Background
Pulse wave velocity (PWV) reflects arterial stiffness and may provide an integrated index of vascular status and cardiovascular disease (CVD) risk. Although the individual components of the metabolic syndrome (MS) are well established cardiovascular risk factors, the impact of the MS and its components on PWV has not been well defined.
Methods
Using an automatic wave form analyzer, heart-femoral (hf) and brachial-ankle (ba) PWVs were measured simultaneously in 364 subjects (age, 44.8±9.3 years). None of the subjects had clinical atherosclerotic CVD, diabetes, or systemic disease. The association between PWVs and the features of the MS, individual and clustered, were analyzed.
Results
By univariate analysis, the individual components of the MS, except for a low HDL-cholesterol level, were associated with increased hfPWV and baPWV. Hypercholesterolemia was also associated with increase in both PWVs. A low HDL-cholesterol level was associated with an increased baPWV. However, by multivariate analysis, none of the components of the MS, except for an elevated blood pressure (BP), was an independent factor affecting hfPWV and baPWV. After controlling for age and gender, hfPWV and baPWV were increased according to the number of MS components present (p<0.001 for both). After controlling for age, gender and BP, the MS was associated with an increased baPWV (p<0.05).
The metabolic syndrome (MS) is characterized by clustering of clinical features including: abdominal obesity, insulin resistance, hyperglycemia, hypertension and dyslipidemia. The MS is associated with an increased risk of cardiovascular disease (CVD) and type 2 diabetes1-3). The age-adjusted prevalence among US adults is reported as 24%4). The prevalence among Korean adults has been reported to be 11~19%5-7). The individual components of the MS are well established risk factors for CVD and diabetes. Components of the MS can, in combination, increase the risk for CVD and diabetes8) as well as the development of atherosclerosis9). Management of the MS may calls for the detection and control of vascular dysfunction before progression to overt clinical disease.
Pulse wave velocity (PWV) reflects arterial stiffness10). A population-based study has shown that aortic stiffness, as assessed by measuring the carotid-femoral pulse wave velocity (cfPWV), has a strong positive association with common carotid intima-media thickness (IMT), severity of plaques in the carotid artery, severity of plaques in the aorta, and the presence of peripheral arterial disease11). Aortic PWV has also been identified as a strong, independent predictor of cardiovascular death in subjects >70 years of age, whereas systolic or pulse pressure has not12). Traditionally, aortic PWV is measured by the applanation tonometric method applied to the carotid and femoral arteries13). Recently, simple oscillometric devices have been developed that can measure blood pressure (BP) and PWV in the extremities. These devices can be used in a large number of subjects, in the clinical setting, for screening of arterial stiffness13, 14). The brachial-ankle PWV (baPWV) measured by the oscillometric method reflects the stiffness of both central elastic and peripheral muscular arteries, and correlates positively with the conventional cfPWV (r>0.7)13, 15, 16). The baPWV has been shown to be a marker of atherosclerotic vascular damage or cardiovascular risk17). The heart-femoral PWV (hfPWV) reflects aortic stiffness as well, and correlates positively with the cfPWV (r=0.82)18).
Although the individual components of the MS are well established cardiovascular risk factors, the impact of the MS and its components on PWV has not been well defined. The present study was performed to evaluate the association between PWVs (hfPWV and baPWV) and the individual and clustered features of the MS in subjects without atherosclerotic CVD or diabetes.
This study enrolled 364 Korean adults aged 30 to 79 years. The medical history, symptoms, and cigarettes smoking history of each subject were confirmed by the consulting doctors. Blood samples were taken after the subjects had fasted overnight. Patients with atherosclerotic CVD, diabetes, or other systemic disease were excluded. Patients who were on antihypertensive medication, lipid lowering medication, or hormone replacement therapy were also excluded. All the subjects included in this study had normal ankle/brachial pressure indices (>0.9) and a normal serum creatinine level (<1.3 mg/dL).
Using the MS definition based on the NECP/ATP III2) and obesity as defined by the WHO West Pacific Region19), subjects having three or more the following criteria were classified as having the MS: 1) obesity: waist circumference >90 cm in men; >80 cm in women or body mass index (BMI) ≥25 kg/m2 2) hypertriglyceridemia: ≥150 mg/dL 3) low high-density lipoprotein (HDL) cholesterol: <40 mg/dL in men; <50 mg/dL in women 4) high BP: ≥130/85 mmHg (the higher BP taken in both arms by oscillometry in a supine position 5) high fasting glucose: 110~125 mg/dL. In addition, Hypercholesterolemia was defined as a total cholesterol ≥220 mg/dL, and smoking was defined as current smoking.
PWVs were measured using an automatic waveform analyzer (VP-2000, Colin Co, Komaki, Japan) that simultaneously records pulse waves, BPs, electrocardiogram (ECG), and heart sounds. The subjects were examined in the morning (9~12 am). The subjects rested in a supine position for at least five minutes. Cuffs with an oscillometric pressure sensor were applied to the extremities, ECG electrodes were attached to both wrists, and a microphone for phonocardiography was placed at the second intercostal space at the left edge of the sternum. Tonometric sensors were placed at the left common carotid and left femoral arteries. Pulse waves were recorded when carotid and femoral pulse waves, ECG, and phonocardiogram were stable. Pulse waves were stored for a sampling time of 10 s with automatic gain analysis and quality adjustment. Recordings were performed two times consecutively. Values at the second recording were used to eliminate error caused by physical stress.
The wave form analyzer automatically determines the time intervals between the second heart sound and the dicrotic notch of the carotid pulse (ΔThc), between the foot of the carotid pulse and the foot of the femoral pulse (ΔTcf), and between the foot of the brachial pulse and the foot of the ankle pulse (ΔTba). The sum of Tcf and Thc is the time for pulse waves to travel from the heart (aortic orifice) to the femoral artery (ΔThf). In addition, the wave form analyzer automatically determines the distances between the 2 recording sites on the basis of the subject's height using the following formulas: the distance from the heart to the femoral artery (Lhf) = 0.5643×height (cm)-18.381; the distance from the brachium to the ankle (Lba) = 0.5934×height (cm)+14.4014. PWVs were calculated automatically according to the following equations: hfPWV = Lhf/ΔThf; baPWV = Lba/ΔTba14, 15, 17, 20). For the analysis, baPWV was used as the mean value for the right and left sides.
To assess the reproducibility of PWVs, two different observers measured PWVs repeatedly in 30 consecutive subjects. The first observer measured PWVs twice at five or more minute intervals. After five or more minutes, a second observer measured PWVs once. At each measurement of PWVs, the cuffs were wrapped again. Tonometric sensors and a microphone for phonocardiography were also positioned again. Pearson's correlation coefficients for intraobserver reproducibility of hfPWV and baPWV were r=0.912 and r=0.976, respectively. The corresponding coefficients of variation were 4.5% and 2.6%. Pearson's correlation coefficients for interobserver reproducibility of hfPWV and baPWV were r=0.802 and r=0.972, respectively. The corresponding coefficients of variation were 5.8% and 3.3%21).
Quantitative values were expressed as mean±SD. The Statistical Package for Social Sciences version 11.5 was used for the analysis. The Student t-test was used for evaluation of the differences in the mean values of PWVs based on the categorical risk factors including the components of the MS, hypercholesterolemia and smoking. Multiple linear regression analysis was performed to determine the independent factors affecting PWVs among age, gender, components of the MS and hypercholesterolemia. The association between the number of components of the MS and the PWVs was assessed by an analysis of co-variance controlled for age and gender. The association between the MS and the PWVs was analyzed by an analysis of co-variance controlled for age, gender and BP. A p<0.05 was considered statistically significant.
The age was 44.8±9.3 years, and BMI was 24.0±3.0 kg/m2. Brachial BP was 129±18/80±12 mmHg. Serum levels of total cholesterol, triglyceride and HDL-cholesterol were: 199±33, 145±95 and 49±12 mg/dL, respectively (Table 1). The proportion of subjects for each component of the MS was: 45.6% for obesity, 42.0% for high BP, 6.0% for high fasting glucose, 35.2% for hypertriglyceridemia and 33.5% for low HDL-cholesterol. Among 364 subjects, there were 85 that had no component of the MS, 104 that had one, 81 that had two, and 94 had three or more (Table 2).
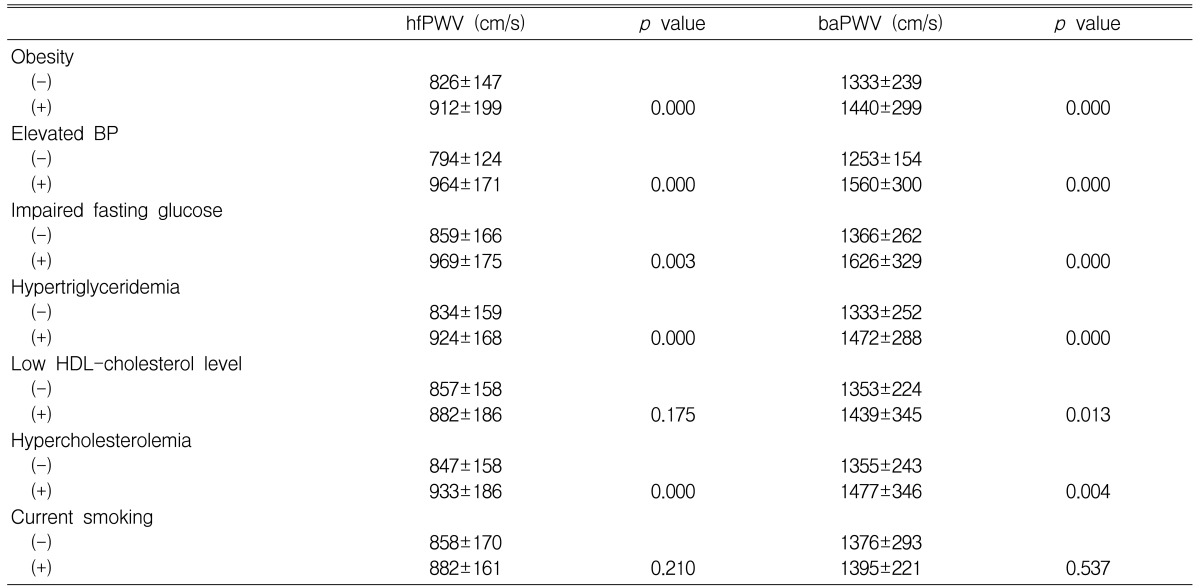
The individual components of the MS, except for a low HDL-cholesterol level, were associated with increased hfPWV and baPWV. Hypercholesterolemia was also associated with an increase in both PWVs. A low HDL-cholesterol level was associated with an increased baPWV. Smoking was associated with neither an increased hfPWV nor an increased baPWV (Table 3).
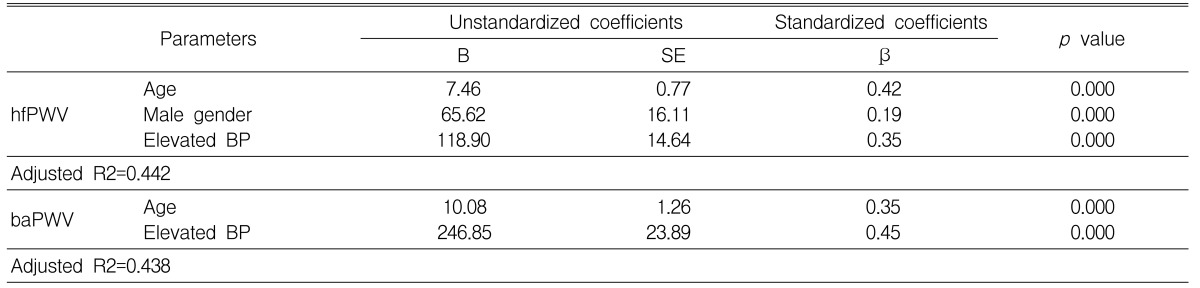
Multivariate analysis showed that the independent factors affecting hfPWV were age, gender and an elevated BP. The independent factors affecting baPWV were age and an elevated BP. When the same analysis was performed using BP, fasting glucose, triglyceride, and total cholesterol as continuous variables, BP remained the only independent factor affecting both PWVs (Table 4).
In subjects with 0, 1, 2, and ≥3 components of the MS, hfPWVs were 790±111, 810±150, 904±168, and 963±173 cm/s, respectively. The corresponding baPWVs were 1252±140, 1,294±198, 1,409±248, and 1,574±339 cm/s. After controlling for age and gender, hfPWV and baPWV were increased according to the number of the components of the MS (p<0.001 for both)(Figure 1).
After controlling for age, gender and BP, hfPWV was not significantly different among subjects with and those without the MS (962±152 vs 832±152 cm/s, p=NS). After controlling for age, gender and BP, baPWV was significantly higher in subjects with the MS compared to those without the MS (962±152 vs 832±152 cm/s, p<0.05) (Figure 2).
At present the prevalence of the MS, associated with the primary clinical outcome of CVD, is lower in Korea than in Western countries5-7), but the prevalence is expected to increase. PWV can be interpreted as an index of arterial stiffness and vascular health. Clinically, PWV is readily measurable using noninvasive techniques22). An increased PWV occurs with a range of established cardiovascular risk factors, including: age, hypertension, diabetes, dyslipidemia, smoking and obesity22-24).
The present study showed that by univariate analysis individual components of the MS, except for a low HDL-cholesterol level and hypercholesterolemia, were associated with an increase in both hfPWV and baPWV. A low HDL-cholesterol level was not related to an increase in the PWV of the central elastic artery, i.e., hfPWV, which could be accounted to a very low correlation coefficient between the HDL-cholesterol level and aortic PWV (r<0.05)25). No association was found between smoking and an increased hfPWV or baPWV; smoking affects largely the PWV of peripheral arteries, such as the femoral-ankle PWV (author's unpublished data). However, by multivariate analysis, none of the components of the MS, except for an elevated BP, was an independent factor affecting hfPWV and baPWV, nor was hypercholesterolemia. It is unclear whether every component of the MS is an independent factor affecting PWV. Regarding obesity, there is controversy as to whether it is associated with an increased aortic PWV24, 26-28). A study by Sutton-Tyrrell et al.29) suggested that abdominal visceral fat is a more important factor affecting aortic PWV than is waist circumference or BMI. There are few studies of the relationship of obesity to baPWV. It is likely that BMI is not an independent variable30), or is only a weak independent variable, for baPWV31). It is also unclear whether other components of the MS, including hypertriglyceridemia and a high fasting glucose level, are independently related to an increased aortic PWV26-28). These components may only weakly affect the baPWV, even if they are independent variables for baPWV31). Therefore, it is conceivable that the individual components of the MS, except for an elevated BP, either do not affect PWV independently or only weakly affect PWV.
PWV increases with an increase in the number of risk factors for atherosclerosis13). We have shown previously that both hfPWV and baPWV increase according to the number of risk factors including: hypertension, obesity, diabetes, hypercholesterolemia, a low HDL-cholesterol level and smoking21). In accordance with the results from our previous study, both hfPWV and baPWV increased with the number of components of the MS. This suggests that a combination of components of the MS is associated with increased PWVs. Since the components of the MS are related to one another, and frequently appear as clustered features, it is necessary to manage the components of the MS together to control increased arterial stiffness.
Age and BP are the main determinants of large artery stiffness23). The prevalence of the MS increases with advancing age4-7). An elevated BP is one of the components of the MS. Since age, BP and gender are important independent variables affecting PWVs, as noted from the present and previous studies, we further analyzed the association of the MS with increased PWVs after controlling for these variables. We found that the MS was associated with an increased baPWV, but not with an increased hfPWV. This suggests that the clustering of the components of the MS may interact synergistically to increase arterial stiffness, even though individual components do not affect arterial stiffness independently. This also suggests that the clustering of the components of the MS may differentially impact arterial stiffness in various arterial regions. Golden et al9) showed that grouping of the insulin resistance syndrome components are associated with excess carotid intima-media thickness (IMT) beyond an additive effect. Scuteri et al32) reported that even after taking into account each component of the MS, the MS was independently associated with increased carotid IMT and stiffness. Thus, our findings are consistent with prior reports9, 32). However, to date, no study has demonstrated an association of the MS with increased PWV at different arterial regions. O'Neal et al.10) suggested that the relative impact of the MS or hyperinsulinemia on arterial structural and functional parameters may be greater in more muscular arteries in the lower limbs than in the more elastic carotid artery, although they did not examine elastic arteries such as the aorta or the carotid artery. Since the present study shows an association of the MS with an increased baPWV, but not with an increased hfPWV, we postulate that the impact of the MS on PWV is greater in peripheral muscular arteries than in central elastic arteries. Additional studies will be required to demonstrate the relative impact of the MS on PWV in different arterial regions.
This study has important limitations. First, the distance for measurement of PWV was calculated automatically from the subject's height and the anthropomorphic data based on the Japanese population. This method has not been validated in the Korean population. Second, before beginning measurements, recommendations to standardize the subject's conditions33) were not followed strictly. Third, BP and PWV may vary according to the time of the day in each subject, although we measured these variables in the morning for each subject.
In spite of these limitations, measurement of PWV as an index of arterial dysfunction may be applicable to subjects with the MS to prevent progression to atherosclerotic CVD. In particular, simply measured baPWV might be useful in clinical practice. Future interventional studies will be required to follow up PWV in subjects with the MS.
In conclusion, the clustering of the components of the MS may interact to synergistically increase arterial stiffness, even though individual components, except for an elevated BP, do not affect arterial stiffness independently.
ACKNOWLEDGMENT
The author thanks Sun-Ok Kang, M.P.H., at Dankook University Hospital for assistance in the data analysis.
References
1. Timar O, Sestier F, Levy E. Metabolic syndrome X: a review. Can J Cardiol 2000;16:779–789PMID : 10863169.

2. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): final report. Circulation 2002;106:3143–3421PMID : 12485966.


3. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433–438PMID : 14744958.


4. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National National Health and Nutrition Examination Survey. JAMA 2002;287:356–359PMID : 11790215.


5. Park JS, Park HD, Yun JW, Jung CH, Lee WY, Kim SW. Prevalence of the metabolic syndrome as defined by NCEP-ATP III among the urban Korean population. Korean J Med 2002;63:290–298.
6. Park SH, Lee WY, Kim SW. The relative risks of the metabolic syndrome defined by adult treatment panel III according to insulin resistance in Korean population. Korean J Med 2003;64:552–560.
7. Lym YL, Hwang SW, Shim HJ, Oh EH, Chang YS, Cho BL. Prevalence and risk factors of the metabolic syndrome as defined by NCEP-ATP III. J Korean Acad Fam Med 2003;24:135–143.
8. Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care 2002;25:1790–1794PMID : 12351479.


9. Golden SH, Folsom AR, Coresh J, Sharrett AR, Szklo M, Brancati F. Risk factor groupings related to insulin resistance and their synergistic effects on subclinical atherosclerosis: the atherosclerosis risk in communities study. Diabetes 2002;51:3069–3076PMID : 12351449.


10. O'Neal DN, Dragicevic G, Rowley KG, Ansari MZ, Balazs N, Jenkins A, Best JD. A cross-sectional study of the effects of type 2 diabetes and other cardiovascular risk factors on structure and function of nonstenotic arteries of the lower limb. Diabetes Care 2003;26:199–205PMID : 12502681.


11. van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke 2001;32:454–460PMID : 11157182.


12. Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol 2001;21:2046–2050PMID : 11742883.


13. Sun K, Daimon M, Watanabe S, Komuro I, Masuda Y. The relation of pulse wave velocity by oscillometric and tonometric methods and clinical application studies. Jpn J Appl Physiol 2002;32:81–86.
14. Kubo T, Miyata M, Minagoe S, Setoyama S, Maruyama I, Tei C. A simple oscillometric technique for determining new indices of arterial distensibility. Hypertens Res 2002;25:351–358PMID : 12135312.


15. Munakata M, Ito N, Nunokawa T, Yoshinaga K. Utility of automated brachial ankle pulse wave velocity measurements in hypertensive patients. Am J Hypertens 2003;16:653–657PMID : 12878371.


16. Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 2003;91:1519–1522PMID : 12804752.


17. Yamashina A, Tomiyama H, Arai T, Hirose K, Koji Y, Hirayama Y, Yamamoto Y, Hori S. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res 2003;26:615–622PMID : 14567500.


18. Yamashina A. In: Ozawa T, Masuda Y, eds. Measurement of pulse wave velocity. Pulse wave velocity. 2002;Tokyo: Medical View, 26–34.
19. WHO West Pacific Region. The Asia-Pacific perspective: refining obesity and its treatment. 2000;2. IOTF.
20. Kimoto E, Shoji T, Shinohara K, Inaba M, Okuno Y, Miki T, Koyama H, Emoto M, Nishizawa Y. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes 2003;52:448–452PMID : 12540620.


21. Kim YK, Lee MY, Rhee MY. A simple oscillometric measurement of pulse wave velocity: comparison with conventional tonometric measurement. Korean J Med 2004;67:597–606.
22. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol 2003;23:554–566PMID : 12615661.


23. Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, Safar M. Influence of age, risk factors and renal disease on arterial stiffness: clinical applications. Am J Hypertens 2002;15:1101–1108PMID : 12460708.


24. Toto-Moukouo JJ, Achimatos A, Asmar RG, Hugues CJ, Safar ME. Pulse wave velocity in patients with obesity and hypertension. Am Heart J 1986;112:136–140PMID : 3728268.


25. Imai Y, Kawazu S. Ozawa T, Masuda Y. Hyperlipidemia obesity. Pulse wave velocity. 2002;Tokyo: Medical View, 56–63.
26. Wildman RP, Mackey RH, Bostom A, Thomson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 2003;42:468–473PMID : 12953016.


27. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Change in arterial stiffness and wave reflection with advancing age in healthy men and women. Hypertension 2004;43:1239–1245PMID : 15123572.


28. Nakanishi N, Susuki K, Tatara K. Clusterd features of the metabolic syndrome and the risk for increased aortic pulse wave velocity in middle-aged Japanese men. Angiology 2003;54:551–559PMID : 14565630.


29. Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension 2001;38:429–433PMID : 11566917.


30. Ohnishi H, Saitoh S, Takagi S, Ohata J, Isobe T, Kikuchi Y, Takeuchi H, Shimamoto K. Pulse wave velocity as an indicator of atherosclerosis in impaired fasting glucose: the Tanno and Sobetsu study. Diabetes Care 2003;26:437–440PMID : 12547876.


31. Tomiyama H, Yamashina A, Arai T, Hirose K, Koji Y, Chikamori T, Hori S, Yamamoto Y, Doba N, Hinohara S. Influence of age and gender on results of noninvasive brachial-ankle pulse wave measurements: a survey of 12517 subjects. Atherosclerosis 2003;166:303–309PMID : 12535743.


Figure 1
Heart-femoral pulse wave velocity (hfPWV) and brachial-ankle pulse wave velocity (baPWV) according to the number of the components of the metabolic syndrome (MS). Values are presented as mean±SD. The p value indicates p for the difference adjusted for age and gender among the groups.

Figure 2
Heart-femoral pulse wave velocity (hfPWV) and brachial- ankle pulse wave velocity (baPWV) by the metabolic syndrome (MS) status. Values are presented as mean±SD. The p value indicates p for the difference adjusted for age, gender and blood pressure between the groups.
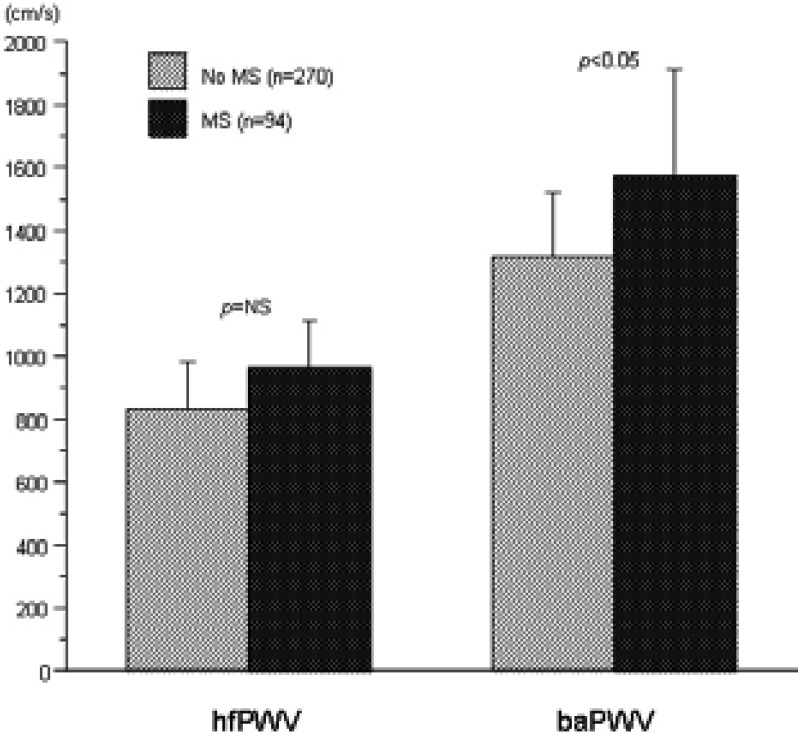
Table 2
Categorical risk factors in subjects

Data are n (%). HDL, high density lipoprotein; MS, metabolic syndrome; Obesity, waist circumference >90 cm (men), >80 cm (women) or body mass index ≥25 kg/m2; Elevated blood pressure (BP), BP ≥130/≥85 mmHg; Impaired fasting glucose, fasting blood glucose 110~125 mg/dL; Hypertriglyceridemia, triglyceride ≥150 mg/dL; Low HDL-cholesterol; HDL-cholesterol <40 mg/dL (men), <50 mg/dL (women); Hypercholesterolemia, total cholesterol ≥220 mg/dL




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



