INTRODUCTION
Phosgene (COCl2) is a colorless oxidant gas which is heavier than air and a lethal exposure dose (LC50) in humans is 500 ppm/min. The gas is only slightly soluble in water and the small amount of phosgene which dissolves is immediately hydrolyzed1). It was originally manufactured as an agent for chemical warfare but is now widely used in the synthesis of chemicals and plastics2ŌĆō6).
In an experimental model of phosgene exposure7), profound lung injury was apparent in 30 min after exposure and, four hours after, histological section showed disruption of alveolar septae and marked interstitial edema. Pulmonary edema following phosgene inhalation appears to develop in three phases8): (a) after an initial injury to the lung, permeability of the air-blood barrier to water increases: (b) a subsequent increase in movement of extravascular fluid: (c) finally, there is a significant increase in extravascular fluid volume (interstitial and alveolar). At present, there, is no specific antidote against phosgene. The early use of glucocorticoids in high doses, administration of oxygen combined with positive end-respiratory pressure, antibiotics and physical rest can be recommended9). Except for some severe cases of acute respiratory distress syndrome (ARDS), the vast majority of phosgene intoxication, including cases with pulmonary edema, have a good prognosis. However, complete recovery from phosgene poisoning may require up to several years10).
We report on six patients with phosgene inhalation injury with a review of the literature.
CASE REPORT
On September 8, 1994 at 15 : 40, fifty-six persons were injured by toxic chemical inhalation. A 400 kg of gas mixture (TDI, toluence, HCl and phosgene) bursted out from the break of a pipe line in a chemical factory located in Yeochon, Korea. At the time of the accident, the atmospheric temperature was 21.1┬░C and there was a wind of about 3m/sec from the south. Six of the victims were transferred to Chonnam University Hospital (CNUH) and the rest were treated in nearby hospitals. Of all, 3 victims, including one who was transferred to CNUH, died of acute respiratory failure.
Case 1
A 25 year-old female presented herself to a local clinic because of exposure to phogene. She had been in excellent health until September 8, 1995, when she was exposed to phosgene. She complained of coughing, chest tightness and dyspnea which were not relieved despite therapy with oxygen. She was transferred to CNUH about six hours after exposure.
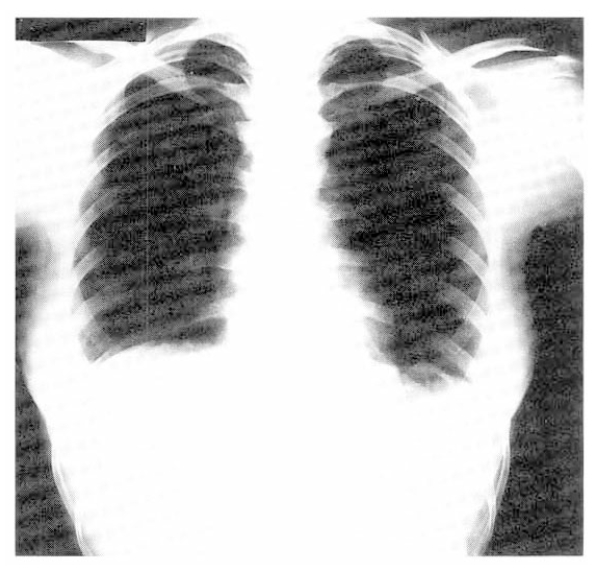
Admitting vital signs were blood pressure 100/60 mmHg. pulse 110/min, respiration 20/min, temperature 36.4┬░C. Examination of the chest revealed bilateral inspiratory and expiratory crackles. The chest X-ray showed diffuse increased haziness on both lung field (Fig. 1). The WBC were 12.7 ├Ś 103/╬╝l, the LDH 409 unit, initial arterial blood gases determinations on room air were, PaO2, 68.9 mmHg, PaCO2, 34.7 mmHg, pH, 7.392.
The patient was treated with a face mask with 8 L/min oxygen. On the second day of admission, she was transferred to the ICU with respiratory insufficiency. She had progressively increasing respiratory symptoms with the respiratory rate rising to 44/min, pulse rate to 114/min and blood pressure decreasing 70/40 mmHg. The WBC were 25.2 ├Ś 103/╬╝l with 95.6% neutrophils and LDH was 665 unit. The arterial blood gases were PaO2, 41.2 mmHg, PaCO2 34.9 mmHg, pH, 7.364. The patient was intubated and ventilated with controlled mode and 8.0 cmH2O of positive-end expiratory pressure (PEEP) was applied. Two hours after ventilation, the patient became cyanotic and oxygen saturation decreased to 60% and a chest X-ray showed pneumothorax on the right thoracic cavity. Chest tube was inserted with a water seal bottle. Five hours after controlled ventilation, arterial blood gases on FiO2 0.6 were PaO2, 60.4 mmHg, PaCO2, 31.3 mmHg, pH, 7.520. Ventilation was controlled with diazepam and vecuronium to reduce anxiety and fighting.
On the third day of ventilation, she was weaned from the ventilator and extubated. The patient received methylprednisolone (500 mg) for 7 days, 2 days of furosemide (120 mg), aminophylline, dopamine and antibiotics from the day of admission.
Case 2
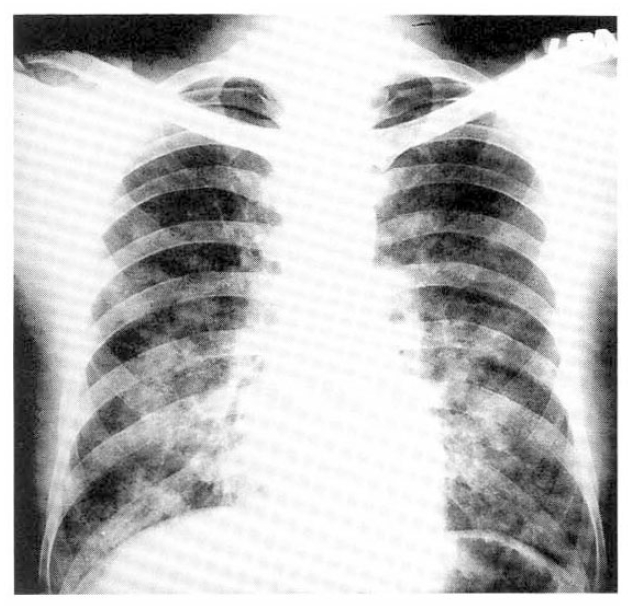
This 37 year-old male was admitted to CNUH about ten hours after phosgene exposure. Physical findings on admission were blood pressure 110/70 mmHg, pulse 154/min, respiration 30/min, temperature 36.4┬░C. Examination of the chest X-ray revealed bilateral diffuse pulmonary edema (Fig. 3). Initial blood gas determinations on room air were, PaO2, 61.8 mmHg, PaCO2, 37.6 mmHg, pH, 7.356.
Ten minutes later, he developed tachycardia and tachypnea despite therapy with oxygen and aminophylline. The arterial blood gases on 10 L/min oxygen were PaO2, 51.9 mmHg, PaCO2, 35.9 mmHg, pH, 7.317. The patient was immediately intubated and ventilated with controlled mode. Arterial blood gases were PaO2, 45.7 mmHg, PaCO2, 40.4 mmHg, pH, 7.301. The blood pressure was 80/50 mmHg. The patient was given fresh frozen plasma, as well as crystalloid solutions and dopamine to stabilize the circulation. After fluid administration, the arterial blood gases were PaO2, 35.2 mmHg, PaCO2, 61.5 mmHg, pH, 7.144. Metabolic acidosis was treated with NaHCO3, as required. However the patient had a progressive metabolic acidosis despite controlled ventilation and fluid administration. The patient died of uncontrolled hypoxemia.
Case 3
The patient was a 30 year-old male who was exposed to phosgene twelve hours prior to admission to a local clinic complaining of dyspnea, coughing and sputum. Auscultation of the chest revealed coarse breath sound with crackles all over the lung fields. A chest X-ray showed bilateral diffuse interstitial infiltration. The WBC were 12.3 ├Ś 103/╬╝l. Arterial blood gases were PaO2, 50.2 mmHg, PaCO2, 37.2 mmHg, pH, 7.401. The patient was transferred to CNUH with worsening dyspnea. The vital signs on admission were blood pressure 130/70 mmHg, pulse 89/min, respiration 28/min and temperature 36.5┬░C. The WBC were 18.0 ├Ś 103/╬╝l with 98% neutrophils. The LDH was 350 unit. Initial blood gas determinations on room air were, PaO2, 55.3 mmHg, PaCO2, 26.7 mmHg. pH, 7.477. Twelve hours after admission, the patient began to have progressively increasing respiratory symptoms with the respiratory rate rising to 55/min. The patient was intubated and ventilated with controlled mode. Ventilation was maintained with diazepam and vecuronium and treated with methylprednisolone and aminophylline. Three hours after ventilation, the fraction of inspired oxygen could be reduced to 0.6. After 24 hours of ventilation with 6 cmH2O PEEP, he developed subcutaneous emphysema extending into the neck and anterior chest. The PEEP could be reduced and subcutaneous emphysema and arterial oxygenation did not get-worse. On the third day, the patient could be weaned from the ventilator and extubated. The patient was treated with methylprednisolone, aminophylline and antibiotics. Pulmonary function tests were performed on the fifth day following exposure and showed a restrictive pattern and a normal diffusion capacity (Table 1).
The three other patients exposed to phosgene were transferred to CNUH and all were in their early forties. Their complaints were also shortness of breath, chest tightness without derangements of vital signs and arterial blood gases. The pertinent findings in the physical examination were bilateral crackles over the lung fields and diffuse bilateral interstitial infiltrates on chest X-ray.
They were treated with supplemental oxygen by external devices, methylprednisolone, aminophylline and antibiotics. Their symptoms were gradually improved in 3 to 5 days of supportive care.
DISCUSSION
Most accidental exposures to phosgene occur during the synthesis of isocyanate, polyurethane resins, dyes and intermediary products used in the pharmaceutical industry. In addition, phosgene may be generated by the thermal decomposition of chlorinated hydrocarbons, as in the cases of poisoning due to chemical paint removers containing methylene chloride which were converted to phosgene after heat exposure3,4). It may also be generated by decomposition of trichloroethylene due to argon-shielded electronic are welding in an atmosphere containing trichloroethylene5) and as a result of activation of a smoke grenade6).
Phosgene inhalation is slowly hydrolyzed to hydrochloric acid and carbon dioxide in 2 to 24 hours. Phosgene appears to cause pulmonary edema by increasing pulmonary vascular permeability rather than by affecting vascular pressure7). Also, it was demonstrated that it produces an emphysematous change in animals exposed to high concentration of phosgene11).
The clinical presentations include initial smarting and watering of the eyes, scratching and reddening of the throat, a dry cough and retrosternal and epigastric sensations of pressure which indicates the inhalation of phosgene concentrations of more than 3 ppm. The cause of these symptoms is probably due to a small degree of hydrolysis of the more highly concentrated phosgene on the moist mucous membranes of the eyes and upper respiratory tract12). Severe mucous membrane irritation in pure phosgene exposure indicates serious toxic phosgene levels. The lung morphology observed after inhalation of phosgene are similar to those observed after oxidant gas exposure. This includes a clinical latent period followed by noncardiogenic pulmonary edema and death at some time after a significant concentration by the time product is attained1). Delayed pulmonary edema with clinical manifestations of dyspnea, cough, chest pain, hemoptysis and cyanosis may occur 2 to 24 hours after exposure. With a moderate phosgene dose, the average clinical latent period lasts 6 to 15 hours13). Our patients reported initial symptoms of coughing, sputum, dyspnea, chest tightness and chest pain followed by pulmonary edema within 12 hours after exposure.
For detecting and following the course of injury from phosgene inhalation, the conventional chest radiography is still perhaps the best non-invasive clinical method in spite of its limited sensitivity. The availability of baseline or pre-exposure films from those exposed to phosgene or other toxic inhalants would enhance the sensitivity of this method8). At the present time, the following parameters can be recommended for early diagnosis of phosgene overexposure12) : (1) Phosgene indicator paper badges to be worn by all persons involved in handling phosgene : (2) Observation of the initial irritative symptoms of the eye and the upper respiratory tract after phosgene inhalation can provide a rough indication of the inhalation concentration and dose : (3) X-ray photographs of the lungs make it possible to detect incipient toxic pulmonary edema at an early stage, during the clinical latent period : (4) A number of additional parameters require further clinical investigation : serum LDH, respiratory parameters (base excess, peak flow, compliance, diffusion capacity), transthoracic electrical impedance.
Among the pulmonary function parameters measured, diffusion capacity was the most sensitive indicator of lung damage after phosgene inhalation. Ghio et al.14) suggested that, as a consequence of phosgene inhalation, lung volumes (total lung capactiy and vital capacity) and diffusing capacity for carbon monoxide (DLco) declined by 24 hours after exposure, while airway function remained unaffected. Differential cell counts on bronchoalveolar lavage fluid, recruited from rats exposed to phosgene, have shown 60 to 70% of polymorphonuclear leukocytes two to three days after exposure15). Twenty four hours after exposure, percent neutrophils, protein, TBA (thiobarbituric acid)-reactive products and leukotriene B4 were all elevated in lavage fluid14).
For the treatment of phosgene poisoning, glucocorticoids and positive pressure ventilation can be recommended, as well as supportive measured such as physical rest, antitussive, buffer solution, sedatives, antibiotics, antispasmodics and possible diuretics16). A vigorous program of diuresis is used to treat the pulmonary edema. Administration of furosemide to produce a negative fluid balance varied from ŌłÆ2 to ŌłÆ5 liters and correlated of well with the reduction in oxygen requirement17). It was demonstrated that dibutyryl cAMP, aminophylline and ╬▓-adrenergic agonists terbutaline and isoproterenol markedly reduced the pulmonary edema caused by phosgene7). Ghio et al.14) showed that cyclophosphamide, 5-lipoxygenase inhibitor AA861 and colchicine also significantly decreased mortality from phosgene. The dose-response study of phosgene and clochicine indicated a beneficial effect of colchicine at doses as low as 0.1 mg/kg. Indomethacine was recently proved to reduce the thromboxane and prostacyclin synthesis without affecting the increase in leukotriene synthesis caused by phosgene18).
If the patient survives, the poisoning, clinical and radiographic edema usually regress within a few days. Blood gases and carbon monoxide diffusion capacity usually normalize within a week. However, exertional dyspnea and increased bronchial resistance may persist for several months1). Complete recovery after phosgene poisoning may require up to several years10).
There are several areas of possible concern from our observations. Firstly, in our two cases, pneumothorax and subcutaneous emphysema were developed in the absence of significant PEEP or fighting against the ventilator. In a previous study11), animals exposed to phosgene had acute bronchiolitis and peribrochiolitis involving terminal and respiratory bronchioles. The epithelium was generally denuded, and there were scattered areas of peripheral atelectasis and moderate over-expansion of terminal air spaces. This explains the liability to develop mediastinal emphysema and in turn, pneumothorax in phosgene lung injury. Therefore, it would be advisable to watch for the evidence of barotrauma which can be avoidable with judicious use of PEEP and sedatives. Secondly, as in the second case presented, it is possible that the pulmonary edema had been aggravated with the use of fresh frozen plasma which was introduced in an attempt to restore the circulatory volume. However, reflecting on the mechanism of pulmonary edema in phosgene poisoning, volume expanders might have worsed the pulmonary edema and gas exchange.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





