 |
 |
| Korean J Intern Med > Volume 37(3); 2022 > Article |
|
Abstract
Acknowledgments
Figure 1
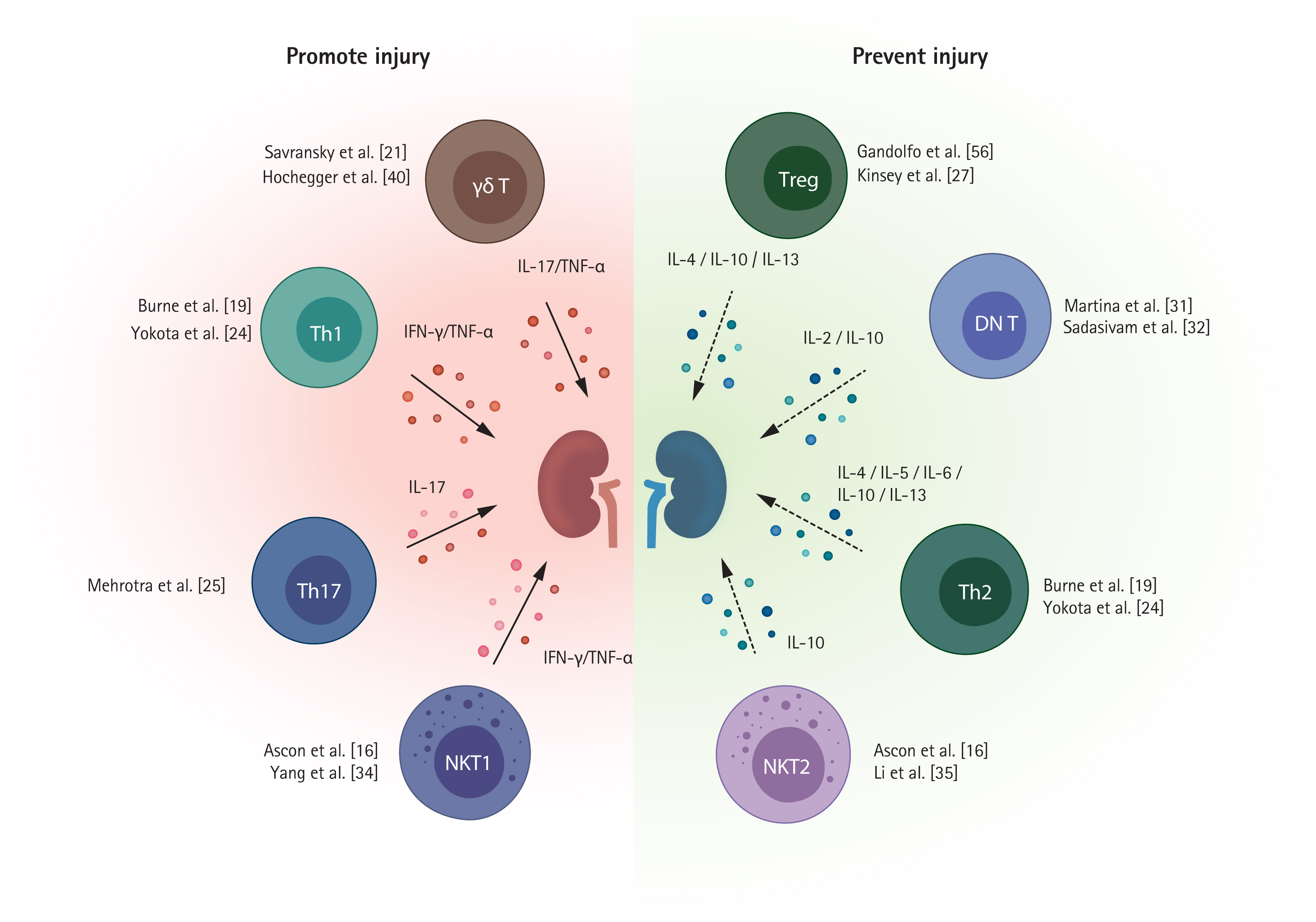
Table 1
| Study | Major findings |
|---|---|
| T cells | |
| Rabb et al. (2000) [18] |
CD4/CD8-deficient mice were protected with reduced neutrophil trafficking T cell-renal tubular epithelial cell adhesion is enhanced after hypoxia-reoxygenation in vitro |
| Burne et al. (2001) [19] |
T cell-deficient mice (nu/nu mice) were protected Adoptive transfer of WT T cells to T cell-deficient mice reconstituted renal injury CD28-deficient T cell transfer to T cell-deficient mice did not reinduce injury phenotype |
| Burne-Taney et al. (2003) [20] |
T cell-deficient mice (nu/nu mice) were protected from whole body IRI T cell-deficient mice (nu/nu mice) showed attenuated expressions of Icam1 after whole body IRI |
| Burne-Taney et al. (2005) [42] | T cell adoptive transfer to Rag1-deficient mice (mice lacking both T and B cells) led to protection |
| Ascon et al. (2006) [16] | CD4+ and CD8+ T cells traffic into post-ischemic kidneys during the early injury phase |
| Savransky et al. (2006) [21] |
TCR αβ-deficient mice were protected TCR αβ-deficient mice showed reduced TNF-α and IL-6 expression in post-ischemic kidneys at 24 hours |
| Satpute et al. (2009) [22] |
T cell-deficient mice (nu/nu mice) reconstituted with T cells having limited TCR repertoire (DO11.10 T cells) were protected from IRI compared to those reconstituted with WT T cells Antigen specific activation of DO11.10 T cells using OVA-CFA abolished its protective effect |
| Jaworska et al. (2015) [23] | Blocking or genetical deficiency of PD-L1 or PD-L2 was protected |
| CD4+ T cells | |
| Burne et al. (2001) [19] |
CD4+ T cell-deficient mice were protected Adoptive transfer of WT CD4+ T cells to T cell-deficient mice reconstituted renal injury Adoptive transfer of CD4+ T cells lacking CD28 or IFN-γ to T cell-deficient mice did not restore injury phenotype |
| Yokota et al. (2003) [24] |
STAT4-deficient mice were protected, whereas STAT6-deficient mice had more severe injury STAT4-deficient T cells showed higher IL-4 expression and lower IFN-γ expression IL-4-deficient mice showed more severe renal injury |
| Lai et al. (2007) [17] |
CD4+ T cells infiltrated post-ischemic kidneys as early as 1 hour S1PR1 agonist treatment reduced CD4+ T cell infiltration and renal injury |
| Mehrotra et al. (2015) [25] | Th17 cells (CD4+ IL-17+ T cells) increased during early injury phase thereafter deceased to the control level at 7 days |
| Mehrotra et al. (2019) [26] |
Store-operative calcium entry channel, Orai1 contributed to IL-17 expression in CD4+ T cells Pharmacologic Orai1 blockade attenuated IL-17+ cell activation and mitigated renal injury |
| CD8+ T cells | |
| Burne et al. (2001) [19] | Mice lacking CD8+ T cells mice were not protected from IRI |
| Jang et al. (2009) [9] |
GF mice have lower percentage of effector memory (CD44high CD62Llow) CD8+ T cells GF mice showed more severe injury with enhanced CD8+ T cell trafficking |
| Tregs | |
| Kinsey et al. (2009) [27] |
Treg depletion using anti-CD25 antibody (PC61) before IRI worsened renal injury with increased IFN-γ producing neutrophil infiltration Rag1 KO mice (mice lacking both T and B cells) that received lymphocytes from Sf mice (mice lacking FoxP3) showed more severe renal injury compared to those that received WT lymphocytes at 24 hours Co-transfer of Sf lymphocytes and Tregs alleviated susceptibility to IRI in Rag1 KO mice IL-10-deficient Treg adoptive transfer did not show protective effect in Rag1 KO mice, whereas WT Treg transfer was protective. |
| Kinsey et al. (2010) [28] |
Adoptive transfer of Tregs prior to IRI showed protective effect Treg depletion in preconditioned mice with anti-CD25 antibody (PC61) abolished protective effect of ischemic preconditioning |
| Kinsey et al. (2012) [29] |
CD73 or A2AR deficiency on Tregs abolished protective effect Pharmacologic A2AR activation on Tregs augmented protective effect and upregulated PD-1 expression on Tregs Adoptive transfer of PD-1 blocking antibody treated Tregs did not show protective effect |
| Kim et al. (2013) [30] |
Treg expansion by IL-2C treatment before IRI ameliorated renal injury Treg depletion by anti-CD25 antibody (PC61) treatment abolished the protective effect of IL-2C |
| Jaworska et al. (2015) [23] | Blocking PD-1 ligands with anti-PD-L1 or PD-L2 antibody abolished protective effect of Treg adoptive transfer |
| DN T cells | |
| Ascon et al. (2008) [7] | DN T cells existed in normal kidneys and post-ischemic kidneys |
| Jang et al. (2009) [33] | Mouse anti-thymocyte globulin treatment increased percentage of DN T cells among total T cells |
| Martina et al. (2016) [31] |
DN T cells have higher expression of IL-10 and IL-27 than CD4+ T cells in steady state DN T cells rapidly increased within 24 hours after IRI IL-10 expression on DN T cells was upregulated following IRI, whereas IL-27 expression was decreased DN T cell adoptive transfer before IRI attenuated renal injury, and IL-10 blocking with anti-IL-10 receptor antibody abrogated protective effect of DN T cells DN T cells were present in human ischemic kidney tissue with considerable proportion |
| Sadasivam et al. (2019) [32] |
PD-1+ and NK1.1+ subsets are two major DN T cell subsets in both human and mice DN T cells required IL-2 for their activation and function in vitro and in vivo PD-1+ subset predominated kidney DN T cells in post-ischemic kidneys |
| γδ T cells | |
| Savransky et al. (2006) [21] | γδ TCR-deficient mice had less structural renal injury |
| Hochegger et al. (2007) [40] |
γδ T cell-deficiency and γδ T cell depletion using anti-γδ TCR antibody showed protective effect γδ T cell-deficiency reduced αβ T cell trafficking to post-ischemic kidneys during early injury phase |
| Gocze et al. (2018) [41] |
Decline of γδ T cells in circulating blood correlated with urinary cell stress biomarker levels in human (TIMP-2 and IGFBP7) γδ T cells increased in post-ischemic kidneys, while circulating γδ T cells decreased at 24 hours after IRI |
| NK T cells | |
| Ascon et al. (2006) [16] | Traffic as early as 3 hours and reduced to baseline level at 24 hours after IRI |
| Li et al. (2007) [35] |
NK T cell depletion using anti-NK1.1 antibody or blocking NK T cell activation by anti-CD1d antibody attenuated renal injury Type I NK T cell-deficient mice (Jα 18−/−) were protected from IRI NK T cells mediated neutrophil infiltration and IFN-γ production in IRI |
| Yang et al. (2011) [34] |
Type II NK T cell activation by sulfatide injection reduced renal injury in WT mice and type I NK T cell deficient mice (Jα 18−/−), but not in NK T cell-deficient mice (CD1d −/−) Adoptive transfer of sulfatide treated NK T cells attenuated renal injury in WT, type I NK T cell-deficient mice, and NK T cell-deficient mice Type II NK T cell mediated protective mechanisms involved in HIF-1α and IL-10 pathways NK T cells (CD3+ Vα24+) were found in kidney tissue from patients with acute tubular necrosis |
| Zhang et al. (2016) [38] | HIF-2α/A2AR axis served protective mechanism by restricting NK T cell infiltration and activation |
| Ferhat et al. (2018) [37] | IL-33 drove recruitment of type I NK T cells and induced INF-γ/IL-17 production |
| Uchida et al. (2018) [39] |
α-Galactosylceramide administration induced AKI and hematuria via NK T cell activation Perforin-mediated pathway and TNF-α/Fas ligand pathways are related to glomerular injury and tubular injury, respectively |
IRI, ischemia-reperfusion injury; WT, wild-type; ICAM-1, intercellular adhesion molecule 1; TCR, T cell receptor; TNF-α, tumor necrosis factor α; IL, interleukin; OVA-CFA, ovalbumin in complete Freund’s adjuvant; PD-L, programmed death ligand; IFN-γ, interferon γ; STAT, signal transducer and activator of transcription; S1PR1, sphingosine-1-phosphate receptor 1; GF, germ-free; Treg, regulatory T cell; KO, knockout; Sf, scurfy; FoxP3, forkhead box P3; A2AR, adenosine 2A receptor; PD-1, programmed death-1; IL-2C, IL-2/anti-IL-2 complex; DN, double-negative; TIMP-2, tissue inhibitor of metalloproteinase 2; IGFBP7, insulin-like growth factor-binding protein 7; NK, natural killer; HIF, hypoxia-inducible factor; AKI, acute kidney injury.
Table 2
| Study | Major findings |
|---|---|
| T cells | |
| Burne-Taney et al. (2005) [64] |
CD4+ T cells infiltration in post-ischemic kidneys at 6 weeks after IRI Upregulation of IL-1 and CCL5 expression in post-ischemic kidneys at 6 weeks after IRI Enhanced IFN-γ secretion of splenic T cells at 6 weeks after IRI |
| Burne-Taney et al. (2006) [68] |
Adoptive transfer of splenic T cells obtained from post-IRI donor mice resulted in albuminuria in normal recipient mice at 12 weeks after transfer Increased activated (CD3+ CD25+) or memory phenotypes (CD4+ CD44+) of T cells in recipient spleens |
| Ascon et al. (2009) [65] |
Activated phenotypes (CD69+) of T cells were expanded at late recovery phase (6 weeks from BIRI, 11 weeks from UIRI) Effector memory (CD44high CD62L−) phenotype T cells were major T cell phenotype at 6 weeks after BIRI or UIRI Inflammatory cytokines and chemokines (Il1b, Il6, Tnfa, Ifng, Cxcl2, and Ccl5) were upregulated in post-ischemic kidneys at 6 weeks after UIRI T cell depletion reduced Ifng expression |
| Ko et al. (2012) [67] |
Upregulation of Ccr5 gene expression on T cells was maintained from early injury to late recovery (4 weeks) CCR5 blockade with anti-CCR5 antibody after IRI attenuated renal injury and reduced renal trafficking of activated phenotype (CD69+) T cells on day 3 after IRI |
| Duraes et al. (2020) [66] |
scRNA-seq analyses of kidney CD45+ cells showed increased T cell cluster at 4 weeks after IRI scRNA-seq analyses of kidney CD4+ T cells showed substantial expansion of Th17 cell cluster during the recovery phase |
| Regulatory T cells | |
| Gandolfo et al. (2009) [56] |
Marked Treg (CD4+ CD25+ FoxP3+) expansion in post-ischemic kidneys on day 3 and day 10 after IRI Adoptive transfer of Tregs (CD4+ CD25+) at 24 hours after IRI reduced structural injury, enhanced tubular regeneration, and reduced TNF-α production in CD4+ T cells on day 10 Treg depletion with anti-CD25 antibody treatment at 24 hours after IRI inhibited tubular regeneration and increased TNF-α and IFN-γ production in CD4+ and CD8+ T cells |
| Kinsey et al. (2010) [28] | IL-10 producing Tregs increased at 7 days after IRI |
| Gandolfo et al. (2010) [69] |
MMF administration at 48 hours post-ischemia reduced Treg expansion and worsened tubular injury on day 10 after IRI MMF administration in T cell-deficient mice did not alter renal outcome |
| Kim et al. (2013) [30] |
Treg expansion by IL-2C administration reduced renal fibrosis on day 28 after IRI Treg depletion with anti-CD25 antibodies abrogated the beneficial effect of IL-2C treatment. |
| Chen et al. (2016) [70] |
mTOR inhibition by rapamycin treatment in CD4+ T cells induced Treg expansion and enhanced expression of IL-10 and TGF-β1 in vitro. Adoptive transfer of rapamycin ex vivo treated Tregs at 24 hours post-ischemia reduced structural renal injuries and a fibrosis marker expression on day 14 after IRI |
| Duraes et al. (2020) [66] |
A set of gene expression in Tregs are highest at late recovery phase Treg expansion by a combination of IL-2C and IL-33 treatment before IRI reduced kidney fibrosis on day 28 from IRI Tregs in fibrotic and regenerative environments had distinct signatures of gene expression |
IRI, ischemia-reperfusion injury; IL, interleukin; CCL5, C-C motif chemokine ligand 5; BIRI, bilateral ischemia-reperfusion injury; UIRI, unilateral ischemia-reperfusion injury; IFN-γ, interferon γ; CCR5, C-C motif chemokine receptor 5; scRNA-seq, single cell RNA-sequencing; Th17, T helper 17 cell; Treg, regulatory T cell; FoxP3, forkhead box P3; TNF-α, tumor necrosis factor α; MMF, mycophenolate mofetil; IL-2C, IL-2/anti-IL-2 complex; mTOR, mammalian target of rapamycin; TGF-β1, transforming growth factor β1.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



