INTRODUCTION
When treating patients with fever of unknown origin who have heart disease or a history of open heart surgery, physicians must consider infective endocarditis (IE) as a differential diagnosis. Recent epidemiological modifications of IE include its mode of acquisition and microbiological profile [1]. The drastic restrictions in indications for IE prophylaxis have been reported unassociated with an increased incidence of IE [23]; however, temporal trend analysis has shown a significant increase in IE in patients without previously detected heart disease [3]. Thorough understanding of the clinical features of these patients is needed to prevent IE, a condition with an unacceptably high mortality rate. Moreover, the relative association of undiagnosed, clinically silent mitral valve prolapse, bicuspid aortic valves, and apparently normal valves with mild valvular dysfunction with the development of IE remains elusive.
IE IN PATIENTS WITHOUT PREVIOUSLY RECOGNIZED PREDISPOSING HEART DISEASE
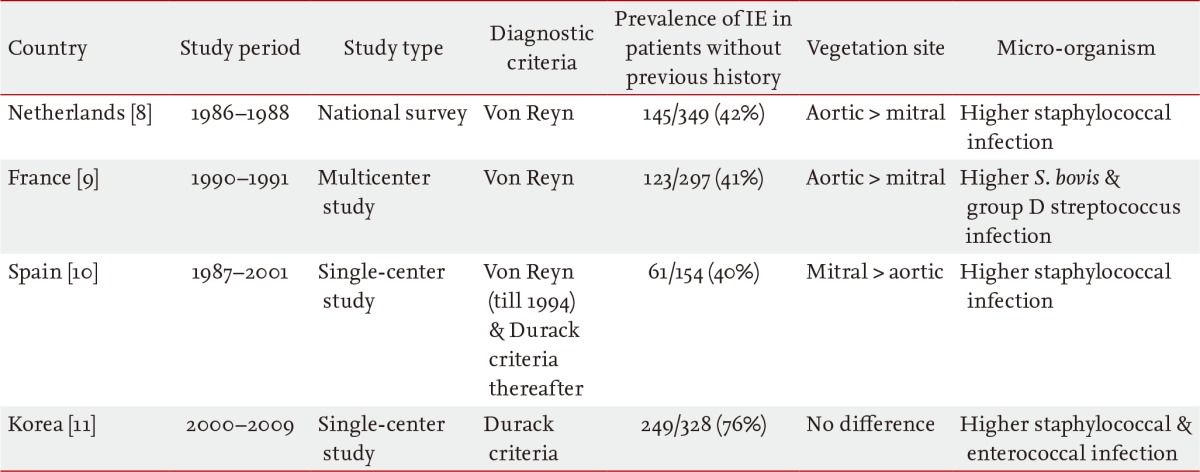
Although blood cultures and cardiac imaging with echocardiography are considered the cornerstones of IE diagnosis, clinical suspicion and the integration of all clinical and laboratory data are also important. Traditionally, IE has been suspected in patients with previously detected heart disease or a history of open heart surgery who develop fever, generalized weakness, have weight loss, and/or evidence of systemic embolism. Endocardial damage due to significant shear stress caused by underlying heart disease that results in non-bacterial thrombotic endocarditis has traditionally been considered a prerequisite for bacteremia and the successful development of vegetation [45]. Based on this paradigm, and despite recent drastic restrictions in its indications, patients with previously detected heart disease or a history of open heart surgery have been the main target of IE prophylaxis [67]. However, even in 20-year-old studies from The Netherlands and France that used the old von Reyn diagnostic criteria [89], ≥ 40% of patients diagnosed with IE had no previous history of predisposing heart disease of any type; IE was the first clinical presentation in these patients (Table 1). Similarly, a study from Spain confirmed this finding, with 40% of patients having no history of heart disease [10]. Groups of patients without previous underlying heart disease have been found to differ in clinical characteristics, including vegetation site, major infecting microorganism, and mortality rate. After excluding intravenous drug abusers and patients with pacemakers, Selton-Suty et al. [9] found that IE affected the aortic valve more frequently, with a higher prevalence of group D Streptococci (bovis) and no difference in mortality. By contrast, Castillo et al. [10] found that in patients with pacemakers, vegetation was more frequent in the right cardiac chamber and the mitral valve than in the aortic valve, and that the prevalence of Staphylococcus aureus was higher and mortality rates were significantly lower.
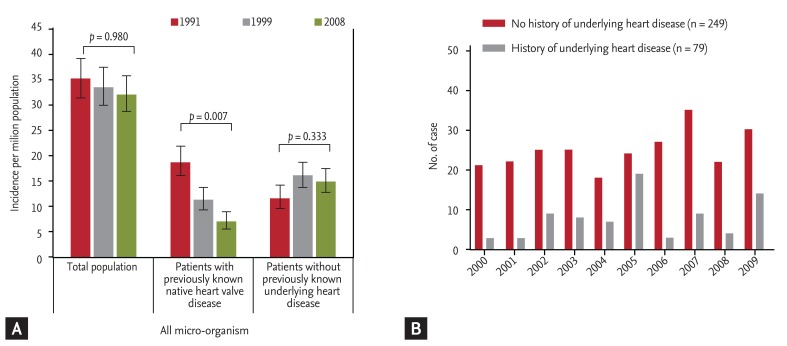
Two recent studies have shown a temporal trend of IE in patients without previously recognized underlying heart disease. Three successive population surveys from France showed that the rate of IE patients without previously detected heart disease increased from 34% in 1991 to 49% in 1999 (p < 0.001) and remained stable at 47% in 2008 (Fig. 1A) [3]. In a recent analysis of 328 consecutive Korean patients with IE in a tertiary referral hospital, 76% (249 patients) had no prior medical history of underlying heart disease at the time of hospitalization for IE [11]. Temporal trend analysis showed that the trend of IE developing in patients without predisposing underlying heart disease did not change during the study period (Fig. 1B). These discrepancies may have been due to differences in inclusion criteria and study settings (a national survey versus an observational study in a single-center). It is interesting that another single-center study by Castillo et al. [10] also showed an increasing tendency during the study period [10].
IE OF APPARENTLY NORMAL-LOOKING VALVES
Although it has been well recognized that a significant proportion of patients with IE have no previously diagnosed predisposing conditions, clinical data regarding underlying unrecognized cardiac lesions associated with the development of IE in these patients are limited. Since imaging modalities such as echocardiography have been utilized to diagnose these conditions for fewer than 20 years, the absence of imaging data has been a critical flaw in many clinical studies, even recent population-based surveys. Undiagnosed mitral valve prolapse or a bicuspid aortic valve have been suggested as major causes of unrecognized cardiac lesions associated with IE development [912]. In a recent report using a modern diagnostic criteria for IE with transesophageal echocardiography being performed in more than 95% of patients, an 'apparently normal-looking valve,' defined as minimally thickened valvular leaflets with normal valvular function or trivial valvular regurgitation, was found to be the second most common underlying condition associated with IE development following mitral valve prolapse [11]. Of 249 patients without prior medical history of underlying heart disease, IE developed in an apparently normal-looking valve in 64 patients (26%), whereas the remaining 185 patients had clinically silent valvular or congenital heart disease with mitral valve prolapse being the most common underlying disease entity (n = 82, 44%). Other underlying disease entities included bicuspid aortic valve (n = 29), rheumatic valvular heart disease (n = 21), calcified aortic stenosis (n = 21), and congenital heart disease (n = 14). This study is not the first to highlight the clinical importance of an apparently normal-looking valve in IE development, as a single-center study from the mid-1980s found that 27% of study subjects with IE had no significant underlying cardiac disease [12].
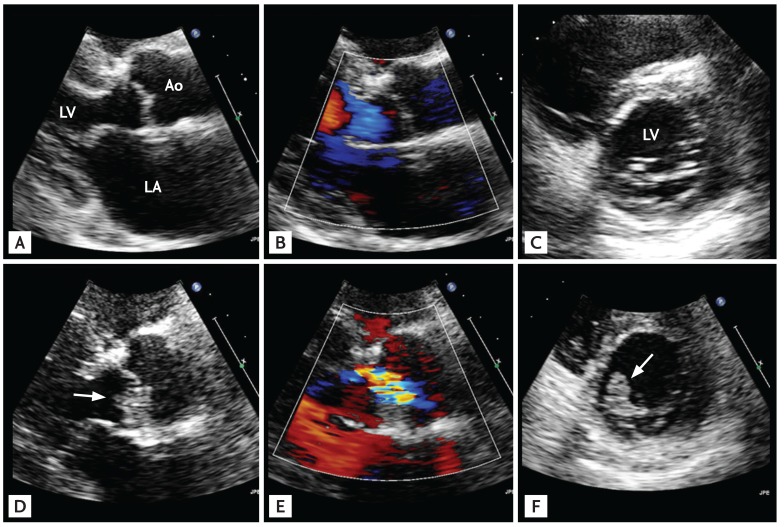
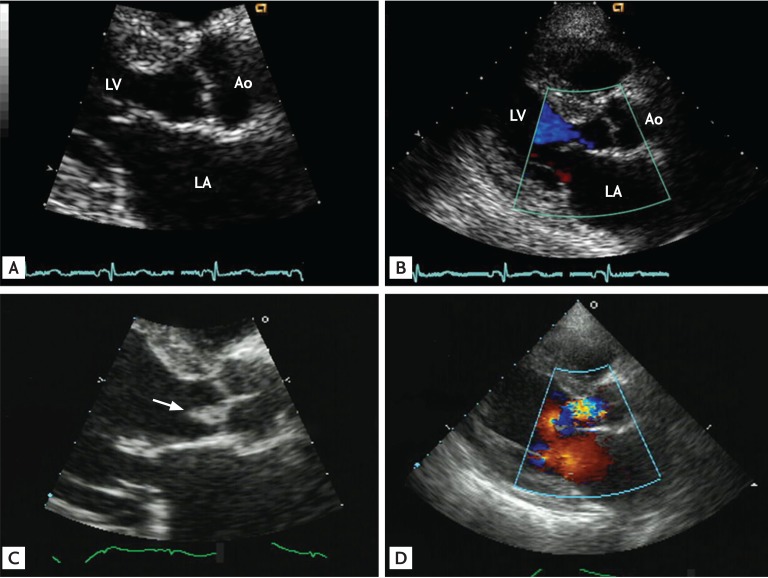
Healthcare associated infective endocarditis (HAIE) is another new epidemiologic trend of IE [13141516], and IE of an apparently normal-looking valve can be associated with both nosocomial (Fig. 2) and non-nosocomial (Fig. 3) HAIE. However, a recent report found that, interestingly, community-acquired IE involving an apparently normal looking valve (Fig. 4) is more frequent than HAIE (59% vs. 41%) [11]. The increasing clinical impact of this type of IE must be considered carefully, because neglecting this increasing proportion of IE patients may negatively impact their mortality rate. Compared to IE in patients with clinically silent valvular or congenital heart disease, patients with IE of apparently normal valves tend to be older with a higher incidence of diabetes and end-stage renal disease on hemodialysis, larger vegetation size, higher European System for Cardiac Operative Risk Evaluation II (EuroSCORE II), more frequent involvement of the aortic and tricuspid valve, and higher rates of IE with staphylococcal and enterococcal infection (Table 2). Surgery was performed less frequently in these patients due to their poorer medical status, which resulted in higher mortality.
Since the 1980s, the prevalence of rheumatic valvular heart disease and congenital heart disease as predisposing factors to the development of IE has decreased rapidly in industrialized countries [812]. Mitral valve prolapse has emerged as the most common underlying cardiac condition leading to IE in developed countries [17]. IE involving an apparently normal-looking valve has also been described as a prototype of IE in intravenous drug abusers, characterized by minimal thickening or deformation of the right-sided tricuspid valve [18]. Few clinical studies have described IE of apparently normal-looking valves in non-drug abusers and it remains unclear whether this pattern should be regarded as a relatively new trend associated with an increased incidence of HAIE. However, one report based on clinical data obtained more than 30 years ago showed that apparently normal-looking valves, as a clinical feature of IE, were more common in non-drug abusers than in drug abusers (59% vs. 41%) [12]. In that study, staphylococcal infections predominated in non-drug abusers with IE of apparently normal-looking valves, whereas streptococcal infections predominated in patients with mitral valve prolapse. The prevalence of co-existing illnesses-including severe vascular disease, metastatic malignancy, and renal failure-was significantly higher (as high as 40%) in non-drug abusers with IE of an apparently normal-looking valve than in patients with mitral valve prolapse and IE. The overall reported incidence of IE in an apparently normal-looking valve in non-drug abusers was 16% in a study performed in the early 1980s [12] and was 20% in a recent study [11], suggesting that clinicians remain unaware of this important finding in IE patients.
The pathophysiologic mechanism involving the development of non-HAIE or community-acquired IE in an apparently normal-looking valve with predominant staphylococcal infection is not yet clearly understood. A recent large population-based survey analyzing IE epidemiology after the recent drastic restrictions in indications for IE prophylaxis clearly showed an increase in the incidence of staphylococcal IE, especially in patients without previously detected underlying heart disease [3]. That finding was thought to be due to the increased number of patients with prosthetic valve IE, pacemaker IE, and diabetes mellitus, all of which are associated with staphylococcal bacteremia. The higher prevalences of non-HAIE and community acquired IE than HAIE in a recent report [11], excluding prosthetic valve and pacemaker IE, suggests that catheter- or device-related blood infections associated with increased healthcare or medical interventions alone cannot explain the contemporary trend in IE patterns. Recently, increased age at the time of diagnosis of left-sided IE is reported to be associated with less valvular impairment (insufficiency and perforation) and a more favorable microbiological profile. Additionally, S. aureus IE is more common in younger patients, suggesting the important role of patients' age in determining clinical characteristics [19]. Age-dependent changes in host-defense mechanisms or underlying cardiac conditions may be important, as may age-dependent increases in clinically silent pre-existent valvular regurgitation without structural abnormalities on two-dimensional echocardiography [2021]. Moreover, age-dependent increases in cardiac valvular abnormalities that met previous IE prophylaxis guidelines have been observed [5]. The rates of mitral and tricuspid regurgitation were similar, with the rate of aortic regurgitation slightly lower, and these findings differed from the affected sites of vegetation in clinical studies. Minor injury to the valvular endocardium may occur more frequently in left-sided cardiac valves inherently exposed to relatively high pressure, making these valves more vulnerable to the development of non-bacterial and/or bacterial thrombotic endocarditis with cumulative bacteremia or repeat intravenous injection of particulate material. A recent report suggested that bacteremia prevention is essential even in patients without previously detected predisposing heart disease to reduce the unacceptably high in-hospital mortality rate in patients with IE [11].
CONCLUSIONS
A large proportion of patients with IE have no previous history of underlying heart disease. Analysis of underlying disease showed that undetected mitral valve prolapse was the most common disease, followed by an apparently structurally normal valve. HAIE developed in less than half of patients with IE in an apparently normal-looking valve. Community-acquired IE involving an apparently normal-looking valve in patients with no history of underlying heart disease has been inappropriately neglected. The higher mortality rate and larger vegetation size observed in these patients warrants repartition of at-risk groups for better targeting of IE prophylaxis to improve clinical outcomes.





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





