Blood eosinophil count and treatment patterns of chronic obstructive pulmonary disease patients in South Korea using real-world data
Article information
Abstract
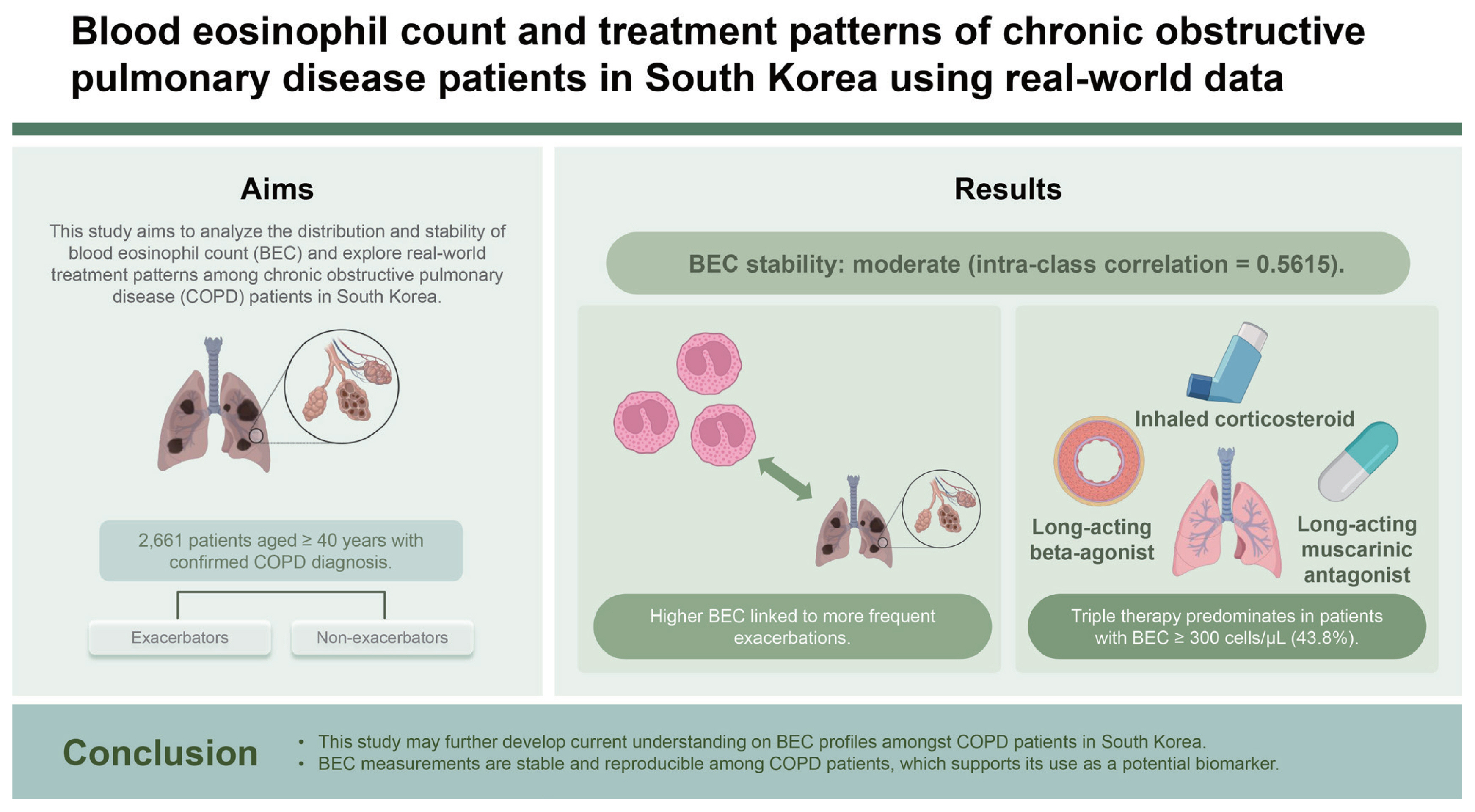
Background/Aims
Chronic obstructive pulmonary disease (COPD) management guidelines have increasingly emphasised the importance of exacerbation prevention, and the role of blood eosinophil count (BEC) as a biomarker for inhaled corticosteroids (ICS) response. This study aimed to describe the distribution and stability of BEC and understand real-world treatment patterns among COPD patients in South Korea.
Methods
This was a retrospective database analysis using data obtained from the KOrea COPD Subgroup Study (KOCOSS) registry between January 2012 and August 2018. KOCOSS is an ongoing, longitudinal, prospective, multi-centre, non-interventional study investigating early COPD amongst South Korean patients. BEC stability was assessed by calculating the intra-class correlation (ICC) coefficient. “Exacerbators” were patients who had a record of ≥ 1 exacerbation in the 12 months prior to the visit.
Results
The study included 2,661 patients with a mean age of 68.6 years. Most patients were male (92.0%). Mean BEC was significantly higher in exacerbators compared to non-exacerbators. Patients with ≥ 2 exacerbations at baseline had a less stable BEC over time (ICC = 0.44) compared to non-exacerbators (ICC = 0.57). Patients with BEC ≥ 300 cells/μL at baseline predominantly received triple therapy (43.8%).
Conclusions
This study may further develop current understanding on BEC profiles amongst COPD patients in South Korea. BEC measurements are stable and reproducible among COPD patients, which supports its use as a potential biomarker.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a common and treatable progressive disease characterised by persistent airflow limitation and respiratory symptoms accompanied by an enhanced chronic inflammatory response in the lungs [1]. COPD is one of the leading causes of mortality and morbidity worldwide [2], and its global prevalence is projected to increase in the coming decades.
In recent years, guidelines for the management of COPD by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) have been updated to include greater emphasis on the importance of exacerbation prevention, and the use of blood eosinophil count (BEC) as a biomarker to potentially identify populations with a greater likelihood of a beneficial response to inhaled corticosteroids (ICS) [3]. The 2023 GOLD Report recommends long-acting beta-agonist (LABA) + long-acting muscarinic antagonist (LAMA) + ICS triple therapy in patients with BEC ≥ 300 cells/μL among high risk patients [3]. Given the potential close link between BEC and ICS response, BEC thresholds can be a useful tool to guide treatment choice in COPD patients. The shift in COPD management towards using BEC as a biomarker of ICS response, as well as the increased emphasis on exacerbation prevention using treatments, warrants further evaluation of the distribution and stability of BEC, and treatment use in the real world. For BEC to be useful as a clinical biomarker, BEC measurements should be stable and reproducible among COPD patients [4]. The implication of variable BEC measurements is that patients may cross BEC thresholds and this may result in patients being assigned to an inaccurate ICS response category. To date, there remains insufficient evidence regarding the stability of BEC and its confounding factors [5].
Until now, most evidence regarding BEC resulted from clinical trials. However, clinical trials cannot represent real clinical practice. Due to the strict inclusion/exclusion criteria, many patients were not included in the trials. Thus, it is mandatory to confirm whether the results of clinical trials are validated in real world study. Moreover, many BEC studies predominantly performed in Western countries. However, there are several unique features in Asian COPD patients [6]. Given the paucity of studies and data sources that evaluate the BEC profiles of COPD patients in Asia, more region-specific studies are needed to better characterise this population. Therefore, this study aimed to describe the distribution and stability of BEC in a clinical setting, and to understand the real-world treatment patterns among COPD patients in South Korea.
METHODS
Data source
Anonymised data were obtained from the KOrea COPD Subgroup Study (KOCOSS), an ongoing, longitudinal, prospective, multi-centre registry investigating the characteristics and disease course of early COPD amongst South Korean patients, to inform guidelines for the early detection and treatment of COPD patients.
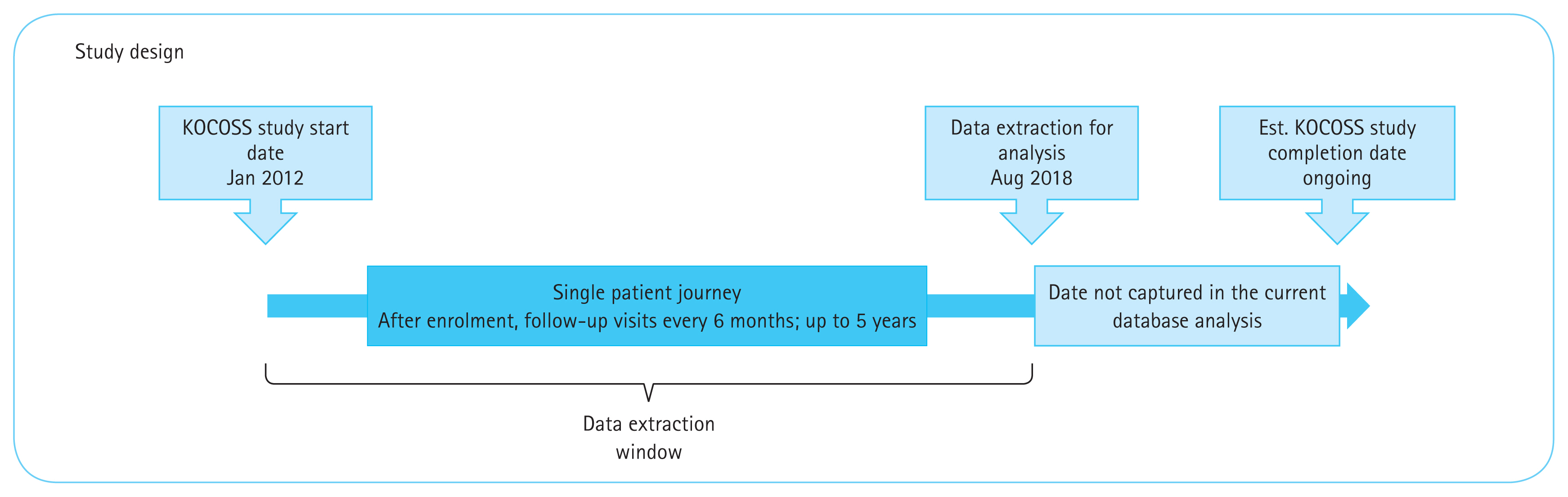
Patient recruitment for KOCOSS began in January 2012 and is ongoing. Patients are enrolled from over 45 tertiary and university-affiliated hospitals across South Korea (Supplementary Table 1) and meet the following inclusion criteria: aged ≥ 40 years, have a confirmed diagnosis of COPD by spirometry (post bronchodilator forced expiratory volume (1 second)/forced vital capacity [FEV1/FVC] < 0.70), and presence of either cough, sputum or dyspnoea. Patients are excluded if they: are unable to complete a pulmonary function test; had a diagnosis of any myocardial infarction or cerebrovascular events within the previous 3 months before enrolment; had systemic steroid use for conditions other than COPD exacerbation within 8 weeks before enrolment; are pregnant; are diagnosed with any rheumatoid disease, malignancy, or irritable bowel disease, or did not consent to study participation. All Patients were enrolled during the stable state. Patients with acute exacerbation were not enrolled during the exacerbation period. Additional eligibility criteria were applied to the data obtained from KOCOSS for selected objectives (Supplementary Table 2). For each of the objectives, patients with missing or incomplete data were excluded for analysis.
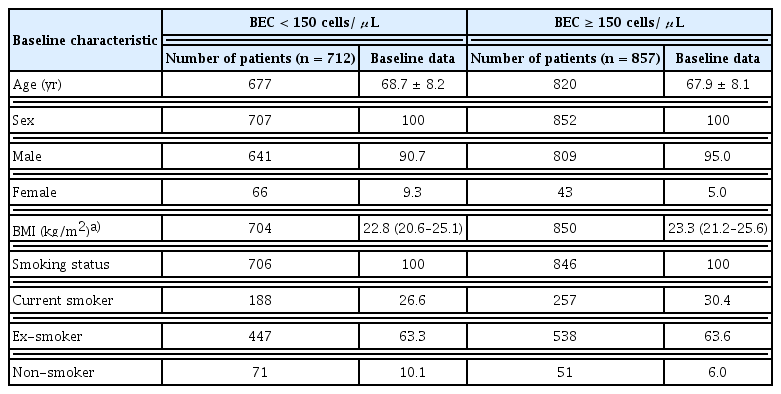
Data are collected at baseline (study entry) and at subsequent visits in 6-monthly intervals for up to 5 years (with a ± 3-month time window for each visit) (Supplementary Table 3). Patient data are collected by physicians or trained nurses using case-report forms (CRF). Baseline characteristics include comorbidities based on patients’ past medical history from the KOCOSS CRF. Comorbidities with > 10% prevalence among the study population are reported. In this analysis, patient demographics were stratified at study entry by BEC level (< 150 or ≥ 150 cells/μL), a commonly used threshold for evaluating eosinophil-associated airway inflammation and exacerbations [7]. Although data on smoking history were also extracted from the KOCOSS database for this study, the definition for each smoking status (current smoker, ex-smoker, and non-smoker) and number of pack years were not specifically defined.
During the conduct of the KOCOSS study, history of smoking was removed as an inclusion criterion due to the presence of COPD patients in South Korea who have never smoked. Additionally, asthma history was removed as an exclusion criterion, to include asthma-COPD overlap (ACO) patients in the registry.
Study design and ethics compliance
This was a retrospective database analysis of patients who enrolled in the KOCOSS between January 2012 and August 2018 (Fig. 1). The KOCOSS study was approved by Konkuk University on the 12th of January 2012 (IRB number: KUH1010338). This study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. This study complied with all applicable laws regarding patient privacy. No direct patient contact or primary collection of individual human patient data occurred. Study results are in tabular form and presented as aggregate analyses that omit patient identification. Patient identifiers were excluded from all publications and reports.
Definitions
Patients’ BEC were recorded in a stable state of COPD (i.e., no symptom-defined exacerbation, or systemic treatment with antibiotics and/or steroids on the day of measurement). Exacerbations were defined in KOCOSS as worsening of respiratory symptoms (cough/sputum/dyspnoea), which required treatment with oral corticosteroids and/or antibiotics. Only moderate (based on the KOCOSS definition and involving unscheduled outpatient department visits) to severe (requiring hospitalisation or visit to the emergency room) exacerbations were considered in this analysis. “Exacerbators” were defined as patients who had a record of ≥ 1 exacerbation in the 12 months prior to the visit. “Non-Exacerbators” were defined as patients who had no record of COPD exacerbation in the 12 months prior the visit. During the follow-up period, patients could transition from being an Exacerbator to a non-exacerbator and vice-versa. To assess treatment patterns, COPD medications and treatment modifications were recorded every visit based on the prescriptions of pulmonology specialists; the use of ICS and triple therapy were recorded as “Yes” or “No”. At the time this study was conducted, treatments examined were based on recommendations from the 2017 GOLD Report. Other definitions are reported in Supplementary Table 4.
PRO assessments
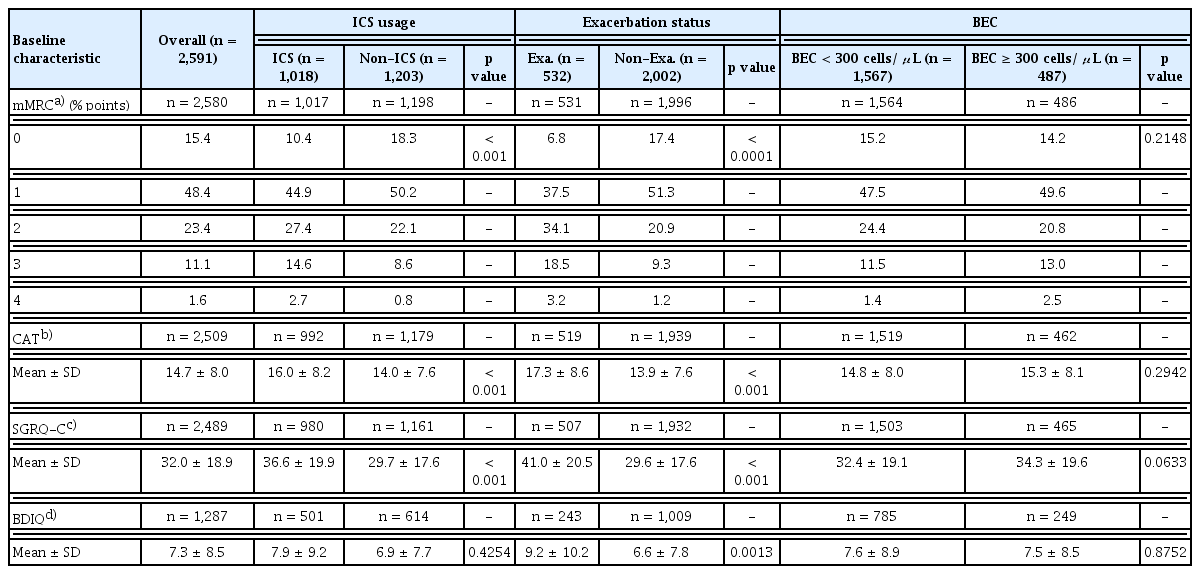
Patients’ quality of life was evaluated with several patient-reported outcome (PRO) assessment tools at baseline and during the follow-up period. COPD impact on patients’ well-being and daily life was measured with the COPD Assessment Test (CAT) and St. George’s Respiratory Questionnaire, COPD version (SGRQ-C). The Modified Medical Research Council Dyspnoea Scale (mMRC) was used to stratify dyspnoea severity in the study population. Lastly, the Beck Depression Inventory Questionnaire (BDIQ) was used to measure the severity of depression in patients at baseline only.
PROs were stratified by ICS usage, exacerbation status, and BEC. Of note, a threshold of BEC ≥ 300 cells/μL was used for stratification instead of BEC ≥ 150 cells/μL due to greater correlation with increased risk of exacerbation given higher baseline severity [8].
Analysis
Following data was collected and analysed: (1) explore the patterns in BEC, stratified by clinical characteristics such as ICS use and exacerbation status; (2) explore the stability of BEC measurements over time; (3) describe patients’ quality of life as evaluated by PROs; and (4) understand the real-world treatment patterns among COPD patients in the KOCOSS cohort.
Statistical analysis
Descriptive analyses were used to evaluate the objectives as described in the preceding section. Descriptive statistics, including mean, standard deviation (SD), median, and inter-quartile range (IQR) are presented for continuous variables, while counts and percentages are presented for categorical variables. Statistical significance in this study was set at p ≤ 0.05.
Stability in BEC during the follow-up period was assessed by calculating the intra-class correlation (ICC) coefficient using the between-patient and within-patient variance (
RESULTS
Patient demographics, stratified by BEC
In total, 2,661 patients were present in the KOCOSS registry at the time of data extraction in December 2018. Patients included for analysis of each objective are reported in Figure 2.
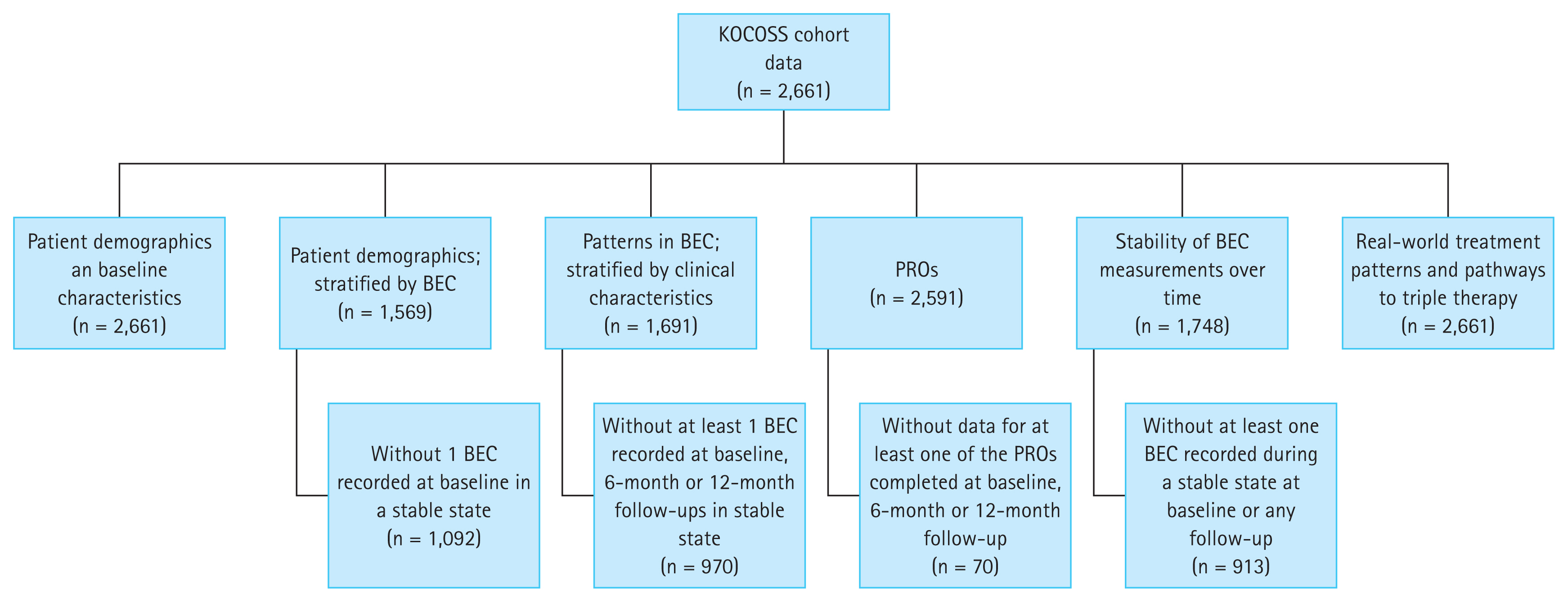
KOCOSS cohort breakdown. Additional criteria by each objective are listed in Table 1. KOCOSS, KOrea COPD Subgroup Study; COPD, chronic obstructive pulmonary disease; BEC, blood eosinophil count; PRO, patient-reported outcome.
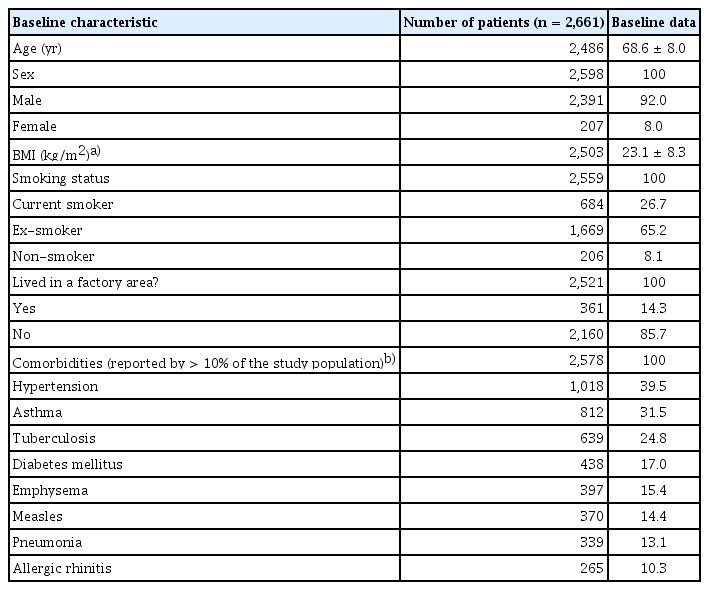
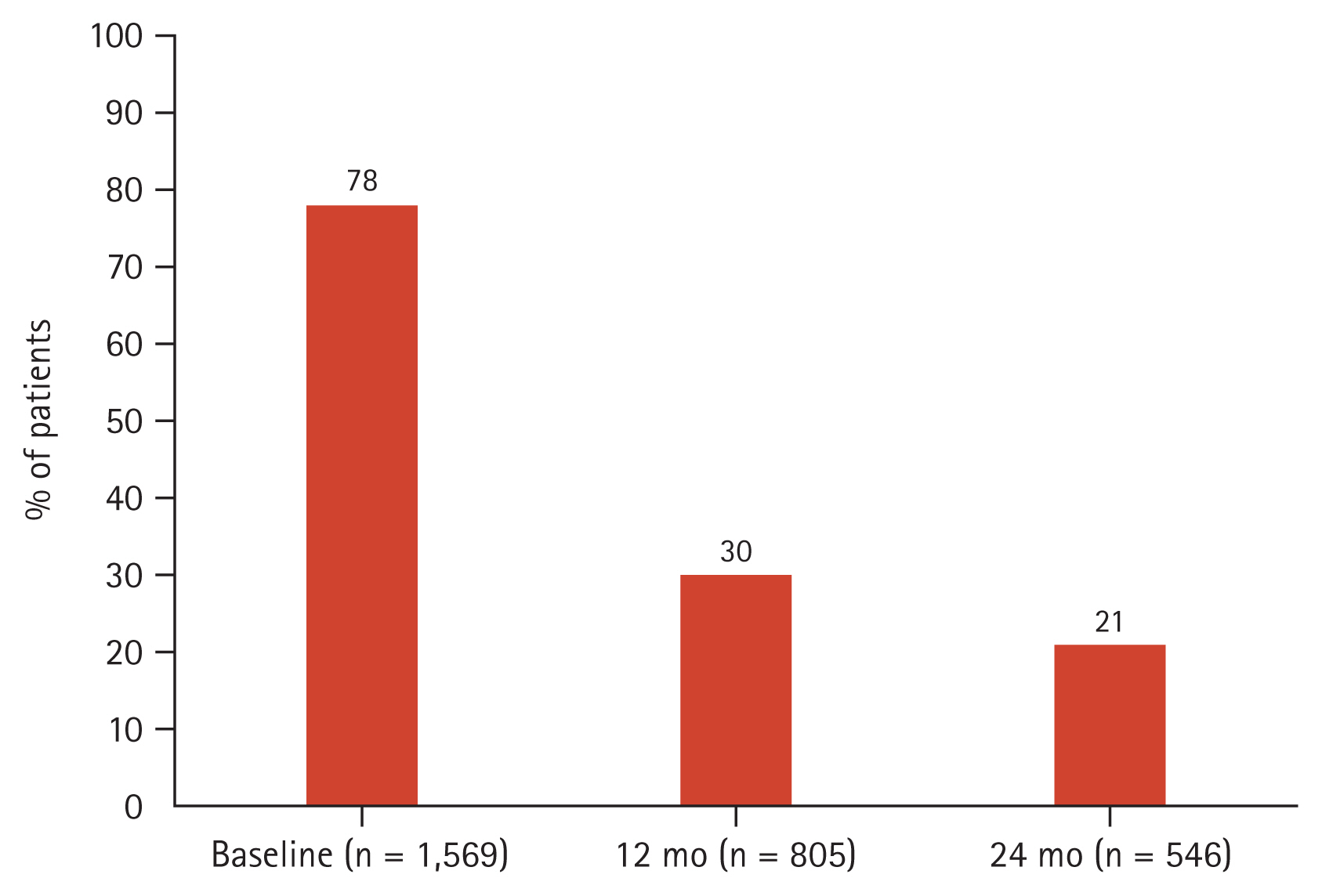
The mean age of patients was 68.6 ± 8.0 years. Patients were mostly male (92.0%) and majority had a history of smoking (91.9%) (Table 1). The most common comorbidities were hypertension (39.5%) and asthma (31.5%). An exacerbation in the previous 12 months prior to enrolment was reported by 21% of patients, and approximately half of the cohort (46%) were on an ICS-containing regimen at entry into the registry. Spirometry results between ICS and non-ICS users are reported in Supplementary Table 5. More than half of the cohort had BEC ≥ 150 cells/μL (55%) at baseline (Fig. 3). Comparing between patients with BEC ≥ 150 cells/μL and patients with BEC < 150 cells/μL, it was observed that patients were similar in age and BMI (Table 2). Patients with a higher BEC (≥ 150 cells/μL) were more likely to be a current smoker compared to those with BEC < 150 cells/μL. Finally, considering the retrospective design of our study which analysed real-world data on patients who may not have undergone multiple testing or follow-up, BEC measurements were not available at all the study timepoints for all patients (Fig. 4).
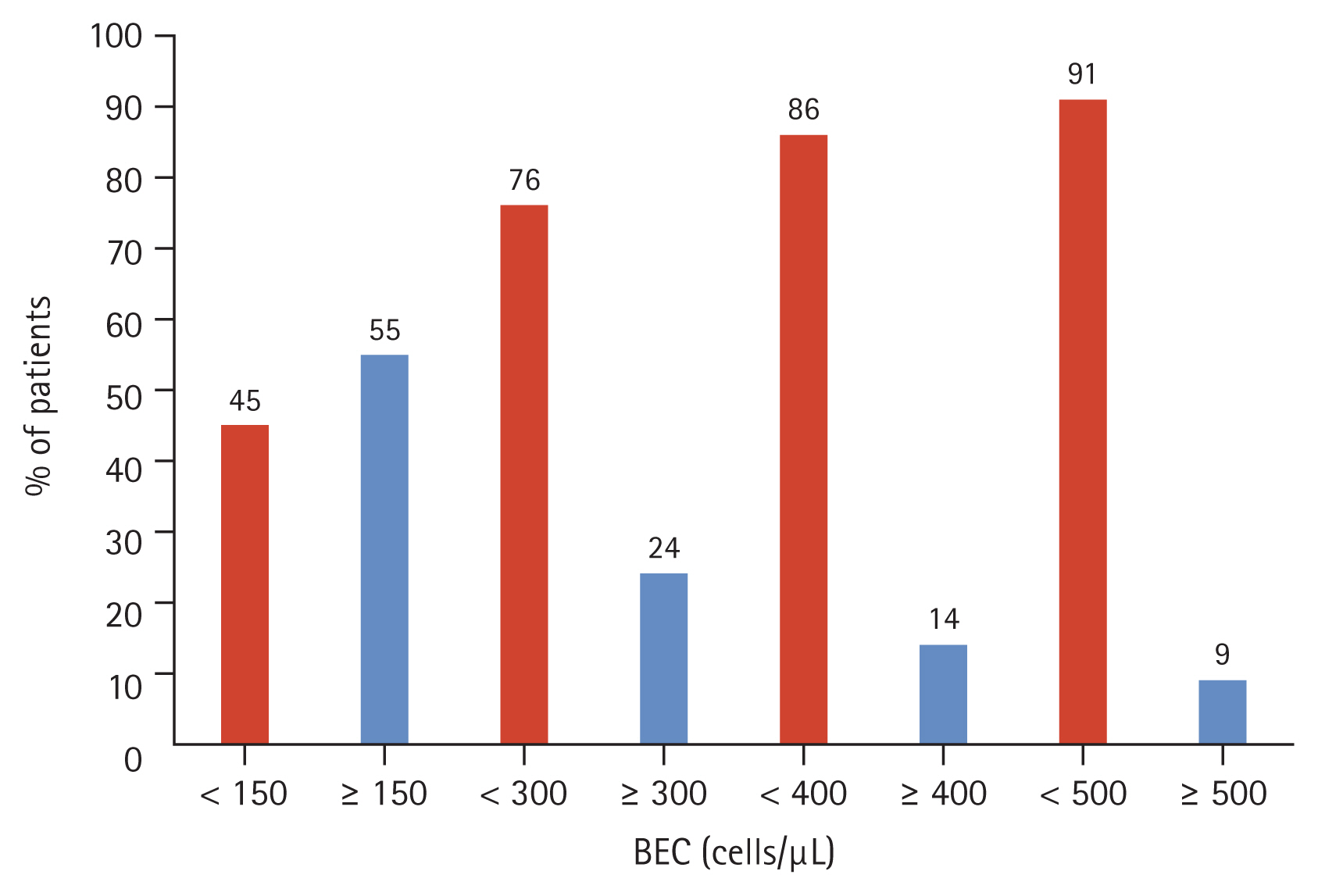
Percentage of patients by BEC threshold (cells/μL), at baseline (n = 1,569). BEC, blood eosinophil count.
Patterns in BEC, stratified by clinical characteristics
The mean BEC for the overall cohort measured at 12-month intervals was similar across timepoints. ICS users had a higher mean BEC than non-ICS users across timepoints (Supplementary Table 6). However, no significant differences were observed between these two groups at any time point (p = 0.1562, 0.3082, and 0.7634 at baseline, 12-month follow- up, and 24-month follow-up, respectively) (Fig. 5).
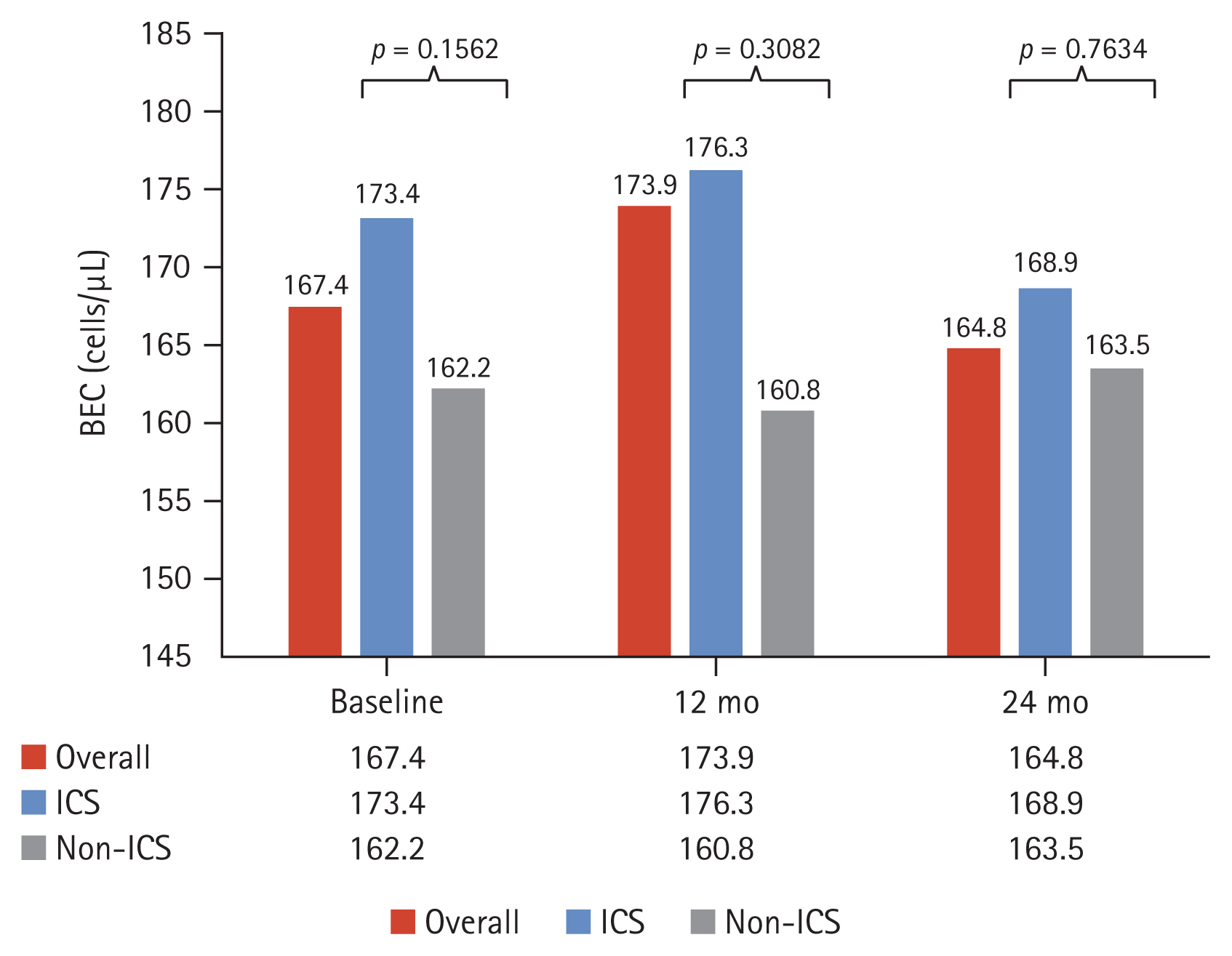
Distribution of BEC (geometric mean) over time, stratified by ICS usage. BEC, blood eosinophil count; ICS, inhaled corticosteroid.
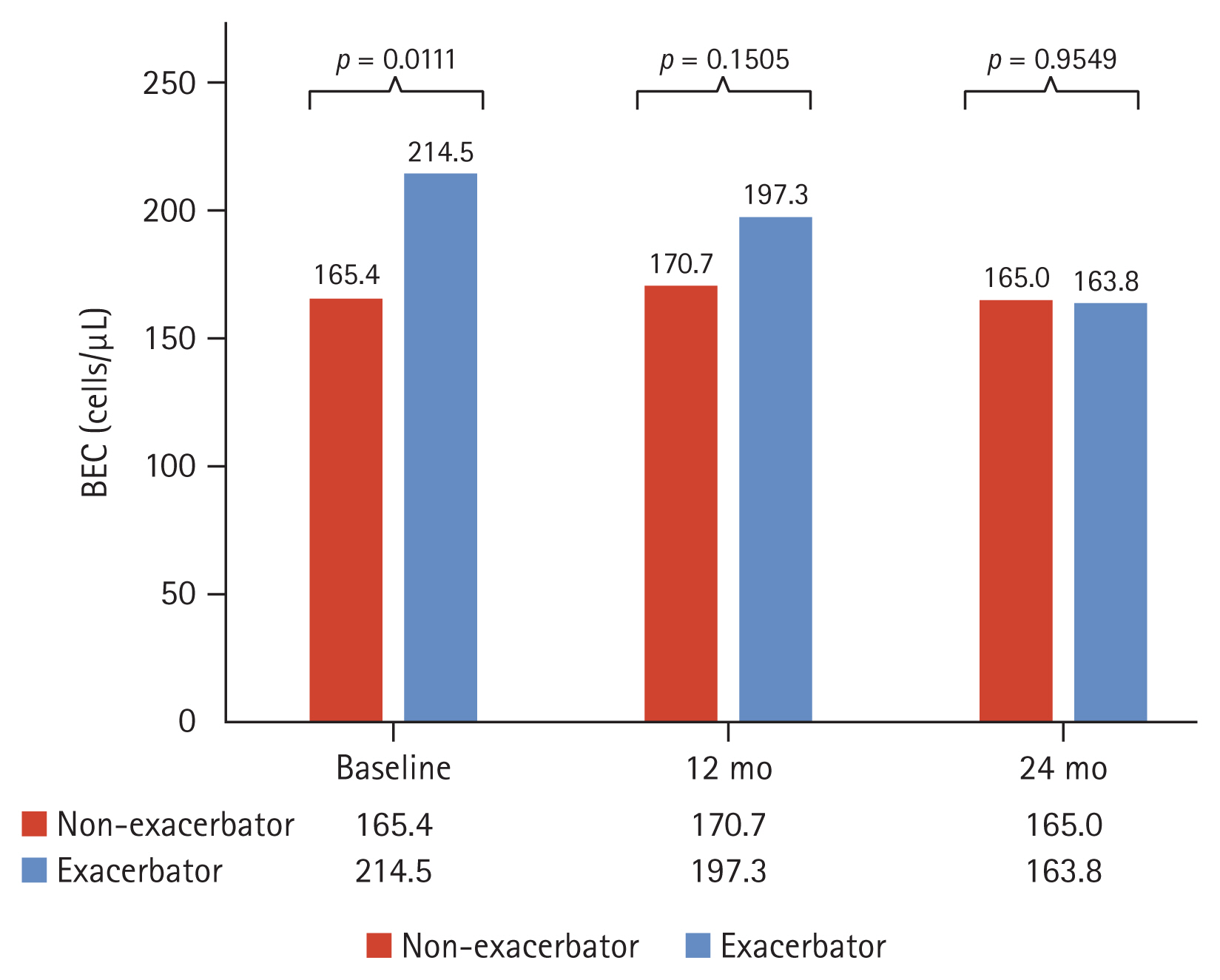
Patients who were exacerbators had a higher BEC than non-exacerbators both at baseline and at the 12-month follow-up (Fig. 6). An exploratory analysis was conducted on the relationship between baseline BEC and exacerbation. For further details, please refer to Supplementary Appendix 1.
Stability of BEC measurements over time
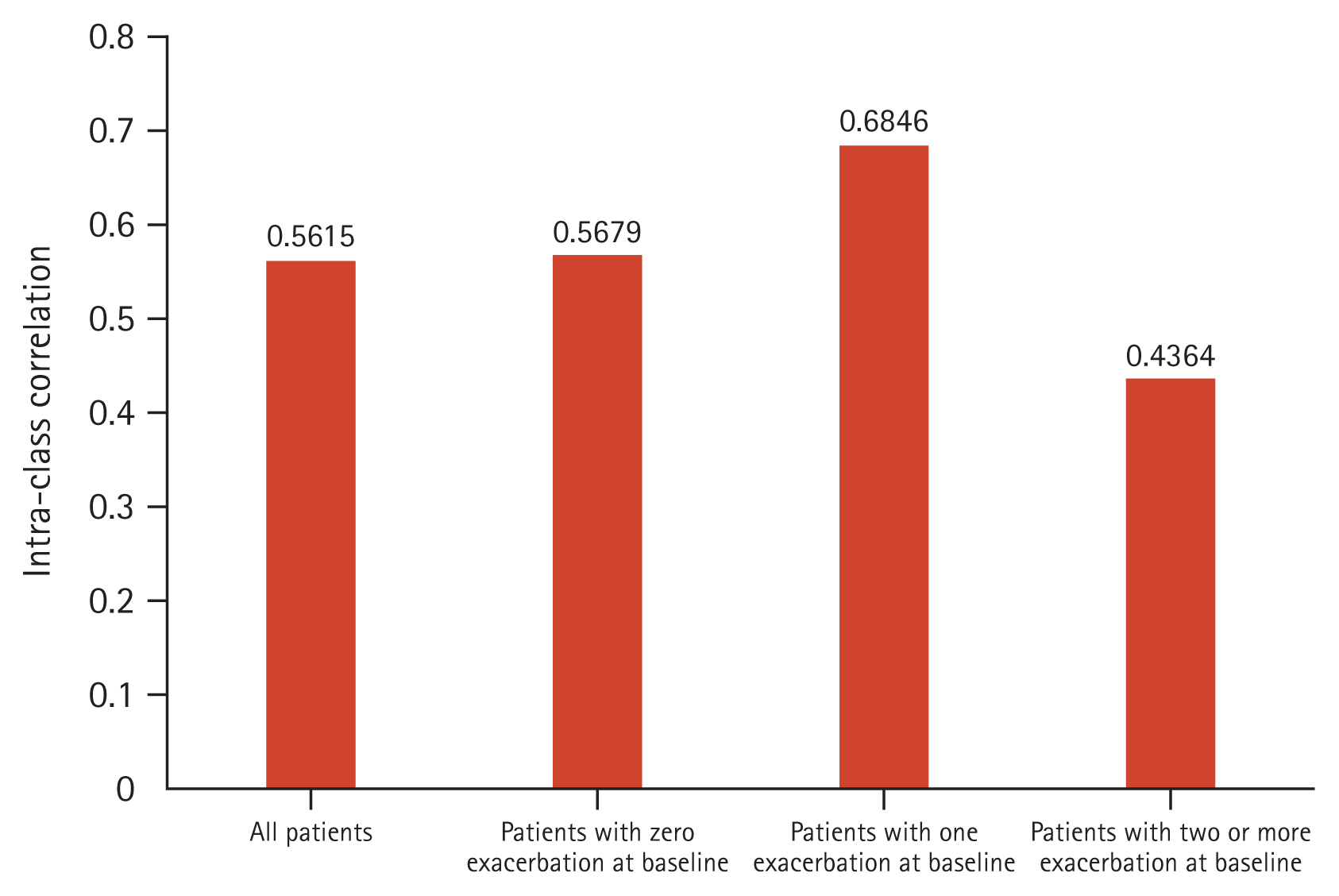
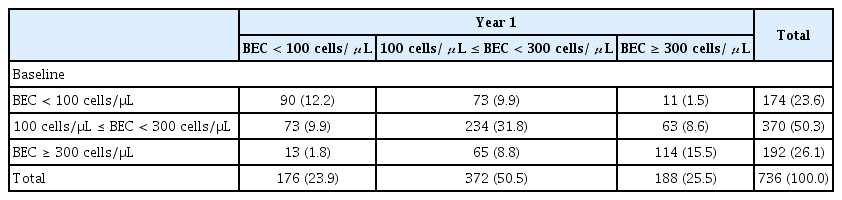
The ICC predominantly lied between 0.40–0.75 (Fig. 7). Patients with ≥ 2 exacerbations at baseline had a less stable BEC over time compared to non-exacerbators (ICC = 0.44 vs. 0.57, respectively) (Fig. 7). Baseline and one year follow up BEC was categorized into three groups. Two consecutive BEC was available in 736 patients. In the baseline, the number (%) of patients with BEC < 100 cells/μL, 100 cells/μL ≤ BEC < 300 cells/μL, and BEC ≥ 300 cells/μL was 174 (23.6%), 370 (50.3%), and 192 (26.1%). The number (%) in year 1 was 176 (23.9%), 372 (50.5%), and 188 (25.5%). About 60% of patients (438/736) remained in the initial group, however, some patients moved to other groups (Table 3). The Cohen’s kappa coefficient was 0.350 (p < 0.001).
Quality of life
Exacerbators at baseline reported significantly worse CAT and SGRQ-C scores compared to non-exacerbators (CAT: 17.3 ± 8.6 vs. 13.9 ± 7.6, p < 0.001; SGRQ-C: 41.0 ± 20.5 vs. 29.6 ± 17.6, p < 0.001) (Table 4). ICS users at baseline reported significantly worse CAT and SGRQ-C scores compared to non-ICS users (CAT: 16.0 ± 8.2 vs. 14.0 ± 7.6, p < 0.001; SGRQ-C: 36.6 ± 19.9 vs. 29.7 ± 17.6, p < 0.001) (Table 4). Patients at baseline with BEC ≥ 300 cells/μL reported worse CAT and SGRQ-C scores compared to patients with BEC < 300 cells/μL (CAT: 14.8 ± 8.0 vs. 15.3 ± 8.1; SGRQ-C: 32.4 ± 19.1 vs. 34.3 ± 19.6). However, there was no significant statistical difference (Table 4).
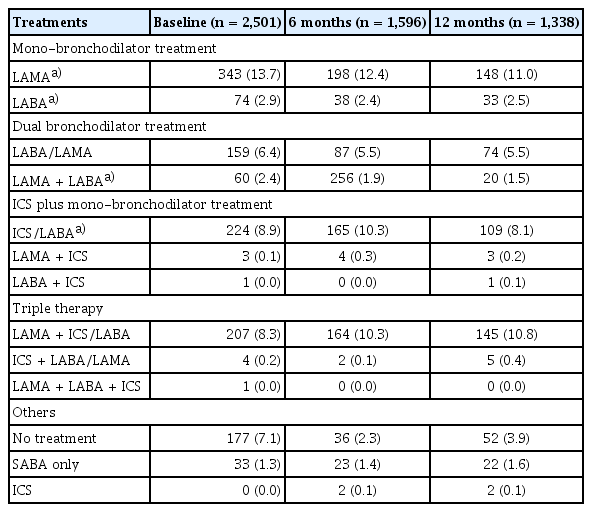
Treatment patterns
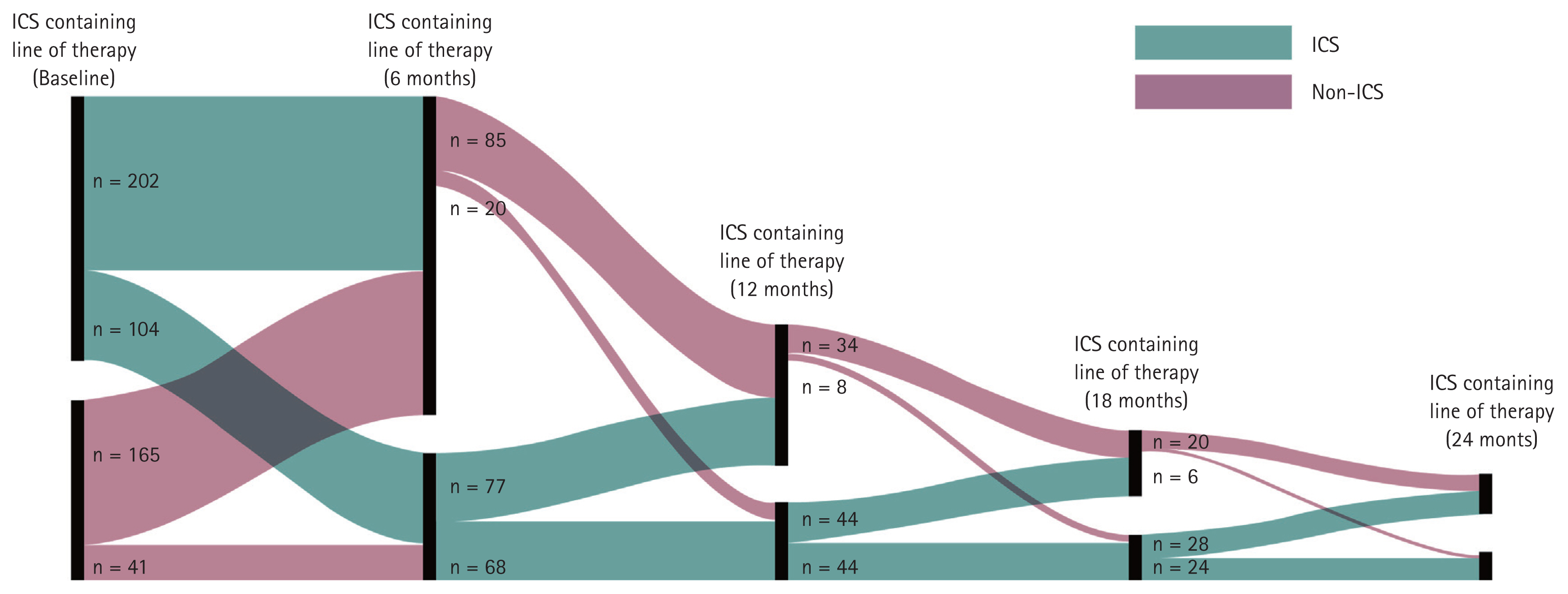
The most prescribed treatments in the overall cohort at baseline were LAMA, followed by ICS/LABA and LAMA + ICS/LABA (triple therapy). This was consistently observed at 6 months post-enrolment (Table 5). Patients most switched to triple therapy from: LAMA; ICS/LABA; LAMA + LABA (Fig. 8). The most common COPD treatments used immediately prior to triple therapy were LAMA and ICS/LABA. Among the cohort, 22% and 21% were treated with LAMA or ICS/LABA, respectively, as their first treatment option before switching to triple therapy as their second treatment option. Similarly, 17% and 14% of the cohort were treated with LAMA or ICS/LABA, respectively, as their second treatment option before switching to triple therapy as their third treatment option (Fig. 8).
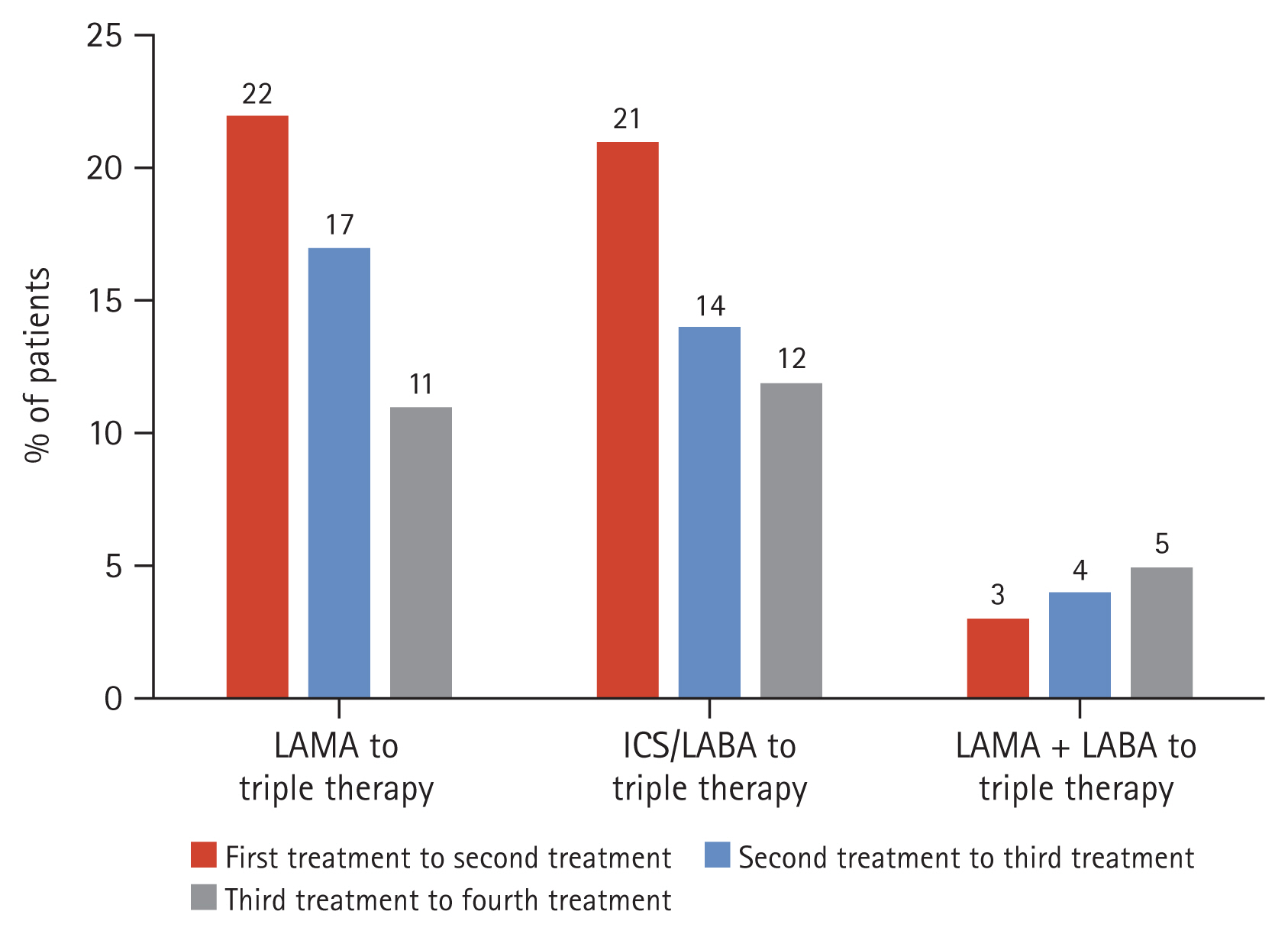
Most common pathways to triple therapy. LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid; LABA, long-acting beta agonist.
Among those who were Exacerbators at baseline, there was an upward trend in the proportion of patients using an ICS-containing regimen as time progressed (baseline to 18 mo) (Fig. 9). Only a small proportion of highly symptomatic patients (CAT > 20) at baseline were prescribed LAMA + LABA treatment (7.8%). ICS/LABA + LAMA (44.2%), ICS/LABA (11.7%) and LAMA (12.3%) were prescribed more than LAMA + LABA treatment. Within the BEC ≥ 300 cells/μL at baseline subgroup, patients were predominantly taking triple therapy (ICS/LABA/LAMA, 43.8%) instead of ICS/LABA (8.2%) or ICS + LABA treatment (0.0%).
DISCUSSION
This study aimed to describe the distribution and stability of BEC and to understand the real-world treatment patterns among COPD patients in South Korea. Most of the 2,661 patients were male, had an average age of 68 years, and were ex-smokers. Hypertension and asthma were the most common comorbidities in the KOCOSS cohort.
Patient demographics, stratified by BEC
Large population-based studies showed that patient characteristics, such as older age, male, high BMI and not being a current smoker, are associated with high BEC [10–12]. However, along with a smaller cohort in France [13], this study was unable to confirm these associations. Further studies will be needed to confirm an association between BEC and patient demographics.
While patient inclusion criteria were adjusted during the study (i.e., the inclusion criteria for patients to have a history of smoking and the exclusion criteria for patients with asthma history were removed), the broadened inclusion criteria resulted in a study population that more accurately reflected the real-world setting. The high proportions of current smokers (26.7%) and ex-smokers (65.2%) reflect the initial inclusion criterion of smoking history. Given that smoking is more prevalent amongst males than females in Korea [14–16], the initial inclusion criterion of smoking history may also explain the high proportion of males (92.0%) observed in our study.
Considering that this was a real-world study, the decrease in percentage of patients with BEC measurements over the study period could be due to patients choosing not to return to tertiary care (study site) but other clinics or hospitals for follow-up, lack of regular follow-up in routine clinical practice, and local testing practices which focus on diagnosis only.
Patterns in BEC, stratified by clinical characteristics
Mean BEC in our study was within the BEC range seen in other countries [17]. The mean BEC was observed to be higher for exacerbators compared to non-exacerbators. Other studies also support an association between higher BEC and increased exacerbation risk [8,18–21]. It may be that patients who require ICS and have exacerbations reflect a more severe COPD profile. However, the specific mechanisms by which higher BEC is associated with increased exacerbations remain to be explored.
Stability of BEC measurements over time
Few studies have explored the reproducibility of BEC levels (i.e., stability of BEC measurements over time) and factors that may influence this. Reproducibility would support the role of BEC as a good biomarker [4]. In our study, BEC stability was 0.5615, suggesting moderate stability overall.
Comparing across subgroups of patients with different baseline exacerbation status, it was observed that the subgroup of patients with ≥ 2 exacerbations at baseline had slightly less stable BEC over time, compared to the non-exacerbators (0.44 vs. 0.57). A study from the United Kingdom supports an association between exacerbations and BEC instability [22]. While there were potential confounders such as treatment regimens, preliminary findings from this KOCOSS analysis suggest a correlation between BEC stability and exacerbations. Further links with treatments, such as ICS, and other outcomes, including hospitalisations or mortality remain to be explored. These data further develop our understanding of BEC as a potential biomarker in COPD that may allow evaluation of disease severity in patients.
Quality of life
COPD reduces breathing capacity and impairs the patient’s ability to carry out daily activities as the disease progresses, adversely affecting the quality of life among patients [23]. In this study, ICS users and Exacerbators reported worse CAT and SGRQ-C scores as compared to non-ICS users and non-exacerbators, respectively.
While older treatment guidelines recommended ICS-containing regimens as the mainstay of therapy [1], the introduction of dual long-acting bronchodilators to the treatment landscape has provided prescribers with a wider range of COPD treatment options, allowing a more tailored management approach that considers the individual patient’s symptoms and exacerbation risk [3]. In our study, worse PRO scores amongst ICS users may reflect older treatment guidelines wherein ICS was commonly prescribed, including in COPD patients with more severe COPD.
Patients with higher BEC also reported worse CAT and SGRQ-C scores compared to patients with lower BEC. However, the difference was not statistically significant, potentially due to the limited number of responses that could be included for this specific analysis. Further studies with larger sample sizes are needed to explore this correlation between BEC and patient’s quality of life.
Treatment patterns
This study showed that patients in South Korea switched to triple therapy from LAMA or ICS/LABA. This result is in line with a United Kingdom study, which reported that ICS + LABA usage was most likely to result in switches to triple therapy [24].
Use of triple therapy was reported in a high proportion (43.8%) of patients with BEC ≥ 300 cells/μL at baseline in our study. In Korea, prescribers follow the Korean Academy of Tuberculosis and Respiratory Disease guidelines, which recommend that LAMA or ultra (24 h) LABA single therapy, or LABA + LAMA or ICS + LABA combination therapy can be administered as first-line treatment for group “Da” patients (analogous to GOLD Group E) [25]. Patients can escalate to triple therapy as second-line treatment if exacerbation persists [25]. Given the multiple routes to triple therapy if prescribers follow the local Korean guidelines for highly symptomatic patients, this may explain the high proportion of patients with BEC ≥ 300 cells/μL who were predominantly taking triple therapy at baseline.
BEC and ICS
There was no significant difference in BEC between patients with and without ICS. Perhaps the use of ICS did not affect the blood eosinophil level. Also, the KOCOSS study began in 2012. In previous GOLD documents, ICS was recommended for patients with an FEV1 < 50%. Blood eosinophils were not recommended as a biomarker for ICS response in those days.
Prescription pattern over time
Blood eosinophil levels are highly associated with ICS response. The aim of the study was to demonstrate changes in BEC over time. Thus, we were also wondering whether there was a change in prescription patterns. Previous studies indicated a trend where the use of ICS-containing regimens increased over time. In this study, we also confirmed that the proportion of ICS-containing regimens has increased over time, although the numbers are limited.
Strengths
This is the largest COPD epidemiology study including BEC data in Asia. Other studies involving smaller populations have been conducted in China (n = 1,566) using data from the acute exacerbation of chronic obstructive pulmonary disease inpatient registry (ACURE) study, as well as in South Korea (n = 629) using data from the COPD in Dusty Area (CODA) registry and the Korean Obstructive Lung Disease (KOLD) cohort [26,27]. Additionally, the longitudinal design of the KOCOSS registry and the full characterisation of the study population (i.e., disease severity, PROs) allow the observation of disease progression and clinical outcomes of Korean COPD patients over time. Lastly, the KOCOSS cohort may have a higher proportion of patients with early-stage COPD as compared to other cohorts, as the registry’s objective was to inform development of future guidelines for the early-detection of COPD patients, as well as the prevention of severe COPD progression.
Limitations
Given the discrete nature of data collection every 6 months, some events (i.e., treatment changes between visits or treatment gaps [treatments stopped until refill at next visit]) may have not been recorded. Due to the infrequency of data measurements (i.e., every 12 mo), the data regarding stability of BEC should be treated with caution. Furthermore, no conclusive evidence was available to demonstrate the statistical significance of the association between BEC and the patient’s quality of life, and between BEC and a more severe COPD profile, likely due to the small subgroup sizes and incomplete data. Although a larger sample size with lower drop-out rate would be advantageous, it should be noted that testing practices may be subject to clinical relevance and guideline recommendations based on available evidence supporting BEC as a biomarker for prognosis. Thus, until BEC measurement becomes part of routine testing, achieving larger sample sizes through real-world data collection may not be feasible.
Changes in prescribing practice were likely influenced by changes to COPD management guidelines over the years. Challenges with accounting for externalities, such as missing follow-up data, led to difficulties with interpreting data from the multivariable fractional polynomial negative binomial model (Supplementary Appendix 1). Although ACO patients were included in the study population to reflect the real-world setting more closely, the high proportion of individuals with co-existing asthma (31.5%) in our analyses potentially limits the generalisability of our conclusions to COPD patients. Due to the use of different asthma diagnosis tools by physicians thus leading to a slightly heterogenous ACO population being represented in the study, the available data for the ACO population were also challenging to interpret (Supplementary Appendix 1).
Conclusions
In this study, we have described the BEC status in Korean COPD patients. This study may further develop current understanding on BEC profiles amongst COPD patients. BEC measurements are stable and reproducible among COPD patients, which supports its use as a potential biomarker.
KEY MESSAGE
1. A fair-to-good BEC stability across patients observed in this study suggests that BEC measurements are stable and reproducible among COPD patients.
2. Mean BEC was higher in exacerbators compared to non-exacerbators.
3. Frequent exacerbator (≥ 2) may have more variable BEC over time compared to non-exacerbator.
Acknowledgments
The authors would like to thank the various parties: Eun-Yeong Cho, employee of GSK during the time of the study for data interpretation and project guidance. Katie Mycock, Daniel Bluff, Olivia Massey of Adelphi Real World for the development of the study report. Medical writing support for the development of this manuscript (in the form of manuscript development, collating author comments, and grammatical editing), under the direction of the authors, was provided by Andrew Lim of Costello Medical Singapore, and was funded by GSK.
Notes
CRedit authorship contributions
Chin Kook Rhee: conceptualization, methodology, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, supervision, project administration; Yu-Fan Ho: conceptualization, methodology, writing - review & editing, project administration; Sumitra Shantakumar: conceptualization, methodology, writing - original draft, writing - review & editing, supervision; Tim Holbrook: conceptualization, methodology, formal analysis, writing - original draft, writing - review & editing; Yein Nam: methodology, formal analysis, writing - original draft, writing - review & editing; Kwang-Ha Yoo: conceptualization, methodology, investigation, formal analysis, writing - review & editing, supervision, project administration, funding acquisition
Conflicts of interest
Chin Kook Rhee: Received consulting/lecture fees from AstraZeneca, Bayer, Boehringer-Ingelheim, GSK, MSD, Mundipharma, Novartis, Sanofi, Takeda, and Teva. Yu-Fan Ho: Employee of, and shareholder in, GSK. Sumitra Shantakumar: Employee of, and shareholder in, GSK. Tim Holbrook: Employee of Adelphi Real World, which received funding from GSK to conduct this research. Yein Nam: Employee of Adelphi Real World during the time of the study, which received funding from GSK to conduct this research. Kwang-Ha Yoo: Received consulting/lecture fees from, AstraZeneca, Boehringer-Ingelheim, Deawon, GSK, Hallym, HanMi, Koron, MSD, Mundipharma, Novartis, Organon, Sanofi, and Teva.
Funding
This study was funded by GSK R&D Limited, 980 Great West Road, Brentford, Middlesex, UK; GSK study identifier Study ID 206975 (PRJ2799).
Data availability
Information on GSK’s data sharing commitments and requesting access to anonymised individual participant data and associated documents can be found at https://www.gsk-studyregister.com/en/.