 |
 |
| Korean J Intern Med > Volume 34(6); 2019 > Article |
|
Abstract
Background/Aims
Non-selective ╬▓-blockers (NSBBs) are used for primary prevention of esophageal variceal hemorrhage (VH) in patients with portal hypertension, but a significant number of patients develop VH while on NSBB therapy. In this study, we sought to determine whether liver volume can predict the risk of primary prophylaxis failure in cirrhotic patients on NSBB therapy.
Methods
A retrospective cohort of 309 patients on prophylactic propranolol was analyzed. Liver volume was measured in portal venous phase images of multidetector computed tomography. Predictors of VH were assessed using a Cox proportional hazards model with competing-risks analysis. A nomogram was developed for estimation of the risk of primary prophylaxis failure.
Results
During a median follow-up of 36 months, 37 patients on propranolol developed VH. Liver volume index, the ratio of measured-to-expected liver volume, was an independent predictor of VH (adjusted hazard ratio [HR], 2.70; 95% confidence interval [CI], 1.37 to 5.33; p = 0.004) as were the presence of large varices and the absence of ascites. A nomogram-based volume score of > 0.6 was predictive of prophylaxis failure (HR, 7.54; 95% CI, 2.88 to 19.73; p < 0.001). Time-dependent receiver operating characteristic curve analysis revealed that a nomogram-based risk score had significantly better discriminatory power than the North Italian Endoscopy Club index in predicting prophylaxis failure at 6 and 8 years.
Esophageal variceal hemorrhage (VH) is a grave complication of liver cirrhosis with portal hypertension. The annual incidence of first VH is 5% to 15% per year, leading to significant mortality [1]. Non-selective ╬▓-blocker (NSBB) therapy and endoscopic variceal ligation (EVL) are the primary tools for prophylaxis of esophageal VH [1,2], and meta-analysis shows that NSBB use reduces the risk of VH [3]. Accordingly, current guidelines recommend NSBBs for cirrhotic patients with increased risk of VH [1,2,4]. However, the risk of bleeding is not completely eliminated by NSBBs and a significant number of patients develop VH during NSBB therapy [5-18]. Therefore, it is necessary to identify patients who have increased risk of primary prophylaxis failure.
The hemodynamic response to NSBBs, i.e., a hepatic venous pressure gradient (HVPG) < 12 mmHg or at least 10% to 20 % reduction from baseline pressure, is the most reliable predictor of the efficacy of prophylaxis [5,8,13,19]. However, HVPG cannot be readily measured in routine clinical practice due to its invasive nature. The North Italian Endoscopy Club (NIEC) index and its variations, composed of scores for Child-Pugh class, size of varices and red wale markings, are validated as significant predictors of first esophageal VH [20-23]. However, it is less well defined whether these indices are still valid in predicting primary prophylaxis failure. Non-invasive measurement of liver/spleen stiffness can predict the presence of clinically significant portal hypertension [1], and several studies have demonstrated promising predictive power for VH [24-26]. However, the heterogeneity of studies with respect to etiology, treatment of underlying disease, prophylactic therapy, type of measurement and cut-off values calls for further validation.
Liver volume measurement has been used to predict operative outcomes in decompensated liver diseases [27-29]. Previous studies reported a correlation between liver volume and functional reserve in cirrhotic patients [30-36]. We also demonstrated that liver volume may be utilized as a predictive marker of hepatocellular carcinoma [37]. From these results, it may be speculated that liver volume may reflect the stage of cirrhosis and possibly portal pressure. However, liver volume has not been studied as a surrogate marker for elevated portal pressure or predictor of VH in cirrhotic patients.
The aim of this study was to determine whether liver volume predicts the risk of esophageal VH in patients with liver cirrhosis who are on propranolol as for primary prophylaxis. Since conventional survival analysis using the Kaplan-Meier method may be inappropriate for an alternative outcome (e.g., deaths) [38,39], we adopted a competing-risk analysis model for the assessment of prophylaxis failure.
This single center retrospective cohort study enrolled consecutive patients aged over 18 years with liver cirrhosis who visited our tertiary referral center in South Korea between April 2003 and October 2015 and received propranolol for the primary prophylaxis of esophageal VH. The electronic medical records were retrieved from a structured chronic liver disease database (BESTCare) [40]. Liver cirrhosis was defined by ultrasonographic findings of coarse liver echotexture with nodularity plus evidence of portal hypertension (splenomegaly, thrombocytopenia or varices). The following were excluded from the study cohort: (1) duration of propranolol prophylaxis < 6 months, (2) previous history of bleeding and/or EVL before propranolol therapy, and (3) lack of contrast-enhanced liver computed tomography (CT) data within 6 months before or after first propranolol dosage. The necessity for CT was determined at the discretion of the attending physician under diverse clinical settings including poor sonic window, abnormal ╬▒-fetoprotein levels or detection of suspicious nodule(s) with ultrasound that warranted further characterization.
The Institutional Review Board of Seoul National University Bundang Hospital approved this study (IRB No: 1608-359-101). All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was waived by the IRB, due to the retrospective observational nature and anonymous analysis of data.
Screening esophagogastroduodenoscopy was recommended for all cirrhotic patients and esophageal varices were graded as small, medium or large [4]. Propranolol prophylaxis was recommended according to the Baveno IV consensus [41]: (1) presence of medium-large esophageal varices or (2) small varices with red color signs or Child-Pugh class C. Propranolol was administered orally at starting dose of 10 to 40 mg per day and adjusted to achieve 25% reduction in resting heart rate a decrease to 55 beats per minute, a maximum dose of 160 mg, or the maximum tolerable dose [41]. Propranolol was not recommended if patients had contraindications such as bronchial asthma, chronic obstructive pulmonary disease, uncontrolled heart failure, sinus bradycardia < 60/minute or heart block greater than first degree.
The primary end point of analysis was the first esophageal VH, defined by hematemesis or melena with endoscopic evidence of recent or active esophageal VH. Deaths were regarded as a competing risk and treated accordingly, since treatment of deaths as simple censored cases may bias the estimated outcomes. Bleeding other than esophageal VH, i.e., gastric VH or portal hypertensive gastropathy, were treated as censored cases. Patients who received prophylactic EVL while on propranolol were also analyzed as censored cases.
Liver volume was measured on the cross-sectional images of the portal venous phase of liver CT by using Image J version 1.50i (Research Services Branch, National Institute of Mental Health, Bethesda, MD, USA; http://imagej.nih.gov/ij) as previously reported with minor modifications [42]. Briefly, image slices were downloaded and boundaries of the liver area were using the Versatile Wand Tool plug in (https://imagej.nih.gov/ij/plugins/versatile-wand-tool/index.html). The inferior vena cava and gallbladder were excluded from selection, whereas intra hepatic portal veins were included in the measured areas. The measured area was summed and multiplied by slice thickness to yield the liver volume.
Since body build may affect liver volume [37,43], normal variance was adjusted by calculating the ŌĆ£liver volume indexŌĆØ as an indicator of hepatic shrinkage:
The formula for liver volume was deduced from the body surface area (BSA): formula liver volume (mL) = 893.485 ├Ś BSA ŌĆō 439.169 (mL) [43].
The BSA was estimated with Du BoisŌĆÖ formula: BSA = 0.007184 ├Ś (weight in kg)0.425 ├Ś (height in cm)0.725 [44].
All statistical analyses were performed using STATA version 14 (StataCorp, College Station, TX, USA) or R package version 3.3.2. Continuous and categorical variables were analyzed using StudentŌĆÖs t test or the Mann-Whitney rank sum test and chi-square test, respectively. In order to adjust for mortality not associated with esophageal VH during follow-up, competing-risks regression analysis was performed using R package mstate and rms. The cumulative incidence of prophylaxis failure and covariate analysis was estimated using the Fine and GrayŌĆÖs proportional subhazards model [45]. The proportionality of cause-specific hazards was confirmed with the log-log curves (STATA stphplot) for categorical variables and Schoenfeld residuals for continuous variables. Comparison of cumulative incidence with competing-risks was made by stcrreg function of STATA. The rms package of R was used to generate a nomogram from the Cox model with multiple outcomes. Comparison of bleeding prediction models was performed using time-dependent receiver operating characteristic (ROC) analysis with competing risks with the ŌĆ£timeROCŌĆØ package of R.
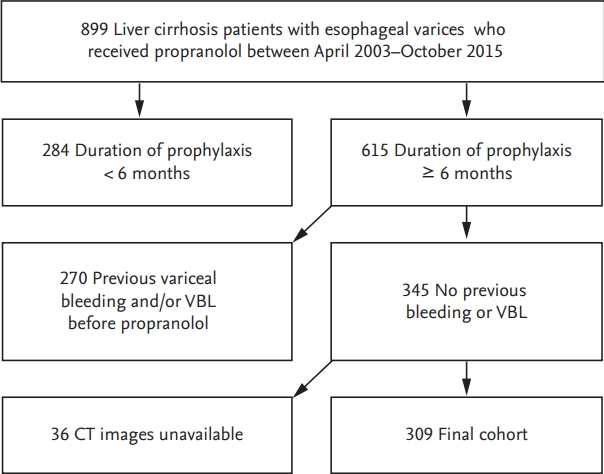
Among 899 patients with liver cirrhosis who received prophylactic propranolol therapy, 590 were excluded and 309 were finally recruited (Fig. 1). Baseline characteristics of the study population are shown in Table 1. The median duration of propranolol prophylaxis was 36 months (interquartile range, 41), and the median dosage of propranolol was 40 mg/day (range, 10 to 240). Eight patients received prophylactic EVL and censored (2.6%). During the study period 37 patients developed esophageal VH (Fig. 2) and 10 patients bled outside of esophagus. The cumulative incidences of prophylaxis failure in the presence of competing risk, i.e., death were 6.2%, 12.0%, 19.2%, 21.9%, and 24.5% at 2, 4, 6, 8, and 10 years, respectively. Patients with prophylaxis failure showed higher frequency of large (F3) varices and red color sign and lower frequency of ascites. Liver and spleen volumes were not significantly different between the two groups. However, liver volume index, an estimated-to-actual liver volume index corrected for patientsŌĆÖ body build, was significantly higher in the prophylaxis failure group, indicating that corrected liver volume was significantly smaller in patients without prophylaxis failure (Table 1).
The liver volume was significantly smaller in decompensated cirrhosis (1,064 ┬▒ 401 mL vs. 1,198 ┬▒ 408 mL in Child B/C vs. Child A cirrhosis, respectively; p = 0.004). The volume index was also lower in Child B/C cirrhosis (0.727 ┬▒ 0.290 vs. 0.802 ┬▒ 0.223, p = 0.011). The liver volume index showed better correlation with Model for End-Stage Liver Disease (MELD) score than simple liver volume (PearsonŌĆÖs correlation coefficient, ŌĆō0.241 vs. ŌĆō0.191, p < 0.001).
Competing-risks regression analysis showed that variceal size, red color signs, absence of ascites and high liver volume index > 1.0 were significant predictors of esophageal VH during prophylactic propranolol therapy, whereas Child-Pugh or MELD scores were not predictive of prophylaxis failure (Table 2). Subgroup analysis showed that the subdistribution hazard ratio was higher in patients with high volume index in non-alcoholic cirrhosis (5.05; 95% confidence interval [CI], 2.14 to 11.90; p < 0.001), but the volume index was not significant predictor in alcoholic cirrhosis (1.19; 95% CI, 0.33 to 4.28; p = 0.788). Covariate analysis by Fine and Gray model showed that large varices, absence of ascites and high liver volume index remained independent predictors of propranolol prophylaxis failure. Neither simple liver volume nor spleen volume predicted bleeding risk.
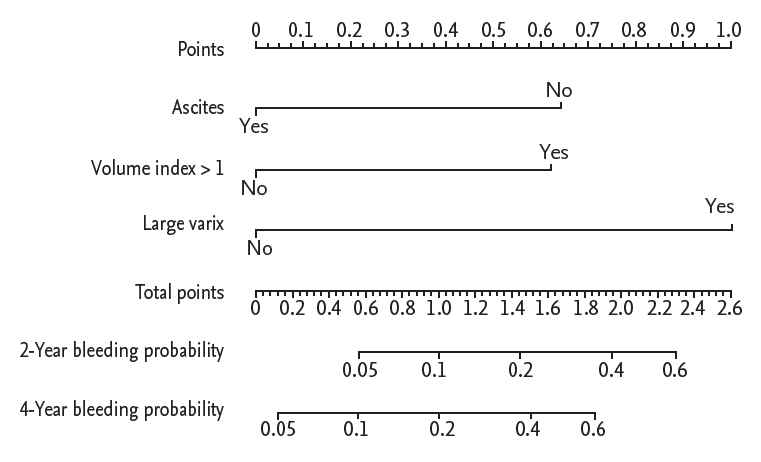
A nomogram was developed from the independent Cox models for predicting propranolol prophylaxis failure (Fig. 3). The nomogram scores for large varices, absence of ascites and high liver volume index were 1, 0.64, and 0.62, respectively. Discrimination analysis showed that patients with a nomogram score > 0.6 showed significantly higher incidence of prophylaxis failure compared to patients with low scores (subdistribution hazard ratio, 7.54; 95% CI, 2.88 to 19.73; p < 0.001) (Fig. 4).
We then compared the performance of the nomogram-based prediction model with that of the previously validated NIEC index and revised NIEC index [20,23]. Time-dependent ROC analysis revealed that the nomogram-based risk score had significantly better discriminatory power compared to that of the NIEC index or revised NIEC index in predicting propranolol prophylaxis failure at 6 and 8 years (Fig. 5).
Current guidelines recommend primary prophylaxis with NSBB for patients with risk factors [1,2,4], but 2.5% to 20% of patients still develop esophageal VH despite NSBB prophylaxis (Table 3). Our cohort showed 6.2% and 12.0% prophylaxis failure rates at 2 and 4 years, respectively, similar to previous reports. If patients at high-risk for prophylaxis failure can be identified in advance, more active intervention such as enhanced surveillance, HVPG-directed NSBB titration or prophylactic EVL may be justified.
Various combinations of clinical and hemodynamic parameters have been reported as predictors of prophylaxis failure (Table 3), but none has been universally accepted. In this study, we found that large variceal size (F3 varix), absence of ascites and liver volume index > 1 were independent predictors of prophylaxis failure in cirrhotic patients on propranolol therapy for esophageal VH prevention. Variceal size but not liver volume has been previously reported as a predictor of prophylaxis failure (Table 3). In this study, high liver volume index and absence of ascites were associated with increased risk of prophylaxis failure.
Our data indicate that propranolol prophylaxis failure is less frequent in patients with more advanced cirrhosis, i.e., small liver volume and/or presence of ascites. Although seemingly counter-intuitive, our data is in line with the recent study from United States veterans hospitals in which presence of hepatic encephalopathy, another indicator of decompensation, was associated with lower risk for prophylaxis failure [18]. The exact mechanism(s) of this finding is not clear. We suppose that although the risk of initial bleeding may be higher in patients with smaller liver volume and/or ascites, these patients may experience greater prophylactic effect of NSBB, i.e., more profound drop in portal pressure, leading to lower risk for bleeding while on NSBB. Since propranolol undergoes first pass metabolism in the liver [46], increasing intra- and extrahepatic portosystemic shunt in leads to increased bioavailability of propranolol, which may in turn potentiate reduction of portal pressure. If small liver volume index can predict portosystemic shunt more accurately compared to Child-Pugh score or MELD score, then volume index may be a better predictor of NSBB response, as indicated in our multivariate analysis. However, this explanation is only speculative because we did not measure the actual portal pressure in our patients. Further studies will be needed to confirm the potentially differential response to NSBB between compensated and decompensated cirrhosis.
There have been concerns regarding the use of NSBB in decompensated cirrhosis with ascites [47,48], advocating the concept of ŌĆ£window hypothesis for ╬▓-blockersŌĆØ: NSBB may be harmful in the end-stage cirrhosis with refractory ascites [49]. However, contradicting results exist and recent meta-analyses indicate that NSBB does not increase mortality in decompensated cirrhosis [50,51]. It is also possible that the favorable hemodynamic effect of NSBB in patients with small volume index may not be necessarily translated to survival improvement. The effect of NSBB on the overall survival of patients with small liver volume and/or ascites may warrant further prospective studies. In the meantime, since prophylactic EVL may not be free of risks, we believe that these patients may choose NSBB for primary prophylaxis, with special attention to cardiac compensatory reserve [49].
Time-dependent ROC analysis with competing-risks showed that our model based on liver volume index had better predictive power compared to that of the conventional and revised NIEC index. The revised NIEC index indicates that the weight of the Child-Pugh score is limited [23]. Serum albumin, bilirubin, and prothrombin time may show fluctuation during the course of cirrhosis, so that the liver volume index may be an alternative marker for severity of cirrhosis. Compared to previous reports of volumetric, our measurement method utilized the semi-automatic selection plug-in of Image J freeware which allows reproducible measurements with ease [42]. Typical measurement of one patient took 5 to 10 minutes in experienced hands by using the edge-detecting tool. Our method can be implemented in any picture archiving and communication systems without additional analysis tools. We also reported that our semi-automatic method show good reproducibility with minimal inter-observer variation [37].
There are several limitations in this study. First, HVPG was not measured in our cohort, so that the correlation between portal pressure and liver volume is speculative at this stage. Further studies are needed to elucidate the relationship between liver volume and portal pressure in cirrhotic patients. Second, since this was a retrospective single-center cohort study, prospective validation studies are needed to determine the predictive power of the volume index in a larger population. Third, we did not access liver and spleen stiffness which may stratify the risks of prophylaxis failure. Examination of the relationship between liver volume and liver stiffness measurement may be warranted with respect to the hemodynamic response to NSBB in further studies. Fourth, the median dosage of propranolol was at the lower recommended end (40 to 80 mg/day) [1]. Competing risks analysis showed that propranolol dosage was not a significant predictor (Table 2), but patients with relatively preserved liver volume may have benefited from higher dose of propranolol.
In conclusion, liver volume index is an independent predictor of first VH and a nomogram-based volume score stratifies the bleeding risks in patients on propranolol prophylaxis.
1. Computed tomography-measured liver volume is an independent predictor of first variceal bleeding in cirrhotic patients on propranolol prophylaxis, along with variceal size and absence of ascites.
2. A nomogram based on liver volume can reliably estimate the probability of first variceal bleeding in cirrhotic patients on propranolol prophylaxis.
3. The nomogram-derived volume score has superior predictive performance compared to conventional predictors such as North Italian Endoscopy Club (NIEC) score and revised NIEC score.
Acknowledgments
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (NRF-2017M3A9E8033225). This work was supported by a grant from the National Research Foundation of Korea funded by the Korean government (NRF 2017R1C1B5017879).
Figure┬Ā2.
Cumulative incidence of esophageal variceal hemorrhage during primary prophylaxis with propranolol. The cumulative incidence was corrected for deaths as a competing event according to Fine-Gray Model.

Figure┬Ā3.
Nomogram for prediction of primary prophylaxis failure. Points of volume index, ascites, and variceal size are read on perpendicular spot on the upper scale and summed to obtain the risk score. The 2- and 4-year probability of primary prophylaxis failure is predicted according to the risk score.
Figure┬Ā4.
Performance of the nomogram for predicting primary prophylaxis failure. Kaplan-Meier analysis showed significantly increased risk for esophageal variceal hemorrhage in patients with risk score > 100 (p < 0.001).

Figure┬Ā5.
Time-dependent receiver operating characteristic (ROC) plot for predicting propranolol prophylaxis failure. Compared to North Italian Endoscopy Club (NIEC) (A) or revised NIEC score (B), the area under curve (AUC) of nomogram score (C) was significantly larger at 6 and 8 years.

Table┬Ā1.
Baseline characteristics
| Variable | Total (n = 309) | Esophageal varix bleeding (n = 37) | Non-esophageal varix bleeding (n = 272) | p valuea |
|---|---|---|---|---|
| Duration of follow-up, mon | 36 (41) | 30 (38) | 36 (35) | 0.188 |
| Age, yr | 58 (14) | 58 (11) | 58 (15) | 0.483 |
| Male sex | 226 (73) | 30 (81) | 196 (72) | 0.245 |
| Etiology of cirrhosis | 0.081 | |||
| ŌĆāHBV | 183 (59) | 17 (46) | 166 (61) | |
| ŌĆāHCV | 25 (8) | 6 (16) | 19 (7) | |
| ŌĆāNon-B, non-C | 101 (33) | 14 (38) | 87 (32) | |
| Alcohol historyb | 123 (40) | 12 (32) | 111 (41) | 0.329 |
| Child-Pugh class | 0.368 | |||
| ŌĆāA | 151(49) | 21 (57) | 130 (48) | |
| ŌĆāB | 134 (43) | 15 (40) | 119 (44) | |
| ŌĆāC | 24 (8) | 1 (3) | 23 (8) | |
| Large varix (F3 vs. F1, 2) | 39 (13) | 13 (35) | 26 (10) | < 0.001 |
| Red colour sign (+) | 113 (37) | 19 (51) | 94 (35) | 0.047 |
| Propranolol dose, mg/day | 40 (60) | 40 (40) | 40 (60) | 0.390 |
| Ascitesc | 200 (65) | 17 (46) | 183 (67) | 0.011 |
| Hepatic encephalopathy | 36 (12) | 7 (19) | 29 (11) | 0.142 |
| Albumin, g/dL | 3.6 (0.9) | 3.6 (0.7) | 3.6 (0.9) | 0.868 |
| Total bilirubin, mg/dL | 1.5 (1.0) | 1.2 (0.8) | 1.5 (1.1) | 0.100 |
| Prothrombin time, INR | 1.25 (0.27) | 1.25 (0.25) | 1.25 (0.26) | 0.300 |
| Platelet, ├Ś 103/mm3 | 82 (50) | 89 (52) | 81 (49) | 0.512 |
| Measured liver volume, mL | 1,062 (520) | 1,108 (480) | 1,028 (528) | 0.202 |
| Measured spleen volume, mL | 416 (375) | 415 (238) | 417 (572) | 0.591 |
| Volume indexd | 0.747 (0.535) | 0.811 (0.372) | 0.737 (0.297) | 0.044 |
Table┬Ā2.
Competing-risks regression analysis for predicting primary prophylaxis failure
|
Univariate |
Multivariate |
|||
|---|---|---|---|---|
| SHR (95% CI) | p value | SHR (95% CI) | p value | |
| Age, yr | 1.02 (0.99ŌĆō1.05) | 0.225 | ||
| Male sex | 2.02 (0.92ŌĆō4.44) | 0.080 | ||
| Etiology of cirrhosis | ||||
| ŌĆāHBV | 0.51 (0.25ŌĆō1.03) | 0.061 | ||
| ŌĆāHCV | 1.19 (0.75ŌĆō1.88) | 0.468 | ||
| Alcohol historya | 1.18 (0.56ŌĆō2.48) | 0.668 | ||
| Child-Pugh class (B/C vs. A) | 0.83 (0.44ŌĆō1.59) | 0.582 | ||
| MELD score | 0.94 (0.88ŌĆō1.01) | 0.085 | ||
| Propranolol dosage | 0.99 (0.98ŌĆō1.00) | 0.175 | ||
| Varix size (large vs. medium/small) | 4.91 (2.55ŌĆō9.43) | < 0.001 | 4.46 (2.20ŌĆō9.05) | < 0.001 |
| Red colour sign | 2.15 (1.13ŌĆō4.08) | 0.019 | 1.49 (0.76ŌĆō2.92) | 0.245 |
| Ascitesb | 0.40 (0.21ŌĆō0.78) | 0.007 | 0.34 (0.18ŌĆō0.65) | 0.001 |
| Hepatic encephalopathy | 1.84 (0.85ŌĆō3.99) | 0.121 | ||
| Development of HCC | 0.79 (0.41ŌĆō1.51) | 0.476 | ||
| Albumin, g/dL | 0.88 (0.56ŌĆō1.37) | 0.569 | ||
| Total bilirubin, mg/dL | 0.74 (0.52ŌĆō1.05) | 0.093 | ||
| Prothrombin time, INR | 0.62 (0.17ŌĆō2.28) | 0.473 | ||
| Platelet, ├Ś 103/mm3 | 1.00 (0.99ŌĆō1.01) | 0.274 | ||
| Liver volume, mL | 1.00 (1.00ŌĆō1.00) | 0.124 | ||
| Spleen volume, mL | 1.00 (1.00ŌĆō1.00) | 0.820 | ||
| Volume index > 1c | 2.81 (1.39ŌĆō5.68) | 0.032 | 2.70 (1.37ŌĆō5.33) | 0.004 |
Deaths were analysed as a competing risk by Fine and Gray model [45].
SHR, subdistribution hazard ratio; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, Model for End-Stage Liver Disease; HCC, hepatocellular carcinoma; INR, international normalized ratio.
Table┬Ā3.
Reported variceal haemorrhage rates during primary prophylaxis with propranolol
| Study | Median follow-up, mon | Proportion of bleeding | Bleeding rate | Predictors of bleeding |
|---|---|---|---|---|
| Groszmann et al. (1990) [5] | 16 | 4% (2/51) | 12.9% at 2 yr | HVPG > 12 mmHg |
| De et al. (1999) [6] | 18 | 7% (1/15) | ||
| Lui et al. (2002) [7] | 21 | 15% (9/66) | 19.4% at 2 yr | Female, NIEC score > 30 |
| Bureau et al. (2002) [8] | 26 | 10% (2/20) | HVPG > 12 mmHg | |
| Schepke et al. (2004) [9] | 34 | 28% (22/77) | 17.6% at 2 yr | Bilirubin, creatinine |
| Jutabha et al. (2005) [10] | 15 | 19% (6/31) | 29% at 1 yr | |
| Psilopoulos et al. (2005) [11] | 28 | 30% (9/30) | 25% at 2 yr | Varix size |
| Thuluvath et al. (2005) [12] | 27 | 7% (1/15) | ||
| Turnes et al. (2006) [13] | 68 | 20% (16/71) | HVPG reduction < 20%, low platelet | |
| Lay et al. (2006) [14] | 35 | 16% (8/50) | 16.9% at 2 yr | |
| Dell'Era et al. (2008) [15] | 32 | 14% (8/57) | 12.6% at 2 yr | |
| Sharma et al. (2009) [16] | 24 | 13% (7/56) | 4%ŌĆō24% at 2 yr | HVPG reduction < 20% |
| Je et al. (2014) [17] | 82 | 10% (32/330) | 2.5% at 3 yr | Varix size, red color sign |
| Shukla et al. (2016) [18] | 12 | 12% (678/5,775) | 11.7% at 1 yr | Age, ascites, medical comorbidity, higher MELD scores. lower daily dose, hemodynamic response |
REFERENCES
1. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017;65:310ŌĆō335.


2. de Franchis R, Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop. Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743ŌĆō752.


3. Simonetto DA, Shah VH, Kamath PS. Primary prophylaxis of variceal bleeding. Clin Liver Dis 2014;18:335ŌĆō345.


4. Jalan R, Hayes PC. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. British Society of Gastroenterology. Gut 2000;46(Suppl 3-4):III1ŌĆōIII15.


5. Groszmann RJ, Bosch J, Grace ND, et al. Hemodynamic events in a prospective randomized trial of propranolol versus placebo in the prevention of a first variceal hemorrhage. Gastroenterology 1990;99:1401ŌĆō1407.


6. De BK, Ghoshal UC, Das T, Santra A, Biswas PK. Endoscopic variceal ligation for primary prophylaxis of oesophageal variceal bleed: preliminary report of a randomized controlled trial. J Gastroenterol Hepatol 1999;14:220ŌĆō224.


7. Lui HF, Stanley AJ, Forrest EH, et al. Primary prophylaxis of variceal hemorrhage: a randomized controlled trial comparing band ligation, propranolol, and isosorbide mononitrate. Gastroenterology 2002;123:735ŌĆō744.


8. Bureau C, Peron JM, Alric L, et al. "A La Carte" treatment of portal hypertension: adapting medical therapy to hemodynamic response for the prevention of bleeding. Hepatology 2002;36:1361ŌĆō1366.


9. Schepke M, Kleber G, Nurnberg D, et al. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology 2004;40:65ŌĆō72.


10. Jutabha R, Jensen DM, Martin P, Savides T, Han SH, Gornbein J. Randomized study comparing banding and propranolol to prevent initial variceal hemorrhage in cirrhotics with high-risk esophageal varices. Gastroenterology 2005;128:870ŌĆō881.


11. Psilopoulos D, Galanis P, Goulas S, et al. Endoscopic variceal ligation vs. propranolol for prevention of first variceal bleeding: a randomized controlled trial. Eur J Gastroenterol Hepatol 2005;17:1111ŌĆō1117.


12. Thuluvath PJ, Maheshwari A, Jagannath S, Arepally A. A randomized controlled trial of beta-blockers versus endoscopic band ligation for primary prophylaxis: a large sample size is required to show a difference in bleeding rates. Dig Dis Sci 2005;50:407ŌĆō410.



13. Turnes J, Garcia-Pagan JC, Abraldes JG, Hernandez-Guerra M, Dell'Era A, Bosch J. Pharmacological reduction of portal pressure and long-term risk of first variceal bleeding in patients with cirrhosis. Am J Gastroenterol 2006;101:506ŌĆō512.


14. Lay CS, Tsai YT, Lee FY, et al. Endoscopic variceal ligation versus propranolol in prophylaxis of first variceal bleeding in patients with cirrhosis. J Gastroenterol Hepatol 2006;21:413ŌĆō419.


15. Dell'Era A, Sotela JC, Fabris FM, et al. Primary prophylaxis of variceal bleeding in cirrhotic patients: a cohort study. Dig Liver Dis 2008;40:936ŌĆō943.


16. Sharma P, Kumar A, Sharma BC, Sarin SK. Early identification of haemodynamic response to pharmacotherapy is essential for primary prophylaxis of variceal bleeding in patients with 'high-risk' varices. Aliment Pharmacol Ther 2009;30:48ŌĆō60.


17. Je D, Paik YH, Gwak GY, et al. The comparison of esophageal variceal ligation plus propranolol versus propranolol alone for the primary prophylaxis of esophageal variceal bleeding. Clin Mol Hepatol 2014;20:283ŌĆō290.




18. Shukla R, Kramer J, Cao Y, et al. Risk and predictors of variceal bleeding in cirrhosis patients receiving primary prophylaxis with non-selective beta-blockers. Am J Gastroenterol 2016;111:1778ŌĆō1787.



19. Villanueva C, Aracil C, Colomo A, et al. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology 2009;137:119ŌĆō128.


20. North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med 1988;319:983ŌĆō989.


21. Rigo GP, Merighi A, Chahin NJ, et al. A prospective study of the ability of three endoscopic classifications to predict hemorrhage from esophageal varices. Gastrointest Endosc 1992;38:425ŌĆō429.


22. Nevens F, Bustami R, Scheys I, Lesaffre E, Fevery J. Variceal pressure is a factor predicting the risk of a first variceal bleeding: a prospective cohort study in cirrhotic patients. Hepatology 1998;27:15ŌĆō19.


23. Merkel C, Zoli M, Siringo S, et al. Prognostic indicators of risk for first variceal bleeding in cirrhosis: a multicenter study in 711 patients to validate and improve the North Italian Endoscopic Club (NIEC) index. Am J Gastroenterol 2000;95:2915ŌĆō2920.


24. Kim BK, Kim DY, Han KH, et al. Risk assessment of esophageal variceal bleeding in B-viral liver cirrhosis by a liver stiffness measurement-based model. Am J Gastroenterol 2011;106:1654ŌĆō1662.



25. Morishita N, Hiramatsu N, Oze T, et al. Liver stiffness measurement by acoustic radiation force impulse is useful in predicting the presence of esophageal varices or high-risk esophageal varices among patients with HCV-related cirrhosis. J Gastroenterol 2014;49:1175ŌĆō1182.



26. Takuma Y, Nouso K, Morimoto Y, et al. Prediction of oesophageal variceal bleeding by measuring spleen stiffness in patients with liver cirrhosis. Gut 2016;65:354ŌĆō355.


27. Higashiyama H, Yamaguchi T, Mori K, et al. Graft size assessment by preoperative computed tomography in living related partial liver transplantation. Br J Surg 1993;80:489ŌĆō492.


28. Henderson JM, Heymsfield SB, Horowitz J, Kutner MH. Measurement of liver and spleen volume by computed tomography. Assessment of reproducibility and changes found following a selective distal splenorenal shunt. Radiology 1981;141:525ŌĆō527.


29. Urata K, Kawasaki S, Matsunami H, et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 1995;21:1317ŌĆō1321.


30. Lin XZ, Sun YN, Liu YH, et al. Liver volume in patients with or without chronic liver diseases. Hepatogastroenterology 1998;45:1069ŌĆō1074.

31. Saygili OB, Tarhan NC, Yildirim T, Serin E, Ozer B, Agildere AM. Value of computed tomography and magnetic resonance imaging for assessing severity of liver cirrhosis secondary to viral hepatitis. Eur J Radiol 2005;54:400ŌĆō407.


32. Zhou XP, Lu T, Wei YG, Chen XZ. Liver volume variation in patients with virus-induced cirrhosis: findings on MDCT. AJR Am J Roentgenol 2007;189:W153ŌĆōW159.


33. Liu P, Li P, He W, Zhao LQ. Liver and spleen volume variations in patients with hepatic fibrosis. World J Gastroenterol 2009;15:3298ŌĆō3302.



34. Li WX, Zhao XT, Chai WM, et al. Hepatitis B virus-induced liver fibrosis and cirrhosis: the value of liver and spleen volumetry with multi-detector spiral computed tomography. J Dig Dis 2010;11:215ŌĆō223.


35. Tong C, Xu X, Liu C, Zhang T, Qu K. Assessment of liver volume variation to evaluate liver function. Front Med 2012;6:421ŌĆō427.



36. Hagan MT, Sayuk GS, Lisker-Melman M, et al. Liver volume in the cirrhotic patient: does size matter? Dig Dis Sci 2014;59:886ŌĆō891.




37. Lee CS, Jung YJ, Kim SS, et al. Liver volume-based prediction model stratifies risks for hepatocellular carcinoma in chronic hepatitis B patients on surveillance. PLoS One 2018;13:e0190261.



38. Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: the importance of multistate models and competing risks analysis. Hepatology 2015;62:292ŌĆō302.


39. D'Amico G, Morabito A, D'Amico M, et al. Clinical states of cirrhosis and competing risks. J Hepatol 2018;68:563ŌĆō576.


40. Yoo S, Lee KH, Lee HJ, et al. Seoul National University Bundang Hospital's electronic system for total care. Healthc Inform Res 2012;18:145ŌĆō152.



41. de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005;43:167ŌĆō176.


42. Dello SA, van Dam RM, Slangen JJ, et al. Liver volumetry plug and play: do it yourself with ImageJ. World J Surg 2007;31:2215ŌĆō2221.



43. Um EH, Hwang S, Song GW, et al. Calculation of standard liver volume in Korean adults with analysis of confounding variables. Korean J Hepatobiliary Pancreat Surg 2015;19:133ŌĆō138.



44. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known 1916. Nutrition 1989;5:303ŌĆō311.

45. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496ŌĆō509.

46. Masubuchi Y, Hosokawa S, Horie T, et al. Cytochrome P450 isozymes involved in propranolol metabolism in human liver microsomes. The role of CYP2D6 as ring-hydroxylase and CYP1A2 as N-desisopropylase. Drug Metab Dispos 1994;22:909ŌĆō915.

47. Serste T, Melot C, Francoz C, et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology 2010;52:1017ŌĆō1022.


48. Mandorfer M, Bota S, Schwabl P, et al. Nonselective ╬▓ blockers increase risk for hepatorenal syndrome and death in patients with cirrhosis and spontaneous bacterial peritonitis. Gastroenterology 2014;146:1680ŌĆō1690.


49. Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of ╬▓-blockers improve survival of patients with cirrhosis during a window in the disease. Gut 2012;61:967ŌĆō969.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



