 |
 |
| Korean J Intern Med > Volume 1(1); 1986 > Article |
|
Abstract
Antibodies against thyroxine (T4) were detected in a patient of systemic lupus erythematosus associated with chronic thyroiditis and a patient with primary myxedema. Both patients were clincally hypothyroid with elevated serum TSH.
Serum T4 values measured with solid phase radioimmunoassay were very high (over 25ug/dl), but were undetected with polyethylene glycol separation method. On the extraction of the sera with ethanol, the concordant low values of T4 were obtained with both radioimmunoassay methods and these values were compatible with clinical findings and serum TSH levels.
Major portion of 125I-T4 binding of sera was found in 7S fraction on Sephadex G-200 column chromatography and T4-binding protein migrated to the gamma-globulin region on cellulose acetate electrophoresis. The 125I-T4 binding of IgGs were competitively inhibited by the addition of unlabelled T4 in both patients.
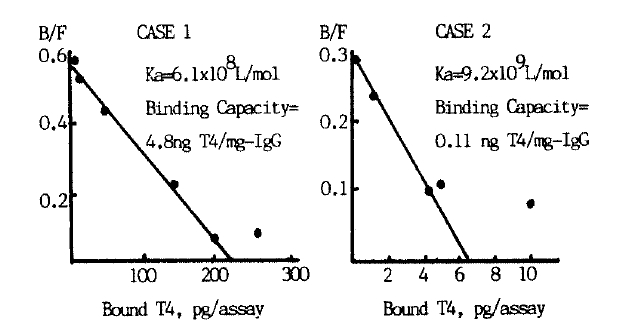
In Case 1, the association constant (Ka) for binding to T4 was 6.1 ├Ś108 l/mol and the binding capacity was 4.8ng T4/mg IgG. The anti-T4 antibody of Case 1 cross reacted with T3 and resulted in falsely high or low T3 values with radioimmunoassay. Ka and the binding capacity of case 2 were 9.2├Ś109 l/mol and 0.11ng T4/mg IgG respectively.
The clinical significance of these antibodies was discussed.
The presence of gamma-globulins capable of binding to thyroid hormones was suggested first by Robbins et al.1) and Premachandra et al2) in certain cases of thyroid carcinoma and HashimotoŌĆÖs disease, and later confirmed by Staeheli et al.3) who also suggested their influences on thyroxine (T4) or triiodothyronine (T3) radioimmunoassay. Most of the antibodies were IgG and specific to T3 or T4 but cross-reactivity with thyroglobulin was also demonstrated in certain cases.12) Radioimmunoassays give spuriously high or low T3 and T4 values in the presence of anti-T3 or T4 antibody according to the separation method and quantity of antibody. The pathophysiologic and clinical significancy of thyroid hormone autoantibodies are still unknown, however recently, Karlsson et al.7) reported cases of hypothyroidism occassioned by such antibodies to expedite studies on their clinical significance. To our knowledge, there was no report of such antibodies to thyroid hormone in Korea, and moreover, this is the first report to demonstrate anti-T4 antibody in the case of systemic lupus erythematosus.
We attested the presence of anti-T4 antibodies in SLE patients with autoimmune thyroiditis, and primary myxedema patients, and also investigated their influence on radioimmunoassays, binding characteristics with T4 and their cross-reactivity with T3.
A 27-year-old woman visited the outpatient clinic of Seoul National University Hospital because of goiter and hypothyroid symptoms of mild degree in Nov. ŌĆś82. 100ug of Synthroid was administered under the impression of chronic thyroiditis. Serum T3 was 476 ng/dl, T4, over 25 ug/dl and TSH was 68.5ull/ml at the time of first visit. Titers of antimicrosomal and antithyroglobulin antibodies were 1:3202 and 1:640,2 respectively. She has been hospitalized because of superimposed symptoms, (e.g., fever, chest pain, edema and dyspnea on Mar. ŌĆś83.) Physical and radiological examination disclosed cardiomegaly, pleural effusion, hepatomegaly and goiter (50gm). A diagnosis of SLE was made with the labolatory findings such as hypoproteinemia, proteinuria, pancytopenia, positive LE cell and elevated serum anti-DNA antibody (2944 uLI/ml). T3 resin uptake was 20%, T3, over 600 ng/dl, T4, over 25 ug/dl and TSH level was over 155 uU/ml at the time of admission. Prednisolone and Cytoxan were administered with clinical improvement including diminished goiter size and decrement of T3, T4, TSH levels (Table 1).
A 35-year-old woman visited the outpatient clinic because of weight gain of 5Kg over 6 months, edema, slurred speech, hoarseness and menorrhagia. Physical examination disclosed typical findings of hypothyroidism including cold, coarse skin and hungup reflex etc., and goiter was absent. T3 resin uptake was 21.7% T3, 63 ng/dl, T4, over 25 ug/dl and TSH was over 160 uU/ml. Titers of antimicrosomal and antithyroglobulin antibodies were both 1:1280.2
Solid phase method: Bound and free forms of T3 and T4 were separated with antibody-coated bead using T3 RIA BEAD, TETRABEAD-1 25 kits (Abbott). Polyethylene glycol (PEG) method: 200 ul of antibody was added to the admixture of 100 ul of patientŌĆÖ sera or T3, T4 stardards and 200 ul of 125I-T3, 125I-T4. The tubes were incubated for 90 minutes at room temperature, and centrifuged subsequently for 15 minutes at 1500g after addition of 1 ml PEG. Supernatant fluid was decanted and pellet was counted with gamma-counter.
One ml of 99.5% ethanol was added to 500 ul of patientsŌĆÖ sera and after 5 minutes of shaking, tubes were centrifuged for 5 minutes at 2000g. 900 ul of supernatant was evaporated under nitrogen to dryness, and the remainder was reconstituted with 300 ul of reference serum (supplied in the kit) that contained no iodothyronine and served as zero standard.
Three hundreds ul of serum samples and control sera containing trace amount of 125I-T4, 125I-T3 were applied on a Sephadex G-200 column (1.5 ├Ś 90cm), equilibrated with 0.02 M PBS buffer (pH 7.6). 3ml of elutes were collected and opical density at 280 nm and radioactivity of each tube were checked with spectrophotometer and gamma-counter, respectively.
Cellulose acetate electrophoresis (barbital buffer, pH 8.6) was carried out in 50 ul of patientŌĆÖs sera and control sera containing trace amount of 125I-T4, 125I-T3. Lanes were cut into 5 sections containing each band was counted with gamma-counter, subsequently.
Fifty ul of 125I-T4 and 100 ul of 3% BSA-0.3% thimerosal were added to 50 ul of patientsŌĆÖ IgG (1 mg/0.1 ml), and the admixtures were incubated for 20 hours at 4┬░C with 50 ul of serially diluted cold T4 and T3. 300 ul of 30% PEG was used to precipitate bound forms and gamma-counting was done after centrifugation for 20 minutes at 2000 g, 4┬░C.
Serum T4 values were over 25 ul/dl in both cases with solid phase method using anti-T4 antibody coated bead, whereas, unmeasurably low in both cases with PEG method for separation of bound and free forms. Nonspecific binding was over 80% with PEG precipitation method, suggesting the presence of abnormal T4-binder besides TBG (Table 2). Serum T3 values were over 600 ng/dl with solid phase method but unmeasurably low with PEG method in Case 1, while, 65 ng/dl with solid phase method and 37 ng/dl with PEG method in Case 2.
Serum T4 values measured after alcohol extraction were 6.5 and 3.2 ul/dl (Case 1 and 2) with solid phase method, and 5.8 and 2.4 ug/dl with PEG method showing significant discrepancies with the values measured without extraction. These values were regarded as ŌĆ£trueŌĆØ T4 levels considering patientsŌĆÖ clinical status and TSH levels. Serum T3 values were 38 ng/dl with solid phase method and 32 ng/dl with PEG method in Case 1 (Table 2). Recovery of T3 and T4 with alcohol extraction was 86%.
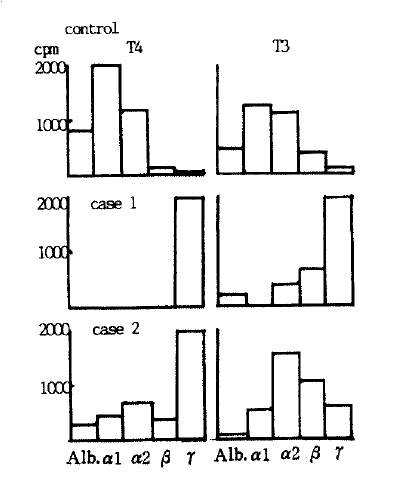
Major portion of 125I-T4 appeared to be bound to immunoglobulin G in both cases whilst, almost all of 125I-T4 was restricted to those fractions containing TBG in normal control sera (Fig. 1). In contiadistinction to 125I-T4, 125I-T3 appeared in the TBG regions, in both patientsŌĆÖ and control sera, suggesting the absence of anti-T3 antibody in patientsŌĆÖ sera.
With control sera, 78.3%, 19.9% and only 0.17% of 125I-T4 were found to be present in the inter alpha-globulin, albumin and gamma-globulin regions, respectively, whereas, 88% (Case 1) and 60.3% (Case 2) were present in gamma-globulin domain with patientsŌĆÖ sera (Table 3). 62% and 31.5% of 125I-T3 were in the gamma-globulin and inter alpha-globulin domain, respectively, in Case 1, but case 2 showed similar distribution pattern of 125I-T3 to normal control sera (Table 3, Fig. 2).
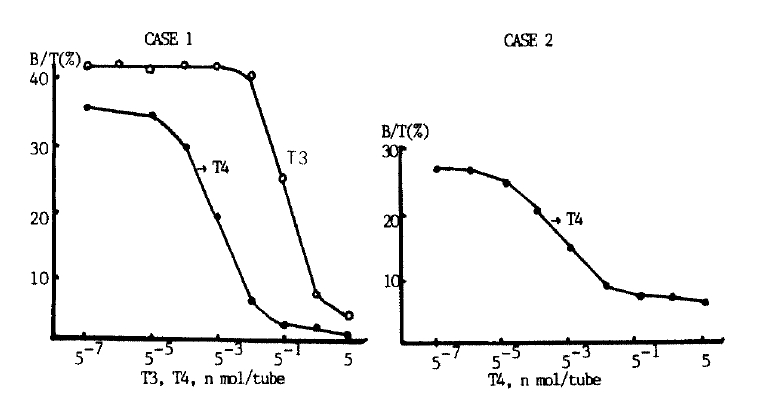
Adding cold T4 inhibited binding of patientsŌĆÖ IgG with 125I-T4 in both cases (Fig. 3). Binding was also inhibited by high concentration of T3 in Case 1 suggesting cross-reactivity of anti-T4 antibody with T3. As shown in Fig. 4, Schatchard analysis gave an association constant of 6.1 ├Ś 108 l/mol and a binding capacity of 4.8 ng T4/mg IgG in Case 1. In Case 2, the association constant was 9.2├Ś109 l/mol to be higher than in Case 1, and the binding capacity was 0.11 ng T4/mg IgG to be smaller.
Most of anti-T3 or T4 antibodies reported so far are found in autoimmune thyroid diseases (e.g., GravesŌĆÖ disease or HashimotoŌĆÖs disease, but also found rarely in thyroid carcinoma,1) normal subject7) and WaldenstromŌĆÖs macroglobulinemia15) etc). These antibodies interfere radioimmunoassay of thyroid hormones to give falsely high or low values according to separation method. That is to say, in case of double antibody method or solid phase method, radioiodinated hormone binds by and large to patientŌĆÖs own antibody instead of test antibody to lower apparent B/F ratio to bring about a spuriously high result. To the contrary, in case of precipitation method using ammonium sulfate or PEG, radioiodinated hormone bound to patientŌĆÖs own antibody co-precipitate to heighten apparent B/F ratio to give spuriously low value.
Our 2 cases were clinically hypothyroid with elevated serum TSH levels, but had elevated T4 levels with solid phase radioimmunoassay, while, they had unmeasurably low values with PEG method. Moreover, nonspecific binding of over 80% with PEG precipitation suggested abnormal T4-binding protein besides TBG, albumin and prealbumin, and it is further substantiated by the findings that T4 levels measured after ethanol extraction showed similar results with both separation methods and matched well with clinical findings and serum TSH levels. These abnormal T4-binding proteins seem to be antibodies to T4 considering 88% (Case 1) and 60.3% (Case 2) of 123I-T4 migrated to gamma-globulin region on electrophoresis and peaks of 123I-T4 coincided with gamma-globulin domain on Sephadex G-200 column chromatography. Moreover, binding of 123I-T4 and these antibodies were specifically inhibited by adding cold T4 to corroborate the nature of these antibodies.
In Case 1, the association constant was 6.1 ├Ś 108 l/mol and the binding capacity was 4.8 ng T4/mg IgG, being substantially larger than that of TBG on Schatchard analysis.
Binding of IgG with 125I-T4 was inhibited by T3 in case 1 to some degree, suggesting cross-reactivity of anti-T4 antibody with T3, and to our knowledge, there has been no report of such cross-reactivity. The findings that the T3 level was high with the solid phase method but unmeasurably low with the PEG method and T3 measured after ethanol extraction matched well with clinical status may suggest the existence of anti-T3 antibody. But there was no evidence of binding of 125I-T3 with IgG on chromatographic findings. Migration of 34.8% and 30.4% of 125I-T3 to gamma-globulin region on electrophoresis might to interpreted in either way, and it is not certain whether it means mere cross-reactivity or presence of another anti-T3 antibody.
The mechanism of genesis of anti-T4 or T3 antibodies is far from clear, although they are nearly confined to autoimmune thyroid diseases,3,8,11ŌĆō14,18) and regarded as a kind of autoantibodies. Most of these antibodies are reported to cross-react with thyroglobulin,4,10,18) and it is also reported experimental animals can produce anti-thyroid hormone antibodies when immunized with thyroglobulin under appropriate condition, suggesting that immunogen of antithyroid hormone antibody would be thyroglobulin and this antibody to thyroglobulin would cross-react with thyroid hormones.18) This assumption is consistent with the findings that most of the patients with these antibodies have high titer of antithyroglobulin antibody as 1:6402 and 1 ;12802 in our cases.
Clinical significance of these anti-thyroid hormone antibodies seems mainly to consist in disturbance of radioimmunoassay for thyroid hormones, but Karlsson et al.7) reported a case of hypothyroidism due to anti-T3 antibody. In our first case, the administration of prednisolone and Cytoxan brought about diminution of goiter size and decrease in TSH levels with improvement of lupus symptoms. This suggests the possibility that the anti-T4 antibody having cross-reactivity with T3 would have induced hypothyroidism in our first case. But we could not exclude the possibility that the decrease in TSH might be due to the improvement of autoimmune thyroiditis because the patient had high titer of antimicrosomal antibody besides antithyroglobulin antibody, and we did not prove that histology of her thyroid was normal with biopsy.
Acknowledgments
We wish to thank Mr. Jae Min Chung and II Taek Suh for excellent technical assistance and Ms. Eun Ja Kwak for preparation of the manuscript.
Fig.┬Ā1.
Sephadex G-200 column chromatography of the control and patientŌĆÖs sera incubated with 125I-T4 and 125I-T3. Abscissa is fraction number.

Fig.┬Ā2.
Distribution of 125I-T4 among proteins in control and patientsŌĆÖ sera after electrophoresis on cellulose acetate.
Table┬Ā1.
Laboratory data of case 1
| Lab. | Date | |||
|---|---|---|---|---|
| 11/11/82 | 4/1/83 | 6/27/83 | 9/22/83 | |
| T3 RU(%) | 19 | 20 | 36.3 | 31.6 |
| T3(ng/dl)* | 476 | >600 | 52 | 92 |
| T4(ug/dl) | >25 | >25 | 17.7 | 13.4 |
| TSH(uU/ml) | 68.5 | 155 | 2.7 | 9.4 |
| MCHA* * | 3202 | 3202 | ŌĆō | 3202 |
| TGHA*** | 6402 | 6402 | ŌĆō | 3202 |
| Anti DNA Ab(uU/ml) | ŌĆō | 2,944 | 64 | 9 |
|
|
||||
| Tx | ||||
| ŌĆāŌĆāT4 | 100ug | ŌĆō | ŌĆō | ŌĆō |
| ŌĆāŌĆāPrednisolone | ŌĆō | 60mg | 70mg | 30mg |
| ŌĆāŌĆāCytoxan | ŌĆō | ŌĆō | 50mg | ŌĆō |
Table┬Ā2.
T4 and T3 values in the whole sera and the ethanol extract of the sera obtained by solid phase and PEG separation methods of radioimmunoassay
Table┬Ā3.
Binding percent of 123I-T4 and 125I-T3 to each fraction of serum protein in cellulose acetate electrophoresis
| 123I-T4 | 125I-T3 | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| NC* | Case 1 | Case 2 | NC* | Case 1 | Case 2 | |
| Alb | 19.8% | 2.3% | 7.7% | 14.6% | 6.3% | 1.9% |
| ╬▒1 | 50.3 | 3.7 | 11.1 | 37.8 | 11.3 | 11.3 |
| ╬▒2 | 28.3 | 2.9 | 12.7 | 34.4 | 20.2 | 33.3 |
| ╬▓ | 1.2 | 2.7 | 8.2 | 10.6 | 27.2 | 23.1 |
| ╬│ | 0.7 | 88.0 | 60.3 | 1.5 | 34.8 | 30.4 |
REFERENCES
1. Robbins J, Rail Je. An unusual instance of thyroxine binding by human serum gamma blobulin. J Clin Endocrinol Metab 16:573. 1956.


2. Premachandra BN, Blumenthal HT. Abnormal binding of thyroid hormone in sera from patients with HashimotoŌĆÖs disease. J Clin Endocr 27:931. 1967.


3. Staeheli V, Vallotton MB, Burger A. Detection of human anti-thyroxine and anti-trilodothyronine antibodies in different thyroid conditions. J Clin Endocrinol Metab 41:669. 1975.


4. Ochi Y, Shiomi K, Hachiya S, Yoshimura M, Miyazaki T. Immunological analysis of abnormal binding of thyroid hormone in the gamma-globulin. J Clin Endocrinol Metab 35:743. 1972.


5. Rubenstein HA, Butler VP, Werner SC. Progressive decrease in serum triiodothyronine concentrations with human aging: radioimmunoassay following extraction of serum. J Clin Endocrinol Metab 37:247. 1973.


6. WU SY, William L, Green . Triiodothyronine (T3)-binding immunoglobulins in a euthyroid woman: effects on measurement of T3(RIA) and on T3 turnover. J Clin Endocrinol Metab 42:642. 1976.


7. Karlsson FA, Wibell L, Wide L. Hypothyroidism due to thyroid-hormone binding antibodies. N Engl J Med 296:1146. 1977.


8. Ikekubo K, Konishi J, Endo K, Nakajima K, Okuno T, Kasagi K, Mori T, Nagata I, Torizuka K. Anti-thyroxine and anti-triiodothyronine antibodies in three cases of HashimotoŌĆÖs thyroiditis. Acta Endocrinologica 89:557. 1978.


9. Ginsberg J, Segal D, Ehrlich RM, Walfish PG. Inappropriate triiodothyronine(T3) and thyroxine(T4) radioimmunoassay levels secondary to circulating thyroid hormone autoantibodies. Clin Endocrinol 8:133. 1978.

10. Premachandra BN, Ginsberg J, Walfish PG. Binding of reverse triiodothyronine to serum immunoglobulins in man and the rabbit. J Clin Endocrinol Metab 50:802. 1980.


11. Inada M, Nishikawa M, Naito K, Oishi M, Kurata S, Imura H. Triiodothyronine-binding immunoglobulin in a patient with gravesŌĆÖ disease and its effect on metabolism and radioimmunoassay of triiodothynine. Am J Med 68:787. 1980.


12. Pearce CJ, Byfield PGH, Edmonds CJ, Lalloz MRA, Himsworth RL. Autoantibodies to thyroglobulin cross reacting with triiodothyronines. Clin Endocrinol 15:1. 1981.

13. Henry RR, Reyes Fl, Faiman C. Hypothyroidism, triiodothyronine antibodies, and hyperprotactinemia. Arch Intern Med 141:953. 1981.


14. Konishi J, Iida Y, Kousaka T, Ikekubo K, Nakagawa T, Torizuka K. Effect of anti-thyroxin autoantibodies on radioimmunoassay of free thyroxin in serum. Clin chem 28:1389. 1982.


15. Trimarchi F, Benvenga S, Fenzi G, Mariotti S, Consolo F. Immunoglobulin binding of thyroid hormones in a case of WaldenstromŌĆÖs macroglobulinemia. J Clin Endocrinol Metab 54:1045. 1982.


16. Neeley WE, Alexander NM. Polyclonal 3, 5, 3, - triiodothyronine(T3) antibodies in a euthyroid woman and their effect on radioimmunoassays for T3. J Clin Endocrinol Metab 57:851. 1983.


17. Moron LA, Meltzer SJ, Bastomsky CH. Thyroid Disease with monoclonal (Immunoglobulin G) antibody to triiodothyronine and thyroxine. J Clin Endocrinol Metab 56:1009. 1983.


18. Beckett GJ, Todd JA, Hughes GJ, Campbell IW. Primary hypothyroidism with grossly elevated plasma total thyroxine and triiodothyronine levels. Clin Endocrinol 19:295. 1983.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



