Submucosal saline injection and mini-probe endoscopic ultrasound to assess endoscopic resectability of colorectal subepithelial tumors
Article information
Abstract
Background/Aims
This study aimed to evaluate the feasibility and outcomes of mini-probe endoscopic ultrasound (mEUS) followed by submucosal saline injection (SSI-mEUS) for assessing the endoscopic resectability of colorectal subepithelial lesions (SELs).
Methods
From January 2020 to December 2023, the medical records of 391 SELs (364 patients) were retrospectively reviewed and categorized into no EUS, mEUS-only, and SSI-mEUS groups according to the procedure used. To compare variables between the SSI-mEUS and other groups, the no EUS and mEUS-only groups were combined into the non-SSI-mEUS group. In the SSI-mEUS group, submucosal cushion thickness was endosonographically measured after the saline injection. Treatment outcomes and histological diagnosis were retrospectively reviewed.
Results
A total of 210 lesions in the no EUS group, 23 in the mEUS-only group, and 125 in the SSI-mEUS group were endoscopically resected. The mean SEL size was larger in the SSI-mEUS than in the non-SSI-mEUS group (6.8 ± 2.6 mm vs. 4.9 ± 2.6 mm, p < 0.001). R0 resection was achieved in 107 of 110 neoplastic lesions (97.3%) in the SSI-mEUS group vs. 159 of 176 neoplastic lesions (90.3%) in the non-SSI-mEUS group (p = 0.046). Not using SSI-mEUS was the only factor associated with indeterminate or positive deep resection margins (odds ratio 3.45, 95% confidence interval 1.19–13.40, p = 0.021).
Conclusions
For colorectal SELs, including those that appear insufficiently elevated during conventional endoscopy, SSI-mEUS enables an objective assessment of the feasibility of endoscopic resection and can predict a high likelihood of achieving a safe and complete resection.
INTRODUCTION
As screening colonoscopy becomes more routine, an increasing number of colorectal subepithelial lesions (SELs) are being incidentally detected [1,2]. If colorectal SELs are suspected to be neoplastic but have not invaded the proper muscle layer of the colorectal wall, therapeutic endoscopic resection may be considered. For example, rectal neuroendocrine tumors (NETs) are among the representative colorectal SELs that can be endoscopically treated [3-5]. However, endoscopic resection of colorectal SELs is challenging for endoscopists, as predicting invasion depth using conventional endoscopy is difficult. Non-lifting of lesions after submucosal injection indicates that endoscopic treatment may be challenging in cases of early colorectal neoplasia and rectal NETs [6-8]. However, most colorectal SELs are typically less lifted after submucosal injection than non-fibrotic and non-invasive colorectal epithelial neoplasia. Moreover, non-lifting is a subjective finding, as it cannot be measured through colonoscopy. Therefore, lifting achieved through submucosal injections does not guarantee histologically complete resection after the endoscopic treatment of colorectal SELs.
Mini-probe endoscopic ultrasound (mEUS) provides sonographic images of the colorectal wall structures and aids in identifying the nature and location of SELs. In a previous study evaluating the role of mEUS followed by submucosal saline injection (SSI-mEUS) [9], endoscopic mucosal resection (EMR) was performed on 18 colorectal SELs that showed endosonographic detachment from the muscularis propria after SSI, resulting in complete resection in 17 cases (94.4%). Accordingly, the authors suggested that detachment of submucosal lesions from the muscularis propria could indicate the endoscopic resectability of SELs. However, aside from that small-sample study, no research has evaluated the feasibility of SSI-mEUS for assessing the endoscopic resectability of colorectal SELs. In this study, we evaluated the feasibility of SSI-mEUS and analyzed the outcomes of colorectal SELs treated with endoscopic resection following SSI-mEUS.
METHODS
Patients and data collection
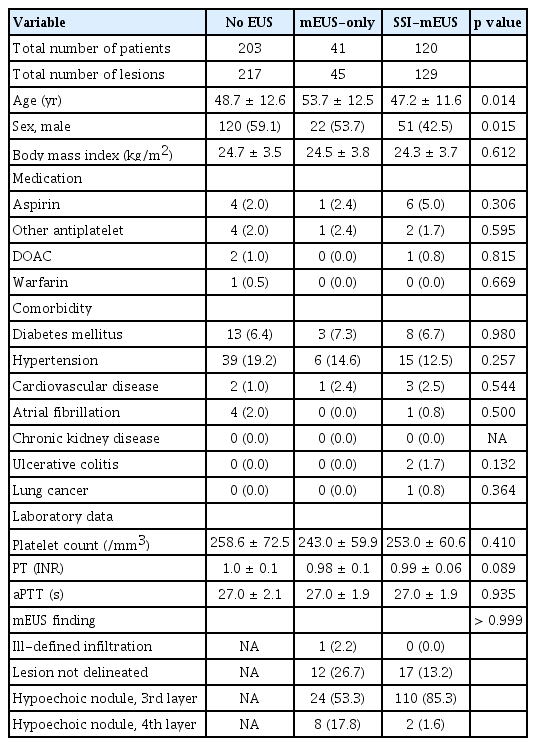
This retrospective study examined data from a single-center database of 391 colorectal SELs (364 patients) treated at Asan Medical Center, Seoul, Korea, from January 2020 to December 2023. All patients provided informed consent before undergoing any procedures. Because mEUS was not reimbursable by the Korean National Healthcare Insurance System, it was performed only on those who provided informed consent for mEUS. A total of 217 SELs (203 patients) were managed without mEUS, while the other 174 SELs (161 patients) were managed with mEUS. SSI-mEUS was primarily performed between October 2022 and December 2023. Thus, 129 SELs (120 patients) were evaluated by SSI-mEUS, while the remaining 45 SELs in 41 patients were evaluated by mEUS alone.
We collected data on lesion characteristics (i.e., estimated size and location) and mEUS findings (including the distance between the deepest SEL margin and the proper muscle layer among those who underwent SSI-mEUS). Treatment outcomes and histological diagnosis derived from the electronic medical records were retrospectively reviewed. Lesions were categorized according to whether mEUS was performed before endoscopic resection to assess endoscopic resectability: no EUS, mEUS-only, and SSI-mEUS. The current study was approved by the Institutional Review Board of Asan Medical Center (approval no. 2024-0919). The requirement for informed consent was waived by the Institutional Review Board in view of the retrospective nature of this study.
SSI-mEUS procedure
Under conscious sedation, a high-definition colonoscope (CF-HQ290AI; Olympus Medical Systems, Tokyo, Japan) was introduced into the colorectum. Upon localization of the target lesion and filling of the space with distilled water, a through-the-channel 20-MHz ultrasonic miniature probe (GF-UM2000 endoscopic system; Olympus Medical Systems) was inserted to obtain endosonographic images. The endosonographically measured lesion size and occupied layers were recorded. For SELs primarily located in the third layer, the status of the interface between the third and fourth layers was recorded. The endoscopist injected 10–32 mL of saline–epinephrine solution using a 22-gauge injection needle (Taewoong Medical Corp., Gimpo, Korea) until the perilesional submucosa was lifted circumferentially. When air inside the catheter is injected along with the saline–epinephrine solution, microbubbles may form and create hyperechoic artifacts in the EUS images, making it difficult to measure the submucosal cushion. To prevent this, the catheter was flushed twice with saline to remove any air before the solution was injected. The volume of the injected solution varied depending on lesion size and degree of scarring from prior procedures. A minimum of 10 mL was injected; additional solution was administered if the submucosal lift was deemed insufficient.
After the submucosal injection, even if a submucosal cushion was formed, optimal EUS images could not be obtained unless the lesion and the EUS probe were horizontally aligned. To address this, the endoscope was maneuvered or injected water volume adjusted to ensure that the EUS probe was horizontally aligned with the lesion-bearing wall before images were obtained. Subsequently, endosonographic images of the lesion and its underlying area were captured. The distance between the base of the lesion and the fourth layer (thickness of the submucosal cushion) was measured endosonographically. When the primary lesion could not be delineated using mEUS (category II), the distance between the second (i.e., muscularis mucosa) and fourth layers was measured and considered the submucosal cushion thickness. Considering the three-dimensional nature of the lesion, submucosal cushion thickness was measured at least twice, and the thinnest and thickest sites were recorded. The SSI-mEUS method is illustrated in Figure 1.
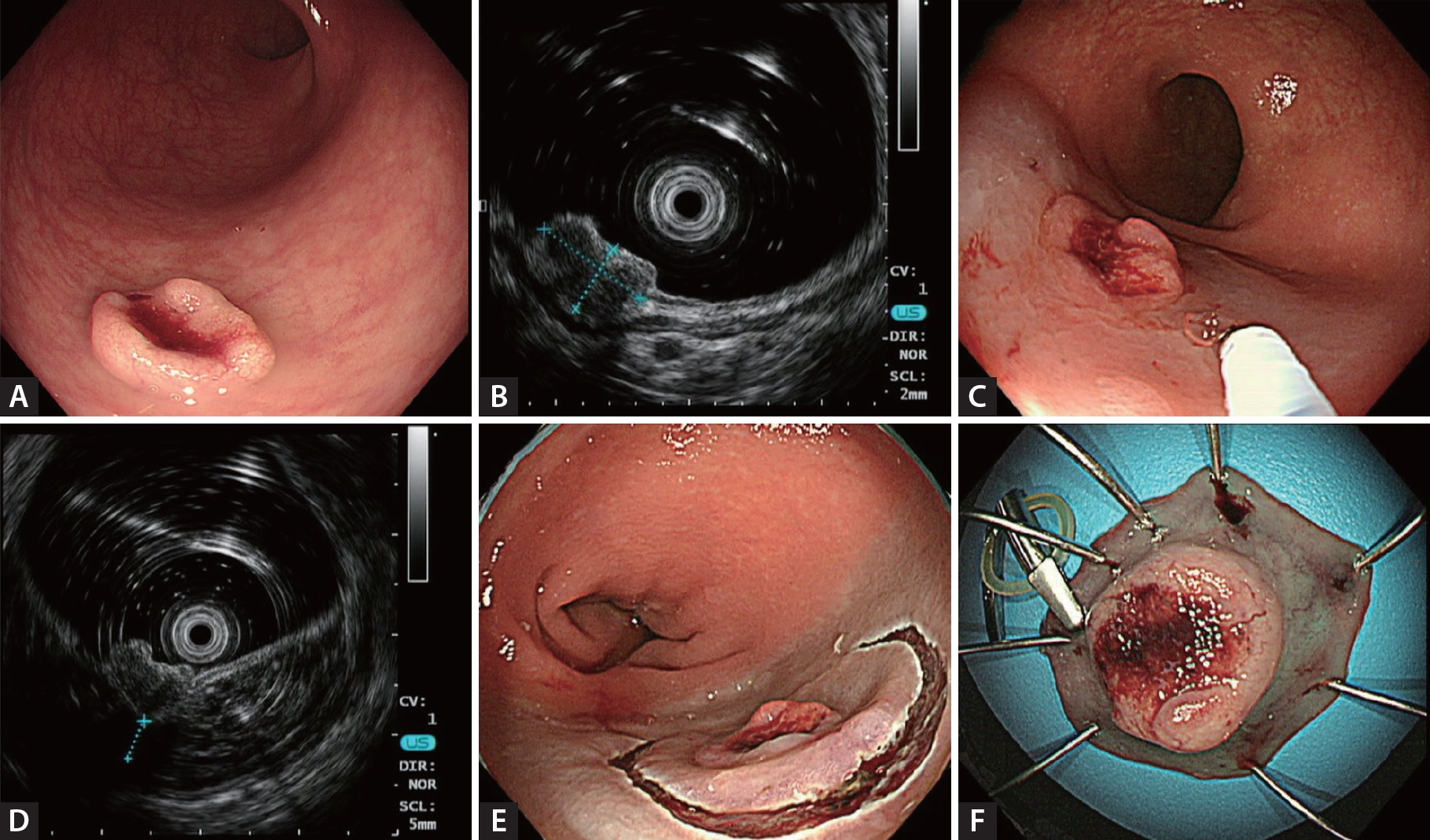
Example mini-probe endoscopic ultrasound image of a colorectal subepithelial lesion. (A) Subepithelial lesion with central ulceration. (B) Mini-probe endoscopic ultrasound image showing a 9.0 × 5.3 mm hypoechoic nodule with a slightly disrupted interface between the third and fourth layers. (C, D) Distance between the third and fourth layers of 4.7 mm after submucosal saline injection. (E, F) Lesion removed by endoscopic submucosal dissection with a final histology of 13 mm, grade 2 rectal neuroendocrine tumor and clear resection margins.
Therapeutic decision-making and endoscopic treatment of colorectal SELs
Based on the endoscopic and mEUS findings, the suspected colorectal SELs were categorized as follows: category I, nodules identifiable on both endoscopy and mEUS regardless of echogenicity; category II, scar changes visible on endoscopy but no identifiable lesion visible on mEUS; category III, neither scar changes nor lesions identifiable on repeated endoscopy and mEUS; and category IV, SELs detected using endoscopy but ill-defined infiltrative lesions detected on mEUS.
Among the category I lesions, if the nodules were located in the third layer (i.e., submucosa) on mEUS and separated from the fourth layer (i.e., muscularis propria) after SSI-mEUS, endoscopic resection was attempted. Transanal excision (TAE) or surgical resection was performed for lesions invading or located within the fourth layer on mEUS as well as those undetached from the muscularis propria in the SSI-mEUS group. Surgical resection was also performed when lymph node metastasis was suspected on radiologic studies. Endoscopic resection was attempted for category II lesions (scars noted only on endoscopy) if the original endoscopic findings or biopsy results from referring endoscopists were suggestive of incomplete resection of neoplastic lesions such as NETs. No treatment was attempted for category III lesions, as neither repeat endoscopy nor mEUS was able to identify the original lesion. For category IV lesions, repeated forceps biopsy and short-term follow-up endoscopy were performed without therapeutic intervention, as the mEUS findings were suggestive of a non-neoplastic or endoscopically unresectable lesion. In the group that did not undergo mEUS, incompletely resected rectal NETs were primarily treated with TAE due to positive deep resection margins, while the remaining lesions were treated with endoscopic resection. The therapeutic flow is depicted in Figure 2.
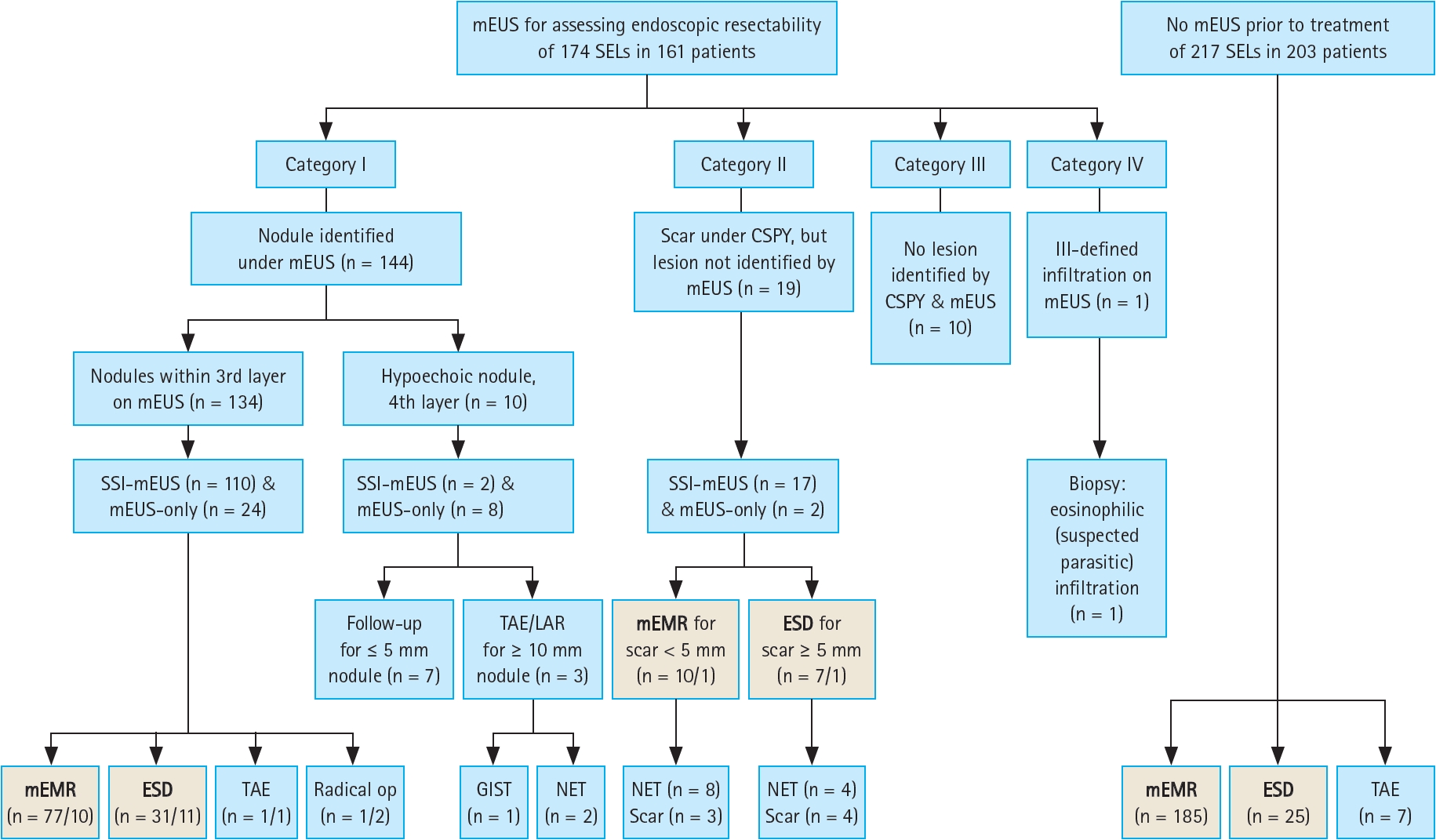
Flow of therapeutic decision-making and endoscopic treatment of colorectal subepithelial lesions. Among the total population, 174 colorectal lesions were assessed by mEUS, of which 144 were identified as nodules (category I), 29 were not delineated (categories II and III), and one had an ill-defined infiltration (category IV). The remaining 217 SELs (203 patients) did not undergo mEUS. A total of 358 lesions were endoscopically resected (written in bold letters in the gray boxes). The number of lesions in the SSI-mEUS group is listed before the slash, while the number of lesions in the mEUS-only group is listed after the slash in the gray boxes. mEUS, mini-probe endoscopic ultrasound; SELs, subepithelial lesions; SSI-mEUS, mEUS followed by submucosal saline injection; CSPY, colonoscopy; ESD, endoscopic submucosal dissection; TAE, transanal excision; LAR, low anterior resection; mEMR, modified endoscopic mucosal resection; GIST, gastrointestinal stromal tumor; NET, neuroendocrine tumor.
Figure 3 presents a simple algorithm for the treatment strategy of colorectal SELs based on SSI-mEUS findings. Endoscopists determine the endoscopic resectability of SELs using SSI-mEUS findings rather than the non-lifting sign. If the lesions remain undetached from the muscularis propria after the saline injection and fail to form an adequate submucosal cushion, TAE or radical resection should be considered. On the other hand, if an adequate submucosal cushion is present, endoscopic resection can be performed. Following the pathological results, further surgery may be considered; if surgery is unnecessary, regular follow-up can be conducted.
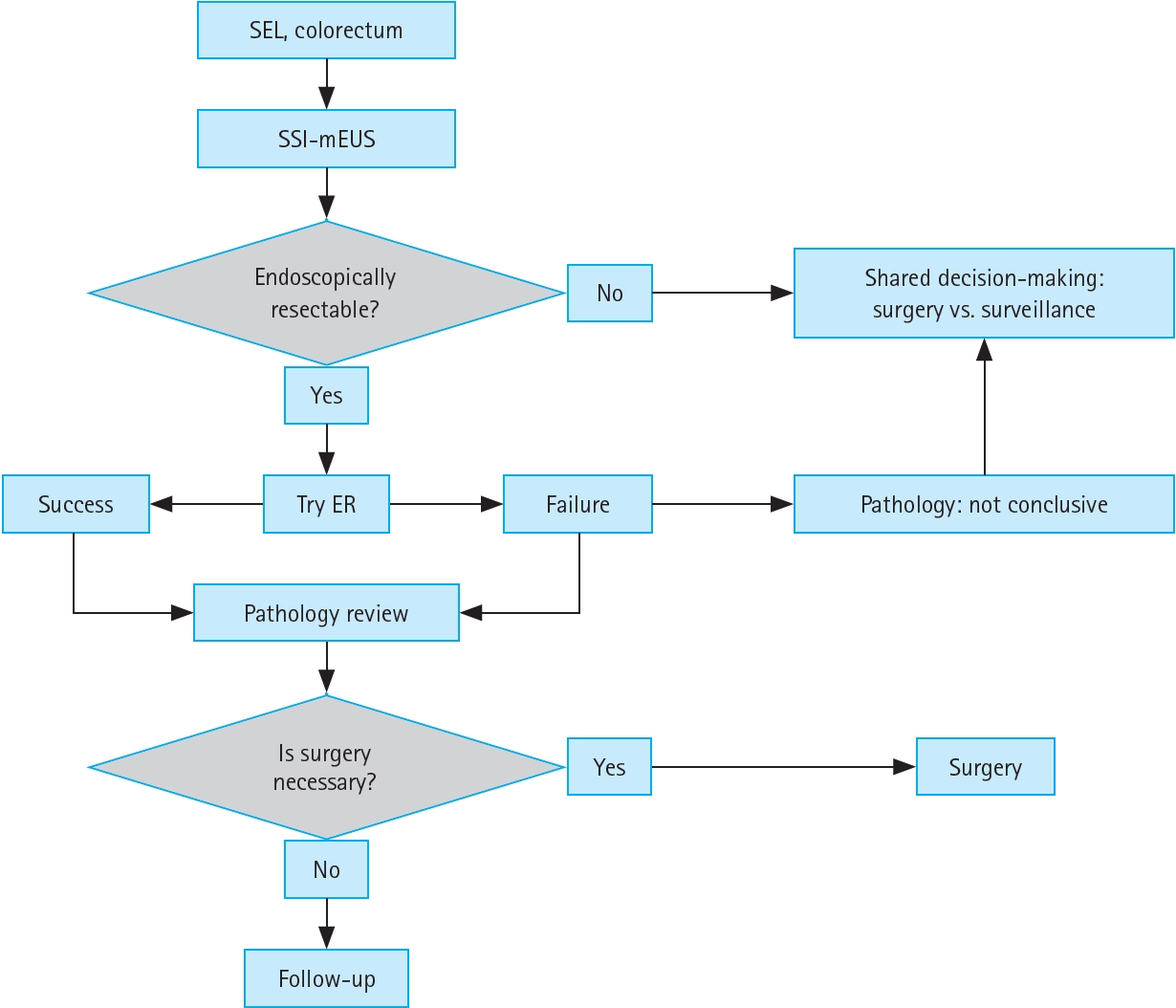
Algorithm for the treatment strategy of colorectal SELs. SEL, subepithelial lesion; SSI-mEUS, mini-probe endoscopic ultrasound followed by submucosal saline injection; ER, endoscopic resection.
For endoscopic resection, two types of modified EMR (mEMR) techniques (Tip-in EMR and precut EMR) or endoscopic submucosal dissection (ESD) were attempted. Each endoscopic resection technique was detailed previously [10-12].
Outcomes and definitions
The primary outcome was the histological complete resection rate defined as the histological absence of tumor cells at the lateral and deep resection margins. All resected specimens were examined by board-certified pathologists specializing in gastrointestinal pathology for the evaluation of resection margins and histological diagnosis. En bloc resection was defined as complete single resection of the targeted lesion irrespective of whether the basal and lateral tumor margins were infiltrated or undetermined.
Using the width of an open forceps as reference, lesion dimensions were assessed during endoscopy and measured directly through mEUS. When the lesions were incompletely lifted (Kato classification c) or not lifted (Kato classification d) by SSI [8], they were categorized as “insufficient lifting.” Despite insufficient lifting, submucosal cushion thickness could still be measured using mEUS.
Intra- and postprocedural complications were defined as peri- or postprocedural perforation or hemorrhage. Intraprocedural bleeding was defined as bleeding that required endoscopic hemostasis using hemostatic forceps or clips during the procedure, while delayed bleeding was defined as bleeding from the resection site that required endoscopic hemostasis or transfusion within 14 days after the endoscopic resection. Perforation was defined as a full-thickness mural defect in the colorectum detected during the endoscopic procedure or radiological evidence of free air under the diaphragm through chest/abdomen radiographs or computed tomography (CT) scans.
Statistical analysis
Continuous variables are presented as mean ± standard deviation, while categorical variables are presented as number and percentage. The no EUS and mEUS-only groups were combined into a new single group, non-SSI-mEUS group. Student’s t-test was used to compare continuous variables, while the chi-squared or Fisher’s exact test was used to compare categorical variables between two groups. One-way analysis of variance with a post hoc (Tukey’s method) test was used to compare the means among three groups. A 2 × 3 chi-squared test was used to analyze categorical variables among the three groups. To identify risk factors of indeterminate or positive deep resection margins in rectal NETs, a univariate analysis of potential factors was performed; variables with values of p < 0.15 in the univariate analysis were included in the multivariate logistic regression analysis. The analytical results are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Values of p < 0.05 were considered statistically significant. All statistical analyses were performed using R software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patients’ baseline characteristics
A total of 391 colorectal SELs (364 patients) were evaluated in this study. Among the total study population, 174 lesions (161 patients) were assessed by mEUS, while the remaining 217 SELs (203 patients) were not subjected to mEUS. The baseline characteristics of the patients with colorectal SELs are shown in Table 1. There were no significant differences in basic characteristics such as body mass index, medications, coagulation test results, or comorbidities among the three groups.
Clinicopathological features of resected SELs and procedural outcomes
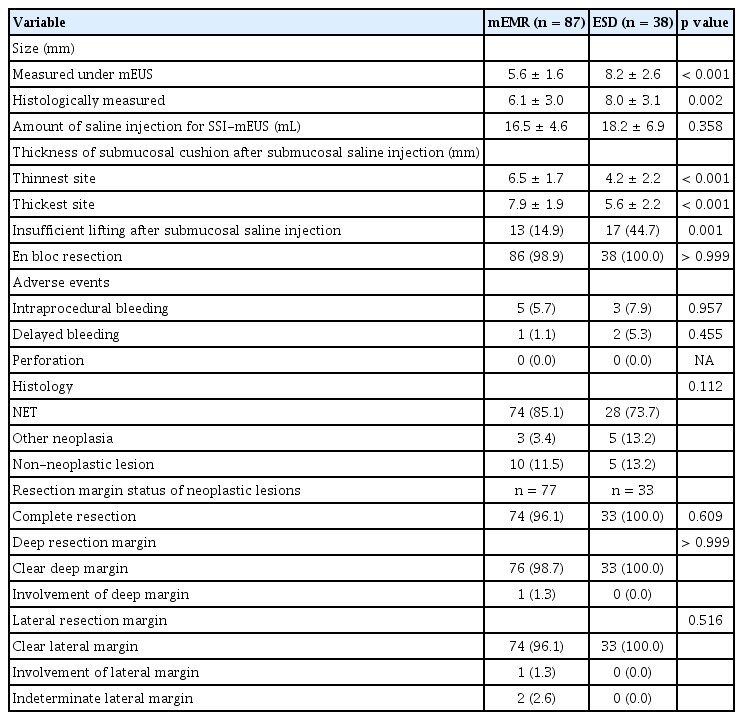
Of the overall study population, 210 lesions in the no EUS group, 23 lesions in the mEUS-only group, and 125 lesions in the SSI-mEUS group were endoscopically resected. To compare the clinicopathological features and procedural outcomes between the SSI-mEUS and other groups, the no EUS and mEUS-only groups were included in the non-SSI-mEUS group. Table 2 compares the endosonographic characteristics, procedural outcomes, and histological diagnoses of the resulting two groups. The mean estimated colorectal SEL size was larger in the SSI-mEUS group than in the non-SSI-mEUS group (6.8 ± 2.6 mm vs. 4.9 ± 2.6 mm, p < 0.001). Three SELs (1.3%) showed a central ulceration or depression on the surface in the non-SSI-mEUS group vs. 10 SELs (8.0%) in the SSI-mEUS group (p = 0.003). Submucosal cushion thickness after the saline injection was 5.8 ± 2.1 mm at the thinnest area and 7.1 ± 2.2 mm at the thickest area on mEUS. Endoscopic resection was attempted for all lesions, including those that were insufficiently lifted. In the non-SSI-mEUS group, mEMR was performed more frequently than in the SSI-mEUS group (84.1% vs. 69.6%, p < 0.001). The following lesions were identified as neoplastic SELs in the overall cohort: 335 cases of rectal NETs (including 110 rectal NETs referred for the salvage treatment due to histologically incomplete primary treatment), 5 of granular cell tumor, 2 of ulcerative colitis-associated dysplasia, and 2 of mucosa-associated lymphoid tissue (MALT) lymphoma.
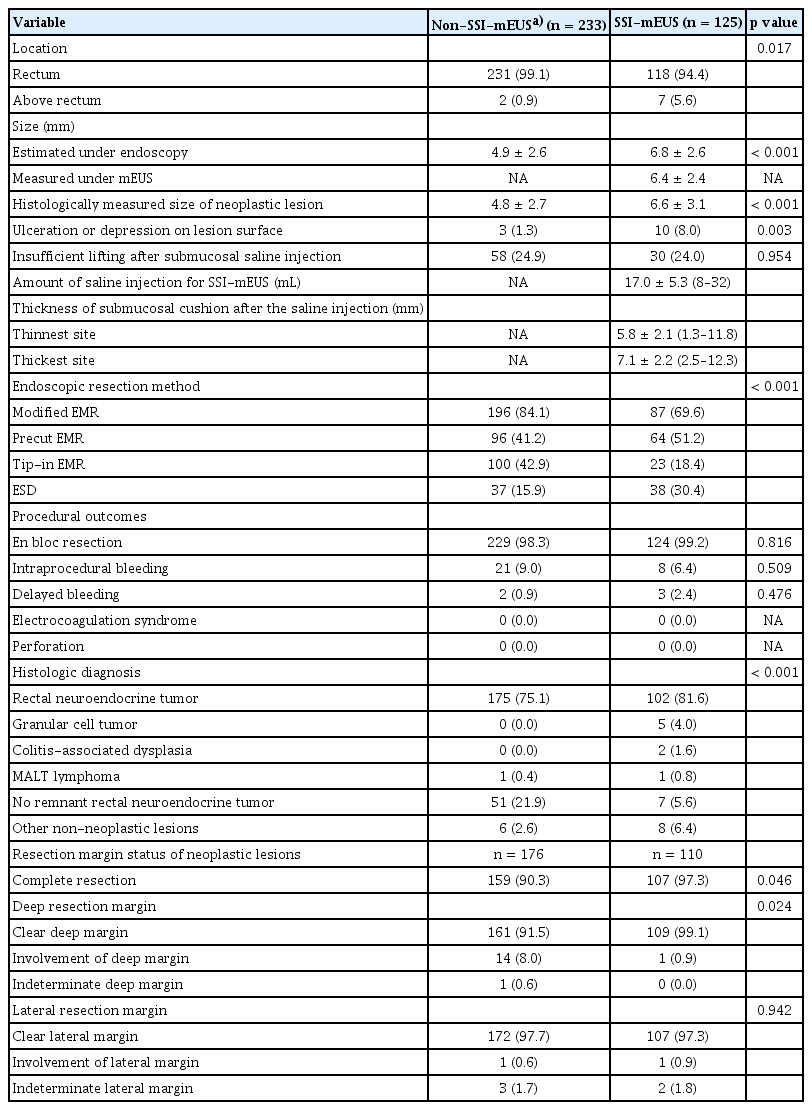
Endosonographic characteristics, procedural outcomes, and histologic diagnosis of endoscopically resected colorectal subepithelial lesions
En bloc resection was unattainable in five cases due to severe fibrosis, thereby necessitating piecemeal resection. No cases of electrocoagulation syndrome or perforation were noted in either group. There were no significant intergroup differences in intraprocedural and delayed bleeding, and all events were successfully managed by endoscopic intervention. R0 resection was achieved in 107 of 110 neoplastic lesions (97.3%) in the SSI-mEUS group and in 159 of 176 neoplastic lesions (90.3%) in the non-SSI-mEUS group (p = 0.046). Detailed information on cases of incomplete resection by group is presented in Supplementary Table 1. All patients in the SSI-mEUS group with positive margins underwent follow-up colonoscopy and abdominal CT; none showed recurrence over a mean follow-up period of 15 months.
ESD vs. mEMR findings in SSI-mEUS group
The mean lesion size was larger for ESD vs. mEMR (8.2 ± 2.6 mm vs. 5.6 ± 1.6 mm, p < 0.001). Submucosal cushion thickness was lower for ESD vs. mEMR (5.6 ± 2.2 mm vs. 7.9 ± 1.9 mm for the thickest site, 4.2 ± 2.2 mm vs. 6.5 ± 1.7 mm for the thinnest site, p < 0.001). Insufficiently lifted lesions were typically more often resected by ESD than by mEMR (44.7% vs. 14.9%, p = 0.001). Thirty SELs that appeared insufficiently lifted on endoscopy demonstrated a sufficient submucosal cushion on mEUS (6.0 ± 2.2 mm for thickest site, 4.5 ± 2.3 mm for thinnest site) and were removed completely by ESD (n = 17) or mEMR (n = 13). En bloc resection was achieved in all lesions treated with ESD vs. all but one lesion treated with mEMR; no significant difference in adverse events or histologically complete resection rates were observed between them (Table 3).
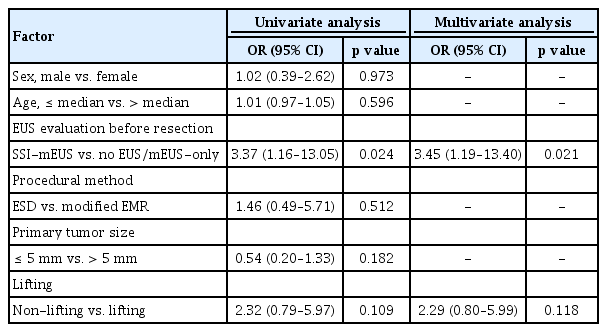
Risk factors associated with indeterminate or positive deep resection margins
The main factor associated with indeterminate or positive deep resection margins in overall colorectal SELs was whether SSI-mEUS was performed before endoscopic resection on the univariate logistic regression analysis. The multivariate logistic analysis also revealed that not performing SSI-mEUS (OR 3.45, 95% CI 1.19–13.40, p = 0.021) was the only factor associated with indeterminate or positive deep resection margins. No significant relationship was observed between endoscopic resection methods, signs of non-lifting, primary tumor size, and indeterminate or positive deep resection margins (Table 4). These results were consistently observed in the rectal NET subgroup. Treatment without SSI-mEUS was the only significant risk factor of indeterminate or positive deep margins in rectal NETs (OR 6.64, 95% CI 1.61–61.07, p = 0.006) (Supplementary Table 2).
Surgical treatment outcomes based on SSImEUS findings
There were two cases among the category I lesions within the third layer in which the SSI-mEUS findings led to surgical treatment instead of endoscopic resection (Supplementary Table 3). Both cases showed hypoechoic nodules in the third layer on mEUS and inadequate submucosal cushions on SSI-mEUS, suggesting proper muscle layer invasion. Surgery confirmed proper muscle layer invasion in both cases. In the second case, 68Ga-labelled DOTA0-Tyr3 octreotide positron emission tomography– CT revealed a tiny presacral nodule with faint uptake interpreted as nonspecific uptake or a small metastatic lymph node. Although laparoscopic ultra-low anterior resection was recommended, the patient opted for local excision with close observation citing concerns about a potential false-positive pelvic lymph node. TAE revealed a grade 2 NET with proper muscle layer and lymphovascular invasion. Despite recommendations for radical surgery, the patient chose follow-up, and no recurrence has been observed at 12 months on endoscopy or rectal magnetic resonance imaging.
DISCUSSION
The current study found that submucosal cushion thickness measured using SSI-mEUS may serve as a new endosonographic parameter for determining the endoscopic resectability of colorectal SELs. The complete resection rate of neoplastic SELs was 97.3% in the SSI-mEUS group, significantly higher than the 90.3% rate of the no EUS and mEUS-only groups (p = 0.046) despite the mean SEL size being larger in the SSI-mEUS group. No severe complications occurred after endoscopic resection in the SSI-mEUS group. Complete endoscopic resection was feasible even for colorectal SELs with insufficient lifting after SSI, as long as SSI-mEUS revealed a sufficient submucosal cushion. However, in two cases, it was difficult to identify submucosal cushion formation using mEUS because the lesions were not detached from the proper muscle layer; therefore, alternatives such as TAE or radical resection should be considered instead of endoscopic resection. In both cases, proper muscle layer invasion of the NETs was confirmed. Although these lesions appeared insufficiently lifted on endoscopy, like other usual submucosal invasion SELs, the absence or insufficient thickness of the submucosal cushion under SSI-mEUS provided an objective indicator favoring surgical or full-thickness resection over endoscopic resection.
A previous study reported a tailored endoscopic resection approach based on the lifting sign after the submucosal injection of small rectal NETs [6]. In this study, conventional EMR was employed for elevated-type tumors or tumors with well-lifting sign after the submucosal injection. The difference in the pathological complete resection rate between the EMR with band ligation group and the conventional EMR group was not statistically significant (93.8% vs. 88.2%, p = 0.441) [6]. The complete histological resection rate of conventional EMR for adequately lifted NETs was 88.2%, relatively higher than those of other studies (71.0–77.4%) [11,13-16], highlighting the importance of the lifting sign as a key factor in achieving complete resection with EMR procedures [6]. The presence of the non-lifting sign after the submucosal injection for colorectal tumors or SELs suggests that endoscopic resection may be technically difficult due to deep submucosal invasion or fibrosis caused by biopsy [6,7,17]. However, judging the endoscopic non-lifting sign is subjective and may vary among individual endoscopists. In contrast, the submucosal cushion between the lesion and proper muscle layer measured on SSI-mEUS provides objective information about SEL resectability, even if they appear insufficiently lifted after the saline injection on endoscopy. Waxman et al. [9] previously suggested that complete SEL detachment from the muscularis propria could be an indicator of its endoscopic resectability in the gastrointestinal tract. This is based on the theory that the submucosal cushion may facilitate wide and deep excision, lowering the risk of perforation by separating the lesion from the muscularis propria, thereby preventing the muscle from being included in the snare resection process. Of 18 colorectal SELs, 17 were resected endoscopically and one rectal NET was removed through TAE due to incomplete lifting from the muscularis propria after the saline injection. In our study, 129 lesions were evaluated using SSI-mEUS. Among them, two were located in the fourth layer on mEUS and SSI-mEUS and subjected to surgical resection. Two lesions in the third layer with insufficient submucosal cushions were removed via TAE and radical surgery. The remaining 125 lesions had a properly formed submucosal cushion on SSI-mEUS; the complete resection rate of 97.3% (107/110) was achieved for neoplastic lesions, all of which were safely removed. Complications included eight cases of intraoperative bleeding and three of delayed bleeding, all of which were successfully managed through endoscopic hemostasis.
Since SELs can appear endoscopically similar, distinguishing between those with malignant potential and benign lesions is challenging. Due to their subepithelial location, biopsies performed using endoscopic forceps often fail to obtain diagnostic tissue [18]. Therefore, additional imaging modalities are necessary to ensure an accurate differential diagnosis. Among the available imaging methods, EUS is the preferred choice for evaluating gastrointestinal SELs because of its superior accuracy in delineating histologic layers and identifying the tumor’s site of origin [1,19]. Based on EUS or fine needle aspiration findings as well as patient preference, endoscopists can decide whether resection or follow-up is appropriate [18]. Asymptomatic benign lesions such as lipomas, lymphangiomas, leiomyomas, and vascular lesions may not require intervention, whereas malignant or premalignant lesions, including NETs, gastrointestinal stromal tumors, ulcerative colitis-associated tumors, and colorectal MALT lymphomas, require endoscopic or surgical resection [18,20-22]. On mEUS, in addition to diagnosing SELs, saline injections can aid in determining whether lesions are suitable for endoscopic resection by visualizing submucosal cushion thickness. Although our study primarily included rectal NETs, the endoscopically resected neoplastic lesions in the SSI-mEUS group included incompletely resected rectal NETs requiring salvage resection, granular cell tumors, MALT lymphomas, and ulcerative colitis-associated dysplasia as well. These findings suggest that various malignant or premalignant lesions, including SELs or SEL-mimicking lesions such as MALT lymphoma and colitis-associated dysplasia, could be indications for SSI-mEUS as a useful tool for assessing the submucosal cushion to determine the feasibility of endoscopic resection. However, the clinical usefulness of SSI-mEUS requires further investigation through additional studies.
Among various colorectal SELs, endoscopic treatment outcomes for rectal NETs have primarily been studied. Histologically complete resection rates of rectal NETs are reportedly 83.3–94.1% with cap-assisted EMR [11,23], 92.5–100% with EMR with band ligation [14,16,23,24], 81.2–96.7% with precut EMR [10,14,25-27], and 87.1–97.7% with ESD [13,16,28,29]. In our study, the complete histological resection rate of rectal NETs using mEMR and ESD after SSI-mEUS was 97.1% (99/102), comparable to those of previous studies reporting high complete resection rates for each method.
In this study, ESD was performed more frequently in the SSI-mEUS vs. non-SSI-mEUS group, as it was more likely to be chosen when the submucosal cushion appeared shallow (30.4% vs. 15.9%, p < 0.001). However, when evaluating risk factors associated with indeterminate or positive deep resection margins in colorectal SELs, endoscopic resection method was not a significant factor. Instead, the only associated risk factor was not performing an SSI-mEUS evaluation prior to the resection. This suggests that SSI-mEUS could be a valuable tool for securing a deep resection margin by enabling visual confirmation of the submucosal cushion and ensuring a sufficiently deep resection. Thus, the widespread implementation of this tool in clinical practice has the potential to significantly enhance the R0 resection rate.
The present study has some limitations. First, its design was retrospective, with a lack of pre-determined criteria for choosing endoscopic resection methods since no reference was available. As we relied on the expertise of endoscopists at a single tertiary referral center, the possibility of selection bias could not be avoided. However, our data showed that the endoscopist chose ESD rather than mEMR for relatively large lesions with a less sufficient submucosal cushion. This decision was made in accordance with a previous study that recommended ESD in cases in which the lesion was large, fibrosis prevented effective suctioning, or the snaring for EMR was unsuccessful [30]. Second, this study was conducted at a highly specialized tertiary referral center and the SSI-mEUS was performed by a single expert endoscopist. While the higher complete resection rate observed in the SSI-mEUS group compared to the non-SSI-mEUS group may suggest that SSI-mEUS contributed to this outcome, it is important to acknowledge that this finding could also reflect the superior skills of the endoscopist performing the procedure. This potential bias is inherent and unavoidable in the study design. However, it is worth noting that all endoscopists at our institution are highly experienced, having performed over 5,000 colonoscopies. Third, the minimally required submucosal cushion thickness was not determined in this study. However, given the 1.5-mm length of the short needle-type knives dedicated to colorectal ESD, a submucosal cushion > 1.5 mm may be required to ensure ESD safety. Finally, the follow-up duration was relatively short to assess the recurrence rate. Therefore, future randomized controlled studies involving multiple endoscopists with varying experience and long-term follow-up periods are essential to verify the effectiveness of SSI-mEUS in guiding therapeutic decision-making of colorectal SELs.
In conclusion, for colorectal SELs, including those that appear insufficiently elevated on conventional endoscopy, a sufficiently thick submucosal cushion created by SSI and measured by mEUS can predict a high likelihood of safe and complete resection. Further studies are required to evaluate submucosal cushion thickness measured by SSI-mEUS to validate its potential as a new endosonographic parameter for determining the feasibility of endoscopic resection.
KEY MESSAGE
1. Therapeutic decision-making for colorectal SELs can be challenging, and the endoscopic non-lifting sign after SSI is not objectively measurable. However, submucosal cushion thickness (the distance between the SEL base and the fourth layer of the colorectal wall) can be measured using SSI-mEUS.
2. The complete resection rate of neoplastic SELs was significantly higher in the SSI-mEUS than in the non-SSI-mEUS group. Even if SELs appear unlifted after SSI, an endosonographically sufficient submucosal cushion indicates the feasibility of endoscopic resection, while an insufficient submucosal cushion indicates the need for surgical resection.
3. Endosonographically measured submucosal cushion thickness may guide the therapeutic decision between endoscopic and surgical resection of colorectal SELs.
Notes
CRedit authorship contributions
Jung-Bin Park: investigation, data curation, formal analysis, validation, writing - original draft; Ji Eun Baek: investigation, data curation, writing - review & editing; June Hwa Bae: investigation, data curation, writing - review & editing; Seung Wook Hong: investigation, data curation, writing - review & editing; Sung Wook Hwang: investigation, data curation, writing - review & editing; Sang Hyoung Park: investigation, data curation, writing - review & editing; Byong Duk Ye: investigation, data curation, writing - review & editing; Jeong-Sik Byeon: investigation, data curation, writing - review & editing; Seung-Jae Myung: investigation, data curation, writing - review & editing; Suk-Kyun Yang: investigation, data curation, writing - review & editing; Dong-Hoon Yang: conceptualization, methodology, data curation, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None