 |
 |
| Korean J Intern Med > Volume 21(1); 2006 > Article |
|
Abstract
Background
The cardio-ankle vascular index (CAVI) is a newly developed arteriosclerotic measurement that has been proposed as an alternative to aortic pulse-wave velocity (PWV). The present study used the CAVI to identify the main factors associated with arteriosclerosis in continuous ambulatory peritoneal dialysis (CAPD) patients.
Methods
Fifteen CAPD patients were enrolled in the study. The CAVI is independent of the pressure and vascular reflection between the heart valve and the ankle. Serum albumin, uric acid, total calcium, phosphorus, lipid levels, high-sensitivity C-reactive protein and homocysteine concentrations in CAPD patients were measured using standard methods. Total body fat mass, truncal and non-truncal fat mass and lean body mass were measured using dual energy X-ray absorptiometry with a Lunar DPX-L scanner.
Results
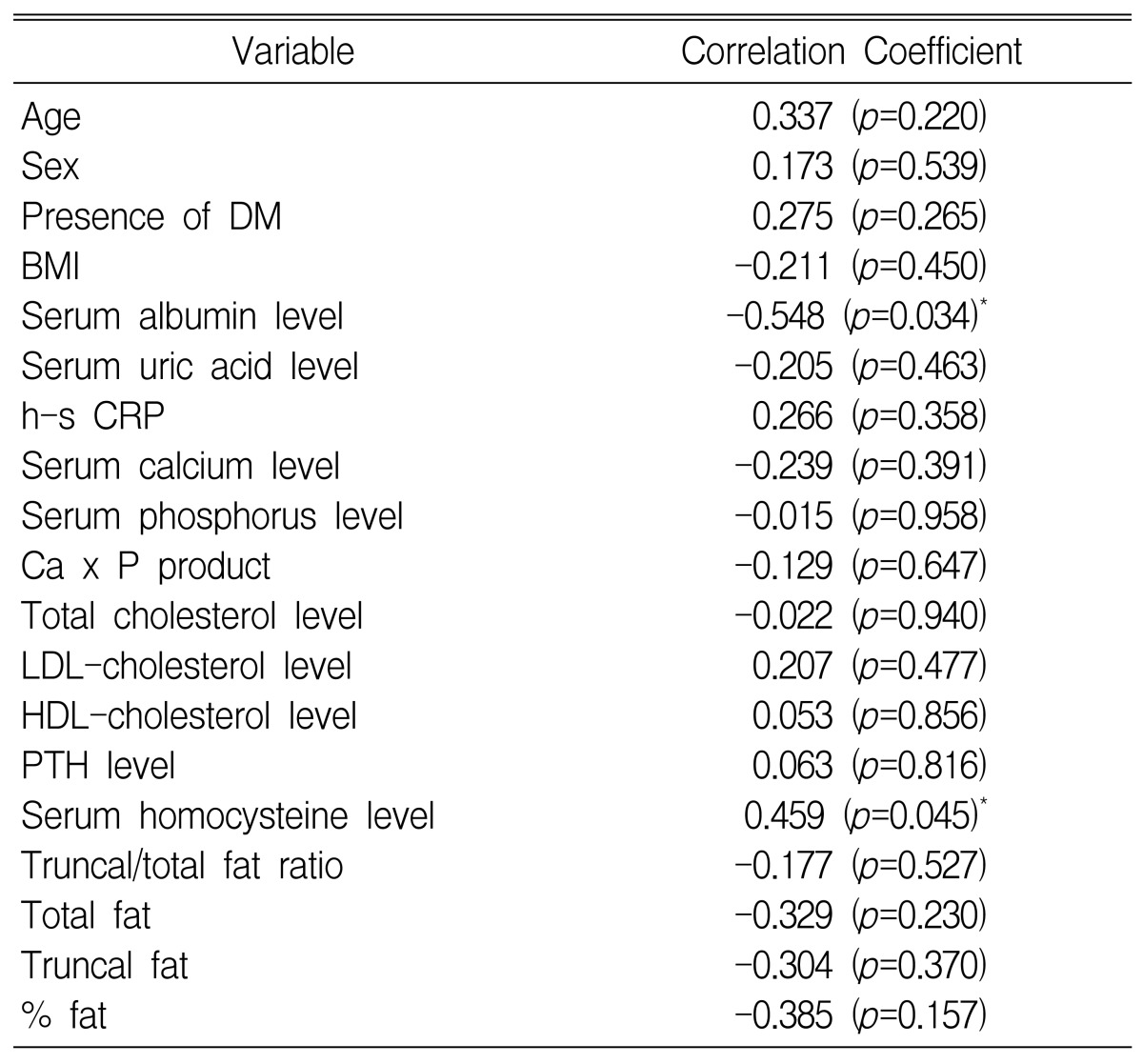
CAPD patients had a mean CAVI of 9.37┬▒3.16 m/sec, which was higher than the general population. The CAVI was negatively correlated with the serum albumin concentration (r=-0.548; p=0.034). Stepwise regression analysis showed that both the serum albumin concentration (╬▓=-0.643, p=0.013) and the serum homocysteine level (╬▓=0.486, p=0.004) were independently associated with the CAVI.
Arterial compliance, an important vessel wall property, reflects the buffering capacity of a vessel. A low arterial compliance caused by arterial stiffening widens pulse pressure amplitude. Increased systolic blood pressure induces left ventricular hypertrophy, whereas decreased diastolic blood pressure impairs coronary blood flow. Wide pulse pressure, which is caused by increased arterial stiffness and early wave reflections, may be a major predictor of cardiac risk, especially coronary mortality1, 2). Recent prospective studies have demonstrated that arterial stiffness and early wave reflections are independent predictors of all-cause and cardiovascular mortality in end-stage renal disease (ESRD) patients and in the general population3, 4)
Arterial wall properties in ESRD patients may be influenced by many factors, including non-specific factors such as age, gender, smoking, blood pressure and diabetes, as well as alterations more specific to ESRD, such as fluid overload, increased endothelin-1, hyperphosphatemia, increased parathyroid hormone activity, increased ADMA concentration, micro-inflammation and blood lipid alterations5). Previous studies have shown that hyperhomocysteinemia is an independent risk factor for an adverse cardiovascular outcome in ESRD6, 7). However, there have been inconsistent reports about the relationship between hyperhomocysteinemia and arterial stiffness in ESRD patients8-10). Using a different method for evaluating arterial stiffness, we previously reported that hypoalbuminemia was related to carotid artery compliance in CAPD patients11). In addition, the relationship between truncal fat mass, IL-6 and high-sensitivity CRP indicates that a chronic inflammatory response, partly related to truncal fat mass, is an important contributor to the atherogenic lipoprotein profile in patients with uremia12). Despite these observations, there have been few reports concerning the relationship between hyperhomocysteinemia, hypoalbuminemia, truncal fat mass and arterial stiffness in patients undergoing continuous ambulatory peritoneal dialysis (CAPD).
While aortic pulse-wave velocity (aPWV) measurement is a useful and standard approach for diagnosing arterial stiffness in any part of the body13, 14), this technique requires a particularly skilled medical technologist for carotid artery measurements. In addition, results are reproducible only if the machinery used is of a very high standard. Therefore, a new arteriosclerotic measurement, the cardio-ankle vascular index (CAVI), has been designed and is being used, particularly in some Japanese clinics15). It is acknowleged that CAVI is compatible with aortic pulse wave velocity16). Brachial artery pulse wave velocity (baPWV) is influenced by blood pressure, but with baPWV, blood pressure compensation is not carried out. Furthermore, the pulse pressure is measured by air pressure15). Therefore any stimulus that exerts pressure on an artery may influence these results. A merit of using CAVI as a measurement of the pressure wave form is its independence from blood pressure16).
The aim of this present study was to use CAVI to identify the main factors affecting arterial stiffness in CAPD patients.
Fifteen CAPD patients were enrolled, and their characteristics are given in Table 1. The group was composed of 7 men and 8 women, with a mean age of 46.1┬▒12.6 years and a mean time receiving CAPD of 35 months (range: 4-120 months). Individuals with the following medical conditions were excluded; cardiac failure (New York Heart Association [NYHA] class II and higher), unstable angina pectoris (NYHA class II and higher), peripheral vascular disease, peripheral pitting edema and pulmonary edema, greater than stage 3 hypertension (systolic blood pressure > 180 mmHg or diastolic blood pressure > 110 mmHg), established infection, intact parathyroid hormone levels > 200 pg/mL, serum total calcium levels > 11 mg/dL or serum phosphate levels > 7 mg/dL. In 6 patients, the primary kidney disease was related to diabetes mellitus, while disease was not diabetes-related in 9 patients.
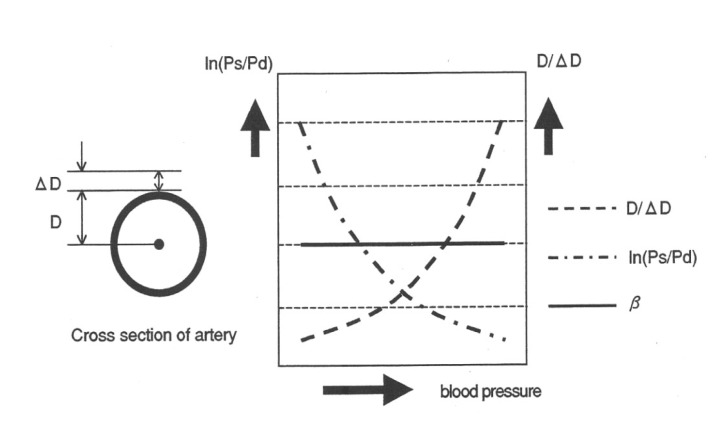
The CAVI is a new arteriosclerotic measurement that is independent of pressure and vascular reflections between the heart valve and the ankle. It is an index for evaluating arterial stiffness; the greater the CAVI, the stiffer the arterial wall. Briefly, in order to calculate the CAVI, the pulse transmission time from the aortic valve to the ankle, and the vascular distance between the heart valve and the ankle artery, are measured. The PWV from the aortic valve to the ankle artery is then calculated using the following equation: PWV = vascular distance between the heart valve and the ankle artery / pulse transmission time from the aortic valve to the ankle.
The CAVI is a pressure-independent index based on the stiffness parameter, and is illustrated in Figure 1, where Ln(Ps/Pd) = the logarithmic pulse pressure (Ps: systolic pressure, Pd: diastolic pressure), and D/D = the reciprocal of the radius percentage change (D: Vascular radius, D: beat-initiated variation in the vascular radius).
As illustrated in Figure 1, increased blood pressure exponentially increases the reciprocal of the percentage change in the radius, while the logarithmic pulse pressure exponentially decreases. Thus, the product of the logarithmic pulse pressure and the reciprocal of the radius percentage change is independent of blood pressure and remains constant. ECG, phonocardiograph, and pressures and waveforms of brachial and ankle arteries were measured and cardio-ankle pulse wave velocity and subsequent CAVI were automatically calculated by CAVI-Vasera (Fukuda Denshi, Tokyo, Japan). The CAVI were calculated as follows:
β = (ln Ps/Pd) (D/ΔD)
D/╬öD = 1/k2 ├Ś PWV2
CAVI = β = (ln Ps/Pd) (D/ΔD) = 1/k2 (ln Ps/Pd) PWV2
After 15 minutes of supine rest, CAVI measurements (15) in this study were obtained in 5 time-continuous measurements by a single observer. A mean value was used as a score. Patients were asked to not smoke, eat or drink for 4 hours prior to CAVI measurements, and were allowed to take their regular medications. Nine of 15 patients were taking anti-hypertensive drugs (calcium channel blockers, ACE inhibitors, beta-blockers, alpha-blockers). Doses of anti-hypertensive or other vasoactive drugs (e.g. calcium-containing phosphorus binders) remained constant during the 1 month period preceding measurements.
Blood phosphorus and total calcium levels were measured in patients using an Hitachi 747 automatic analyzer, with normal ranges considered as being 1.9-4.4 mg/dL and 8.2-10.4 mg/dL, respectively. Intact parathyroid hormone was measured using a commercial immunoradiometric assay (Diagnostic Products Corporation, Los Angeles, CA, USA), with the normal range considered to be 12-72 pg/mL. Serum albumin was measured using an Hitachi 736-740 autoanalyzer (Hitachi Ltd., Tokyo, Japan), and high-sensitivity C-reactive protein was measured using the N High Sensitivity CRP kit (Dade Behring, Marburg, Germany) which has a lower limit of measurement of 0.15 mg/L. Serum total cholesterol, LDL-cholesterol, HDL-cholesterol and triglyceride levels were measured enzymatically using an Hitachi 736-740 autoanalyzer. Total homocysteine was determined using high-performance liquid chromatography according to Ubbink et al8). Total body fat mass, truncal and non-truncal fat mass, and lean body mass were analyzed using dual energy x-ray absorptiometry (DEXA) with a Lunar DPX-L scanner (Lunar Corp, Madison, WI, U.S.A.)
Statistical analyses were performed using the Mann-Whitney U test (SPSS Windows, version 9.0). Relationships between different parameters were assessed using the Spearman rank correlation coefficient. A stepwise regression analysis was performed to assess the contribution of separate variables to the CAVI. Results are expressed as meanSD or median (range). Differences were considered statistically significant if the associated p value was less than 0.05.
We found that CAPD patients had a mean CAVI of 9.37┬▒3.16 m/sec, which was higher than the 7.10┬▒0.50 m/sec observed in the general population15) (Table 1).
In the patient group, systolic blood pressure, diastolic blood pressure, mean arterial pressure and brachial artery pulse pressure were 162┬▒18 mmHg, 98┬▒10 mmHg, 130┬▒20 mmHg and 67┬▒24 mmHg, respectively (Table 1). The serum albumin, uric acid, calcium, phosphorus, Ca x P product, total cholesterol, LDL-cholesterol, high-sensitivity CRP and homocysteine levels were 3.5┬▒0.4 gm/dL, 6.5┬▒1.2 mg/dL, 8.8┬▒1.0 mg/dL, 5.0┬▒1.2 mg/dL, 43.0┬▒10.0 mg/dL, 209.9┬▒49.9 gm/dL, 113.1┬▒28.8 gm/dL, 1.9┬▒1.9 mg/dL and 25.3┬▒5.6 mol/dL, respectively. The truncal/total fat ratio average was 0.56┬▒0.05 (Table 1).
Analysis of these data showed that the CAVI was negatively correlated with serum albumin concentration (r=-0.548; p=0.034) (Figure 2, Table 2). Stepwise regression analysis showed that both serum albumin concentration (╬▓=-0.643, p=0.013) and serum homocysteine level (╬▓=0.486, p=0.004) were independently associated with changes in the CAVI (Table 3).
The present study showed that the CAVI was higher in CAPD patients than in the general population, and that changes in the CAVI were independently associated with both hypoalbuminemia and hyperhomocysteinemia.
It is generally accepted that the serum albumin level is associated with atherosclerosis, and that hypoalbuminemia is a predictor of vascular events and cardiovascular mortality in ESRD patients17, 18). Traditionally, many authors have believed that hypoalbuminemia in ESRD patients was due to inflammation or malnutrition17). In addition, there has been a tendency to link poor nutrition, serum albumin levels and atherosclerosis with processes causing inflammation17). However, the effect and influence of hypoalbuminemia per se on functional changes, such as arterial compliance and large vessel stiffness, have not been widely studied. One recent report18) showed that only hypoalbuminemia was predictive of vascular morbidity, regardless of nutritional status. That study suggested that hypoalbuminemia itself was pathogenically associated with vascular disease, but dissociated this effect from overall protein malnutrition18). Our previous report suggested that common carotid artery compliance was positively correlated with serum albumin concentration, and that common carotid artery intima-media thickness was negatively correlated with serum albumin levels. In addition, serum albumin concentration was independently linked to common carotid artery compliance in that report11). In the present study, we excluded patients with severe malnutrition, as well as those with a level of established infection who showed a high-sensitivity CRP concentration greater than that observed in normal controls. The data presented in this current study show no relationship between serum high sensitivity C-reactive protein levels and the CAVI in CAPD patients. However, the CAVI was independently associated with hypoalbuminemia, independent of CRP-related inflammatory status. This finding suggests that the serum albumin level itself is related to changes in arterial stiffness. The mechanism by which hypoalbuminemia may cause increased arterial stiffness has not been elucidated. One scenario may be that hypoalbuminemia causes a hypercoagulable state19) which depletes L-arginine stores and subsequently reduces nitric oxide production19), thus affecting the mechanisms by which nitric oxide causes equipotent relaxation of arterial smooth muscle20) and inhibits platelet aggregation21). This hypothesis remains untested, and future investigations will be required in order to fully understand the relationship between hypoalbuminemia and arterial stiffness.
In ESRD patients, hyperhomocysteinemia occurs more frequently than any other conventional risk factor22). However, the belief that the total homocysteine level (tHcy) is an independent risk factor for atherothrombotic disease in ESRD patients may deserve further scrutiny. Although several prospective studies showed an association between high tHcy concentration and atherosclerosis in ESRD patients6, 7), a recent study failed to show a causal relationship when using surrogate end points for atherosclerosis and thrombosis23). In terms of arterial stiffness in ESRD patients, similar confounding results have been reported. van Guldener et al. reported that although plasma homocysteine levels decreased after treatment with folic acid, no significant changes occurred in compliance, distensibility coefficients or the stiffness index of the common carotid artery8). In addition, Smilde et al. concluded that homocysteine did not explain a variation in intima-media thickness and only marginally contributed to the variation in distensibility coefficients in the common carotid artery and in compliance coefficients in the common femoral artery9). In contrast, some studies found lower-limb PWV was positively and independently correlated with plasma total homocysteine10) and plasma endothelin, and that acute hyperhomocysteinemia induced a reduction in arterial distensibility and compliance24). In our present study, the CAVI as a parameter of arterial stiffness was independently associated with serum homocysteine levels. The role of hyperhomocysteinemia in atherosclerosis and increased arterial stiffness is not fully known, although harmful effects of homocysteine on vascular endothelium25), interactions with other atherogenic factors (e.g. within the redox thiol system or with other cardiovascular risk factors23)) and a pivotal role for oxidative stress are suggested to be involved23). Serum homocysteine levels may be altered by serum albumin concentration. Vychytil et al. found that levels of the free form of homocysteine were significantly greater in peritoneal dialysis patients with lower serum albumin levels than those with higher serum albumin levels26). The present data are consistent with the possibility that combined hypoalbuminemia and hyperhomocysteinemia synergistically act to increase arterial stiffness.
It has become increasingly evident that fat tissue is an active endocrine organ that may produce several adipokines, including proinflammatory substances such as IL-1, TNF and plasminogen activator inhibitor type 127). Recently, a relationship between body fat mass and inflammatory markers from fat tissue was proposed as a contributor to inflammation in CAPD patients28). Increased body fat mass is a common finding in patients commencing PD using glucose-based solutions, and it may become excessive in some patients29). Axelsson et al. suggested that truncal adiposity, IL-6 and high-sensitivity CRP levels were well correlated with the extent of truncal adiposity12). Microinflammation is an independent risk factor for increased aortic PWV30). However, our present results show that no significant relationship between truncal fat mass and the CAVI.
The design of the present study (cross-sectional) meant that it was unable to address whether hypoalbuminemia or hyperhomocysteinemia per se reflect vascular events and mortality in CAPD patients. Also, the small number of patients enrolled limits the statistical power of the study. In another limitation of this study, it is known that CAVI differs depending on whether or not arteriosclerosis is present in the carotid artery. However, we did not evaluate the carotid artery status. Therefore, to further explore the relationship between serum albumin and homocysteine levels and arterial stiffness in CAPD patients, future studies ideally will have larger and more homogenous subject groups, compared to our present study. While this study did not examine patient volume status using reliable and objective methods, CAPD patients have a relatively stable volume status compared to hemodialysis patients, and this study excluded patients with peripheral pitting edema and pulmonary edema. Therefore, we believe the results obtained in this study were not influenced by volume status of the patients.
In conclusion, this present study shows that the CAVI is independently associated with serum albumin and homocysteine levels, suggesting that independent of other factors, hypoalbuminemia and hyperhomocysteinemia may increase arterial stiffness in CAPD patients.
References
1. Lang RM, Fellner SK, Neumann A, Bushinsky DA, Borow KM. Left ventricular contractility varies directly with blood ionized calcium. Ann Intern Med 1988;108:524ŌĆō529PMID : 3248127.


2. Fang J, Madganan S, Cohen H, Alderman MH. Measures of blood pressure and myocardial infarction in treated hypertensive patients. J Hypertens 1995;13:413ŌĆō419PMID : 7629401.


3. Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on survival in end-stage renal disease. Circulation 1999;99:2434ŌĆō2439PMID : 10318666.


4. London GM, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME. Arterial wave refections and survival in end-stage renal failure. Hypertension 2001;38:434ŌĆō438PMID : 11566918.


5. London GM. Cardiovascular disease in chronic renal failure: pathophysiologic aspects. Semin Dial 2003;16:85ŌĆō94PMID : 12641870.


6. Moustapha A, Naso A, Nahlawi M, Gupta A, Arheart KL, Jacobsen DW, Robinson K, Dennis VW. Prospective study of hyperhomocysteinemia as an adverse cardiovascular risk factor in end-stage renal disease. Circulation 1998;97:138ŌĆō141PMID : 9445164.


7. Mallamaci F, Zoccali C, Tripepi G, Fermo I, Benedetto FA, Cataliotti A, Bellanuova I, Malatino LS, Soldarini A. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int 2002;61:609ŌĆō614PMID : 11849403.


8. van Guldener C, Lambert J, ter Wee PM, Donker AJ, Stehouwer CD. Carotid artery stiffness in patients with end-stage renal disease: no effect of long-term homocysteine-lowering therapy. Clin Nephrol 2000;53:33ŌĆō41PMID : 10661480.

9. Smilde TJ, van den Berkmortel FW, Boers GH, Wollersheim H, de Boo T, van Langen H, Stalenhoef AF. Carotid and femoral artery wall thickness and stiffness in patients at risk for cardiovascular disease, with special emphasis on hyperhomocysteinemia. Arterioscler Thromb Vasc Biol 1998;18:1958ŌĆō1963PMID : 9848890.


10. Blacher J, Demuth K, Guerin AP, Safar ME, Moatti N, London GM. Influence of biochemical alterations on arterial stiffness in patients with end-stage renal disease. Arterioscler Thromb Vasc Biol 1998;18:535ŌĆō541PMID : 9555858.


11. Oh DJ, Lee KJ. The relation between hypoalbuminemia and compliance and intima-media thickness of carotid artery in continuous ambulatory peritoneal dialysis patients. J Korean Med Sci 2005;20:70ŌĆō74PMID : 15716606.



12. Axelsson J, Rashid Qureshi A, Suliman ME, Honda H, Pecoits-Filho R, Heimburger O, Lindholm B, Cederholm T, Stenvinkel P. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr 2004;80:1222ŌĆō1229PMID : 15531669.


13. Schimmler W, Hooffacker M. Spontaneous changes in blood pressure and aortic pulse wave velocity in normotensive subjects (results of a long-term study in 183 men). Basic Res Cardiol 1975;70:521ŌĆō530PMID : 1203046.


14. Bercu BB, Haupt R, Johnsonbaugh R, Rodbard D. The pulse wave arrival time (QKd interval) in normal children. J Pediatr 1979;95:716ŌĆō721PMID : 490238.


15. Yambe T, Yoshizawa M, Saijo Y, Yamaguchi T, Shibata M, Konno S, Nitta S, Kuwayama T. Brachio-ankle pulse wave velocity and cardio-ankle vascular index. Biomed Pharmacother 2004;58(Suppl 1):S95ŌĆōS98PMID : 15754845.


16. Wakabayashi I, Masuda H. Association of acute-phase reactants with arterial stiffness in patients with type 2 diabetes mellitus. Clin Chim Acta 2006;365:230ŌĆō235PMID : 16199026.


17. Beddhu S, Kaysen GA, Yan G, Sarnak M, Agodoa L, Ornt D, Cheung AK. Association of serum albumin and atherosclerosis in chronic hemodialysis patients. Am J Kidney Dis 2002;40:721ŌĆō727PMID : 12324906.


18. Cooper BA, Penne EL, Barlett LH, Pollock CA. Protein malnutrition and hypoalbuminemia as predictors of vascular events and mortality in ESRD. Am J Kidney Dis 2004;43:61ŌĆō66PMID : 14712428.


19. Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and ,ortality in end-stage renal disease. J Am Soc Nephrol 1996;7:728ŌĆō736PMID : 8738808.


20. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biologic activity of endothelium-derived relaxing factor. Nature 1987;327:524ŌĆō526PMID : 3495737.


21. Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987;2:1057ŌĆō1058PMID : 2889967.


22. Bostom AG, Lathrop L. Hyperhomocysteinemia in end-stage renal disease: prevalence, etiology and potential relationship to arteriosclerotic outcome. Kidney Int 1997;52:10ŌĆō20PMID : 9211341.


23. Suliman ME, Stenvinkel P, Barany P, Heimburger O, Anderstam B, Lindholm B. influence of hypoalbuminemia, malnutrition, inflammation, and diabetes mellitus. Am J Kidney Dis 2003;41(3 Suppl 1):S89ŌĆōS95PMID : 12612961.


24. Arcaro G, Fava C, Dagradi R, Faccini G, Gaino S, Degan M, Lechi C, Lechi A, Minuz P. Acute hyperhomocysteinemia induces a reduction in arterial distensibility and compliance. J Hypertens 2004;22:775ŌĆō781PMID : 15126920.


25. Sydow K, Hornig B, Arakawa N, Bode-Boger SM, Tsikas D, Munzel T, Boger RH. Endothelial dysfunction in patients with peripheral arterial disease and chronic hyperhomocysteinemia: potential role of ADMA. Vasc Med 2004;9:93ŌĆō101PMID : 15521698.


26. Vychytil A, Fodinger M, Papagiannopoulos M, Wolfl G, Horl WH, Sunder-Plassmann G. Peritoneal elimination of homocysteine moieties in continuous ambulatory peritoneal dialysis patients. Kidney Int 1999;55:2054ŌĆō2061PMID : 10231471.


27. Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology 2003;144:2195ŌĆō2200PMID : 12746274.


28. Suliman M, Axelsson J, Heimburger O, Quershi AR, barany P, Pecoits-Filho R. Relationship between circulating levels of interleukin-6 and fat mass in patients with end-stage renal disease [Abstract]. J Am Soc Nephrol 2003;14:530A. PMID : 12538755.


29. Nordfors L, Heimburger O, Lonnqvist F, Lindholm B, Helmrich J, Schalling M, Stenvinkel P. Fat tissue accumulation during peritoneal dialysis is associated with a polymorphism in uncoupling protein 2. Kidney Int 2000;57:1713ŌĆō1719PMID : 10760107.


30. Stompor T, Rajzer M, Sulowicz W, Dembinska-Kiec A, Janda K, Kawecka-Jaszcz K, Wojcik K, Tabor B, Zdzienicka A, Janusz-Grzybowska E. An association between aortic pulse wave velocity, blood pressure and chronic inflammation in ESRD patients on peritoneal dialysis. Int J Artif Organs 2003;26:188ŌĆō195PMID : 12703883.


Table┬Ā3
Stepwise regression analysis with the CAVI as a dependent variable

Selected variables: age, sex, presence of diabetes mellitus, serum albumin concentration, high-sensitivity C-reactive protein level, serum uric acid level, serum total calcium and phosphorus concentrations, calcium x phosphorus product, total/HDL cholesterol, LDL/HDL cholesterol, parathyroid hormone level, body mass index, serum homocysteine level, truncal/total fat ratio, total fat, truncal fat, % fat.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



