Assessment of small fiber neuropathy and distal sensory neuropathy in female patients with fibromyalgia
Article information
Abstract
Background/Aims
We investigated sudomotor dysfunction, small fiber neuropathy (SFN), and their clinical significance in female fibromyalgia patients.
Methods
Fibromyalgia patients and healthy controls (HCs) were recruited. Clinical and laboratory data were measured. Electrochemical skin conductance (ESC) values of hands and feet were assessed by SUDOSCAN. Additionally, several other methods were employed, including nerve conduction study (NCS), electromyography (EMG), and questionnaires. Spearman correlation coefficient was calculated to identify factors associated with ESC values of SUDOSCAN.
Results
Twenty-two female fibromyalgia patients and 22 female HCs were recruited. The fibromyalgia group had lower EQ5D and higher Toronto Clinical Neuropathy scores than the HC group. Most of the EMG/NCS findings of motor and proximal sensory nerves were comparable between the fibromyalgia and HC groups, whereas sensory nerve action potential amplitudes of distal sensory nerves were significantly lower in the fibromyalgia group. Mean ESC values of hands and feet were significantly lower in the fibromyalgia group than in the HC group (57.6 ± 16.2 vs. 68.8 ± 10.3 μS, p = 0.010 for hands, 64.9 ± 11.5 vs. 72.0 ± 8.2 μS, p = 0.025 for feet, respectively). Moderate to severe SFN was more common in the fibromyalgia group (68.2%) than in the HC group (68.2 vs. 50%, p = 0.019). Fibromyalgia disease duration was significantly correlated with the ESC values of hands/feet, and tricyclic antidepressant (TCA) responders had higher ESC values than non-responders.
Conclusions
SFN was commonly detected in fibromyalgia patients who had normal EMG/NCS findings and was more severe in fibromyalgia patients with longer disease duration. SUDOSCAN may predict response to TCA therapy.
INTRODUCTION
Fibromyalgia is a disease of widespread musculoskeletal pain with various somatic symptoms [1,2]. The absence of definite pathological findings makes the diagnosis of fibromyalgia difficult, and physicians should rule out all other potential diagnoses before confirming fibromyalgia. Central sensitization is the most emphasized pathological component of fibromyalgia, and fibromyalgia is characterized by nociplastic pain, which is resistant to traditional pain killers, e.g., non-steroidal anti-inflammatory drugs or opioids. Certain drugs that modulate neurotransmitters, such as pregabalin, duloxetine, and milnacipran, are recommended for fibromyalgia treatment [3]. Additionally, combining pharmacological therapy with cognitive behavioral therapy or exercise may help ameliorate symptoms of fibromyalgia [3]. However, many fibromyalgia patients do not experience sufficient therapeutic effects [4]. A better understanding of the pathophysiology of fibromyalgia is needed to help discover novel therapies for intractable fibromyalgia cases.
According to the revised definition of the International Association for the Study of Pain, the pain caused by fibromyalgia is classified as nociplastic pain (rather than nociceptive or neuropathic pain) [5]. However, some studies have shown involvement of small nerve fiber damage in fibromyalgia patients [6–8]. SUDOSCAN (Impeto Medical, Paris, France) is a non-invasive and rapid tool to evaluate sudomotor dysfunction and autonomic neuropathy by assessing thin unmyelinated C fibers of sweat glands [9]. Diabetic peripheral neuropathy is the most frequent complication of type I and II diabetes mellitus, which causes neuropathic pain. The detection of small fiber neuropathy (SFN) by SUDOSCAN can precede the abnormal findings revealed by nerve conduction study (NCS) and electromyography (EMG) in patients with diabetic peripheral neuropathy [10]. Hyperalgesia and allodynia are common pain characteristics of fibromyalgia and can mimic, and thus be confused with, neuropathic pain. However, previous studies did not demonstrate a definite connection between NCS/EMG findings and fibromyalgia pain [11,12]. Therefore, evaluating SFN (sudomotor dysfunction) by SUDOSCAN and determining whether SUDOSCAN findings have clinical importance in fibromyalgia patients may increase our knowledge on the pathophysiology of fibromyalgia.
In the present study, we assessed electrochemical skin conductance (ESC), cardiac autonomic neuropathy risk score (CAN-RS), NCS, and EMG in fibromyalgia patients and healthy controls (HCs). Additionally, we assessed variables associated with ESC (i.e., the severity of SFN) to determine the clinical significance of SFN in fibromyalgia patients.
METHODS
Patients
Fibromyalgia patients and HCs were recruited from a single university-based hospital, Bucheon St. Mary’s Hospital. The inclusion criteria for fibromyalgia patients were as follows: (1) fulfilled all 1990, 2010, and 2016 American College of Rheumatology classification criteria for fibromyalgia [1,2,13], (2) over 18 years of age, and (3) disease duration of less than 5 years. Fibromyalgia patients who had concomitant inflammatory arthritis (e.g., rheumatoid arthritis or spondyloarthritis), other systemic autoimmune diseases (e.g., systemic lupus erythematosus, primary Sjogren’s syndrome, systemic sclerosis, inflammatory myositis, or mixed connective tissue disease), or cancer were excluded. As a control group, age- and sex-matched HCs were included. HCs who had potentially pain-inducing disorders such as osteoarthritis, inflammatory arthritis, systemic autoimmune diseases, cancer, and psychological disorders (e.g., major depression disorder or somatoform disorder) were excluded. Both fibromyalgia patients and HCs underwent SUDOSCAN, EMG, and NCS on the day of enrolment. All enrolled patients with fibromyalgia were naïve to fibromyalgia medication, which was started after the aforementioned examinations were performed. A good response to tricyclic antidepressants (TCAs) was defined as a decrease in the fibromyalgia impact questionnaire (FIQ) by more than 30% or a score below 40 after 1 month of TCA administration. Other fibromyalgia medications were allowed in cases of non-response to TCA therapy. The study was conducted in accordance with the Declaration of Helsinki (1964, and its later amendments). Written informed consent was obtained from each participant before enrolment. The study was approved by the Institutional Review Board of the Catholic University of Korea, Bucheon St. Mary’s hospital (approval number: HC15DISI0040).
Demographics, questionnaires, and laboratory data of enrolled patients
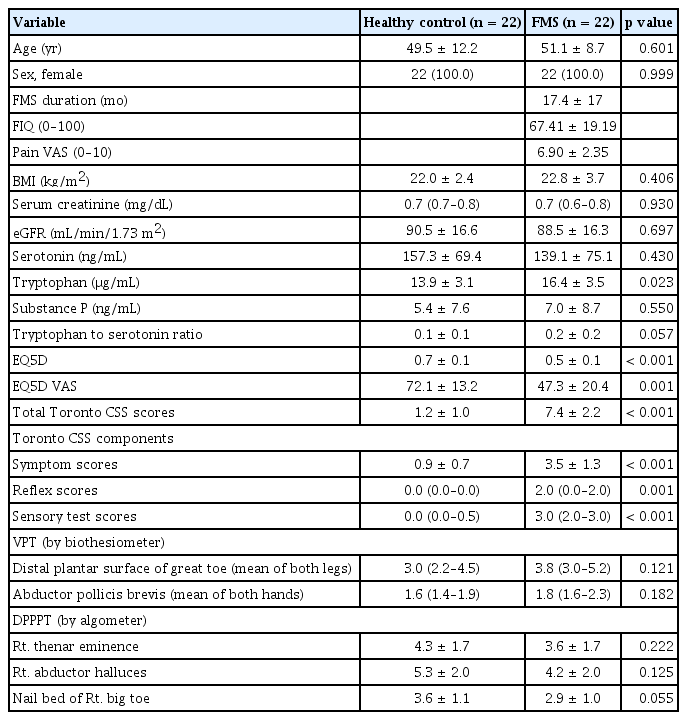
The clinical, demographic, and laboratory data of fibromyalgia patients and HCs were collected at the time of enrolment. The age, sex, body mass index (BMI), serum creatinine level, and estimated glomerular filtration rate of fibromyalgia patients and HCs were collected. To assess quality of life, EQ5D and EQ5D visual analogue scale were used [14]. Several conventional tools for evaluating neuropathy other than NCS, EMG, and SUDOSCAN were also used. The Toronto Clinical Neuropathy Score was used to measure the symptom degree of sensorimotor polyneuropathy [15]. Vibration perception threshold by biothesiometer and deep pressure pain perception threshold by algometer were measured in the distal plantar surface of great toe/abductor pollicis brevis or thenar eminence/abductor halluces/nail bed of first toe. Serum levels of neurotransmitters (serotonin, substance P, and tryptophan) were measured with enzyme-linked immunosorbent assay for all participants on the day of enrollment. The FIQ [16] and fibromyalgia disease duration were only collected in fibromyalgia patients.
NCS and EMG examination
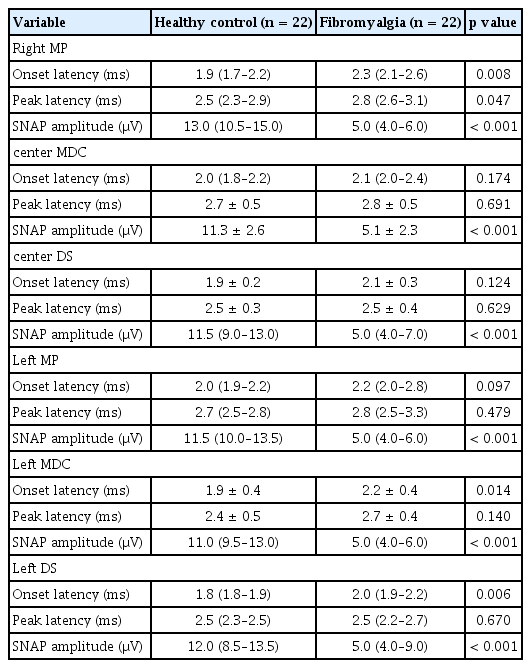
Viking Electromyography System (Nicolet-Viasys Biomedical Inc., Madison, WI, USA) was used for NCS with the following settings: 20 Hz for the lower filter, 3 kHz for the upper filter for sensory studies, 5–10 kHz for motor studies, and a pulse duration of 0.1 ms [9]. NCS tests included the sural sensory, superficial peroneal sensory, median sensory, ulnar sensory, radial sensory, tibial motor, and median motor nerves of one side. The minimum F-response latencies from both tibial nerves and H-reflex latencies from tibial nerve stimulation were collected. EMG needling was done by inserting the monopolar needle into selected lower extremity muscles (tibial and median motor nerves). NCS of bilateral medial plantar (MP) sensory, dorsal sural (DS) sensory, and medial dorsal cutaneous (MDC) sensory nerves were also conducted to evaluate distal sensory neuropathy, which is known to show abnormal findings in early diabetic neuropathy [17]. All NCS and EMG tests were performed by experienced physicians.
SUDOSCAN
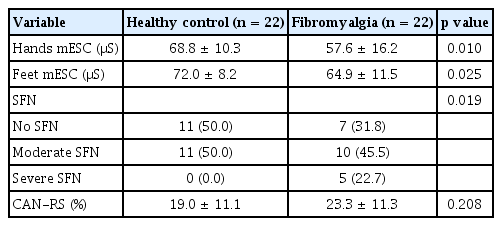
SUDOSCAN was performed in fibromyalgia and HC groups to measure ESC of hands/feet and CAN-RS on the same day that NCS and EMG were performed. A separate examiner who was blind to the patient’s information performed SUDOSCAN before NCS and EMG. SUDOSCAN is a simple and non-invasive procedure that takes only 3 minutes. The detailed procedure of SUDOSCAN was described in a previous study [9]. ESC, expressed in microsiemens (μS), was calculated by analyzing electrochemical currents generated in response to low-voltage stimulation. Lower ESC indicated sweat gland dysfunction (sudomotor dysfunction), which could be interpreted as positive for SFN. Patients were divided into three groups according to the following cut-off values of the mean ESC (mESC) for hands and feet: (1) no SFN (hands mESC ≥ 60 μS and feet mESC ≥ 70 μS), (2) moderate SFN (hands mESC 40–60 μS and feet mESC 50–70 μS), and (3) severe SFN (hands mESC < 40 μS and feet mESC < 50 μS) [18]. The CAN-RS is presented as percentage and automatically calculated by the algorithm included in the SUDOSCAN device software. ESC, BMI, and age are used for calculating the CAN-RS, and a higher CAN-RS indicates a higher risk of cardiac autonomic abnormalities.
Statistical analysis and data management
deviation or median and interquartile range after a normality test of each continuous variable was performed by the Shapiro–Wilk test. Categorical variables were presented as number and percentage, and significant differences between the fibromyalgia and HC groups were determined by the chi-square or Fisher’s exact test. Correlations between ESC versus fibromyalgia disease duration and serum neurotransmitter levels were measured by the Spearman correlation coefficient. p values < 0.05 were considered statistically significant. All analyses were performed using SPSS statistics software (version 23; IBM Corp., Armonk, NY, USA).
RESULTS
Comparison of demographic, laboratory, and clinical data between fibromyalgia and HC groups
A total of 44 participants were enrolled, of which 22 were fibromyalgia patients, and 22 were HCs. All enrolled participants were female. The mean ages of the HC and fibromyalgia groups were 49.5 ± 12.2 years and 51.1 ± 8.7 years, respectively. BMI, serum creatinine, and estimated glomerular filtration rate were comparable between the HC and fibromyalgia groups. Regarding serum neurotransmitter levels, the fibromyalgia group exhibited non-significantly lower serotonin levels (139.1 ± 75.1 ng/mL vs. 157.3 ± 69.4 ng/mL, p = 0.430), significantly higher tryptophan levels (16.4 ± 3.5 μg/mL vs. 13.9 ± 3.1 μg/mL, p = 0.023), and non-significantly higher substance P levels (7.0 ± 8.7 ng/mL vs. 5.4 ± 7.6 ng/mL, p = 0.550) than the HC group. EQ5D and EQ5D visual analogue scale scores were significantly lower in the fibromyalgia group than in the HC group, which implies lower health-related quality of life in fibromyalgia patients. Both the total score and each component of the Toronto Clinical Neuropathy Score (symptom, reflex, and sensory test scores) were significantly higher in the fibromyalgia group. The vibration perception threshold and deep pressure pain perception threshold values did not significantly differ between HC and fibromyalgia groups. All demographic, laboratory, and clinical data are summarized in Table 1.
NCS and EMG findings
The NCS of proximal sensory nerves (including the right side sural sensory nerve and right superficial peroneal sensory nerve) demonstrated lower sensory nerve action potential (SNAP) amplitude in the fibromyalgia group than in the HC group (11.9 ± 7.1 μV vs. 16.8 ± 6.1 μV, p = 0.021, for the right sural sensory nerve and 9.3 ± 4.4 μV vs. 13.9 ± 3.7 μV, p < 0.001, for the right superficial peroneal sensory nerve). The SNAP amplitudes of the right median sensory wrist and right median sensory palm in the fibromyalgia group tended to be lower than those in the HC group, but without statistical significance. The EMG findings regarding onset latency (ms), distal compound muscle action potential amplitude (mV), conduction velocity (m/s), and F-waves on the right tibial motor and right median motor nerves were comparable between the HC and fibromyalgia groups (Table 2). These NCS and EMG findings demonstrate that neuropathies on proximal sensory and motor nerves are not observed more definitely in fibromyalgia than in HC patients.
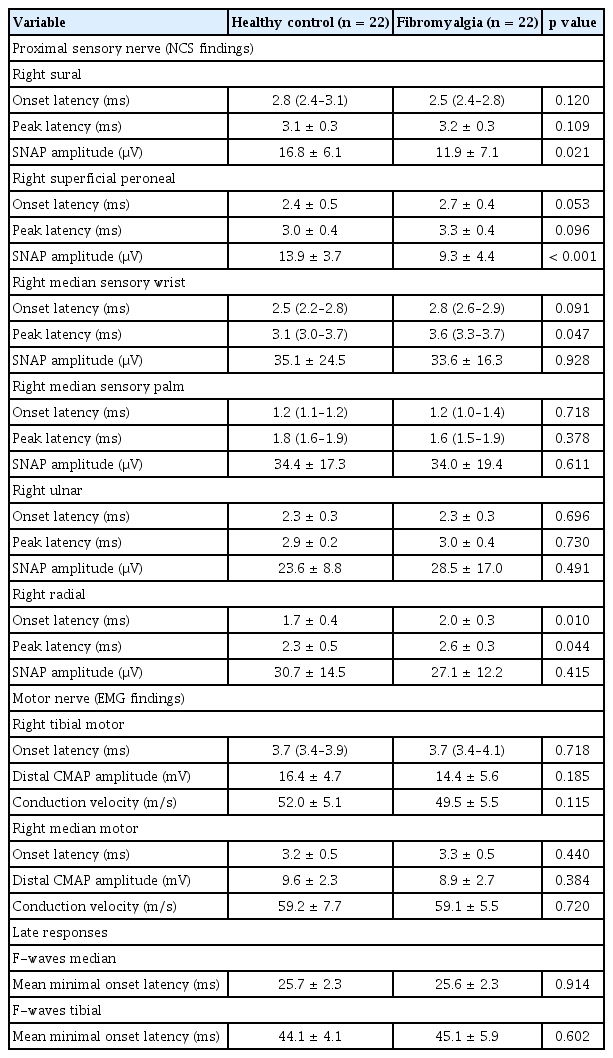
Comparison of the nerve conduction results of sensory, motor nerves, and late responses in the healthy control and fibromyalgia groups (right side)
Next, we performed NCS on distal sensory nerves because most asymptomatic and early neuropathies first occur on distal sensory nerves prior to proximal sensory or motor neuropathies [17,19]. The SNAP amplitudes of bilateral distal sensory nerves, including MP, DS, and MDC nerves, were significantly lower in the fibromyalgia group than in the HC group. Additionally, onset latencies of right MP, left MDC, and left DS nerves were significantly higher in the fibromyalgia group (Table 3). This implies that distal sensory neuropathies are more frequently found in fibromyalgia patients than HCs, even in fibromyalgia patients without definite neuropathies on proximal sensory or motor nerves.
Comparison of SUDOSCAN findings
The mESC values were calculated in bilateral hands and feet. In the HC and fibromyalgia groups, the hands mESC values were 68.8 ± 10.3 μS and 57.6 ± 16.2 μS (p = 0.010), and the feet mESC values were 72.0 ± 8.2 μS and 64.9 ± 11.5 μS (p = 0.025), respectively. In the fibromyalgia group, ten patients (45.5%) exhibited moderate SFN, and five patients (22.7%) exhibited severe SFN. In the HC group, 11 individuals (50.0%) exhibited moderate SFN and none exhibited severe SFN. The CAN-RS values exhibited a tendency to be higher in the fibromyalgia group than in the HC group; however, this was not statistically significant (Table 4).
Correlation between ESC and fibromyalgia disease duration and serum neurotransmitter levels and comparison of ESC value between TCA responder and non-responder
Next, we evaluated correlations between hands/feet mESC and fibromyalgia disease duration and serum neurotransmitter levels in fibromyalgia patients. Spearman correlation coefficients revealed that hands/feet mESC was negatively correlated with fibromyalgia disease duration (Rho = −0.636, p = 0.006, and Rho = −0.726, p < 0.001, respectively, Fig. 1). Additionally, hands/feet mESC was negatively correlated with the tryptophan-to-serotonin ratio (Rho = −0.464, p = 0.039, and Rho = −0.564, p = 0.010, respectively), and serum serotonin levels were positively correlated with hands/feet mESC (Rho = 0.484, p = 0.031, and Rho = 0.512, p = 0.021, respectively). The total FIQ score, widespread pain index, and pain VAS score did not show a significant correlation with the mESC score of the hands or feet. Only the waking component of the FIQ and symptom severity score showed a significant correlation with hand mESC values (Supplementary Table 1).

Correlation between mESC values of hands/feet and disease duration of fibromyalgia. mESC, mean electrochemical skin conductance.
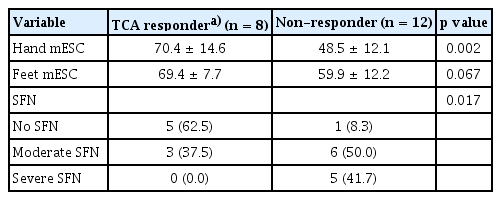
We could only evaluate the TCA treatment responses of 20 out of 22 fibromyalgia patients, because follow up data was lacking for two fibromyalgia patients. Among the 20 patients, eight patients were TCA responders and 12 patients were non-responders. The mESC values of hands and feet were significantly higher in TCA responders, and the non-responder group showed a higher prevalence of moderate to severe SFN (11/12, 91.7%) than the TCA responder group (3/8, 37.5%, Table 5).
DISCUSSION
In the present study, SFNs were more frequently observed in fibromyalgia patients than in HCs. ESC was significantly correlated with disease duration and serum serotonin levels in fibromyalgia patients. These findings suggest that SFN may be a causative factor of pain in fibromyalgia. Additionally, NCS showed that distal peripheral sensory neuropathy may precede proximal sensory or motor nerve neuropathy in fibromyalgia. This pattern is similar to that observed in diabetic peripheral neuropathy.
The pathogenesis of fibromyalgia is still not fully understood, and the symptoms of fibromyalgia are diverse and heterogenous among fibromyalgia patients [20]. Wide-spread musculoskeletal pain is the major symptom of fibromyalgia [21], and several pharmacological and non-pharmacological treatments have shown therapeutic improvement in fibromyalgia patients [3]. However, many fibromyalgia patients still do not achieve meaningful improvement after trying current treatments, which warrants the development of novel approaches [21]. For this purpose, it is most important to understand the pathophysiology of fibromyalgia. The International Association for the Study of Pain has recently defined nociplastic pain as distinct from nociceptive and neuropathic pain, as many chronic pain syndromes (including fibromyalgia) cannot be explained in terms of the conventional concept of pain [5]. Central sensitization, i.e., amplification of pain-related neural signaling within the central nervous system that induces pain hypersensitivity, is the most likely cause of pain in fibromyalgia, whereas neuropathic pain is not considered a definite pathological component of fibromyalgia [20,22]. Targeting neurotransmitters of central nervous system pain signaling, such as serotonin by selective serotonin reuptake inhibitors (SSRIs) or serotonin and norepinephrine reuptake inhibitors (SNRIs), has limited therapeutic effects in fibromyalgia. SSRIs did not reduce pain in 67.4% of fibromyalgia patients, and SNRIs resulted in only modest pain relief for which the standardized mean difference between SNRIs and placebo was only −0.23 [23,24].
Recently, some studies demonstrated the presence of SFN in fibromyalgia patients. Pickering et al. showed that ESC of the dominant hand was significantly lower in fibromyalgia patients than in HCs [6]. Another study revealed that 20% of fibromyalgia patients had decreased ESC, and that these patients had higher central sensitization inventory/hospital anxiety and depression scale scores and needed more analgesics than fibromyalgia patients without SFN [18]. In the present study, mESC was also significantly lower in fibromyalgia patients than in HCs, and definite SFN defined by mESC was more frequent in fibromyalgia patients than in HCs, in agreement with previous studies [6,18]. Additionally, we showed discrepancies between NCS/EMG and SUDOSCAN findings. Fibromyalgia patients did not show distal sensory or motor neuropathy but did exhibit sudomotor dysfunction more frequently than HCs. This suggests that SFN may contribute to the pathophysiology of fibromyalgia and should be considered when managing fibromyalgia symptoms.
SFN occurs in the early stage of type II diabetes mellitus in patients with peripheral neuropathy, and SFN can already be detected even when NCS/EMG findings are normal [17,19]. Skin punch biopsies together with microscopic quantification of epidermal sensory and autonomic nerve fiber densities around sweat glands are one of the most widely used objective tests for diagnosing SFN [25,26]. However, these procedures are invasive and can induce complications. SUDOSCAN is a non-invasive, time-efficient (approximately 3 min), and convenient method for evaluating sudomotor function by measuring sweat gland reactions to low-voltage stimulation [9]. ESC values obtained with SUDOSCAN showed fair predictive value for sweat gland nerve fiber density (area under curve = 0.73, sensitivity = 64%, specificity = 77%) [27] and significant correlation with epidermal nerve fiber density (Rho = 0.73, p < 0.001) and sweat gland nerve fiber density (Rho = 0.64, p < 0.001) [28]. These findings indicate that SUDOSCAN is a reliable alternative to skin punch biopsy.
SFN (A alpha and C fibers) differs from large fiber neuropathy (A delta fibers) in several aspects. The predominant symptoms of SFN are pain, paresthesia, autonomic signs, and temperature loss, whereas large fiber neuropathy causes a loss of vibration perception, position sense, and deep tendon reflexes [29]. Several pharmacological and non-pharmacological therapies, such as mexiletine, recombinant human nerve growth factor, body vibration, pulse electromagnetic fields, stellate ganglion blockage, and dorsal root ganglion stimulation, have shown therapeutic potential for SFN [30]. However, these therapies have not been validated for fibromyalgia with SFN. Future studies should assess the potential of these therapies for fibromyalgia. In the present study, disease duration of fibromyalgia was significantly correlated with mESC values of hands/feet, indicating that small fiber damage may occur with progression of fibromyalgia. Also, TCA responders showed more preserved SUDOSCAN findings (mESC), suggesting that SUDOSCAN might be a promising method for predicting treatment responses of fibromyalgia patients. Furthermore, distinguishing between fibromyalgia patients with and without SFN may enable precision medicine for fibromyalgia treatment.
NCS and EMG are the most widely used methods to detect peripheral sensory and motor neuropathy. One study showed that only 15% of fibromyalgia patients showed abnormal NCS findings, of which most comprised focal entrapment syndrome but not generalized polyneuropathy [12]. Another study based on EMG demonstrated that pain was independent of motor nerve activity in fibromyalgia patients [11]. This suggests that fibromyalgia pain is more similar to nociplastic than neuropathic pain [5,31]. In the present study, NCS of proximal sensory nerves and EMG of motor nerves did not reveal significant differences between the fibromyalgia and HC groups, except for some values regarding sural/superficial peroneal/median/radial nerves. However, the NCS of distal sensory nerves, including MP, DS, and MDC nerves, showed consistently lower SNAP amplitudes in the fibromyalgia group than in the HCs (Table 3). This is consistent with the SUDOSCAN results, which showed more severe sudomotor dysfunction in the fibromyalgia group. Altogether, these findings suggest that distal sensory neuropathy precedes proximal sensory and motor nerve neuropathy.
Furthermore, the present study measured serum levels of pain-related neurotransmitters and demonstrated a correlation between neurotransmitters and ESC for the first time. Serotonin and substance P are well-known neurotransmitters for nociception and central sensitization [32,33]. Substance P is released from nociceptors and increases pain sensitivity [34], whereas decreased serotonin levels are associated with several chronic pain disorders [35]. Similar to other chronic pain disorders, fibromyalgia is characterized by increased substance P levels and decreased serotonin levels [36,37]. Tryptophan is a precursor peptide for serotonin, and indoleamine 2,3-dioxygenase 1 (IDO1) is the key enzyme for tryptophan metabolism [38]. IDO1 activity is increased in patients with chronic pain, and IDO1 upregulation increased the tryptophan-to-serotonin ratio in a rat model [39]. Furthermore, IDO1 inhibitors reduced pain in an animal model [40]. In the present study, serotonin levels and the tryptophan-to-serotonin ratio were significantly correlated with hands/feet mESC. Dumolard et al. [18] evaluated ESC and the degree of central sensitization with a questionnaire (central sensitization inventory) and demonstrated that fibromyalgia patients with lower ESC had more severe scores. This study [18] together with our current study sugest that central sensitization and SFN are associated with fibromyalgia, and that central sensitization markers (e.g., serotonin level) can also represent SFN markers, and vice versa, in fibromyalgia patients.
There are some limitations in the present study. First, the sample size was relatively small. Recently, Dumolard et al. [18] performed a SUDOSCAN study on 265 fibromyalgia patients but did not include HCs. Another study on SFN and fibromyalgia only used questionnaires, which is a more subjective approach than SUDOSCAN [7]. The present study is the first study to evaluate both SFN and serum neurotransmitter levels in fibromyalgia patients. Another strength of the present study is that NCS/EMG was performed on the same day as SUDOSCAN, revealing that SFN can be present in fibromyalgia patients with normal NCS/EMG findings. Second, the design of the present study was cross-sectional and did not include follow-up data. Comparing the prognoses and clinical responses to treatment between fibromyalgia patients with or without SFN may enforce the clinical significance of SUDOSCAN results in fibromyalgia patients.
In conclusion, the present study showed that SFN and distal sensory neuropathy are often detectable in fibromyalgia patients, even in patients who do not have definite NCS/EMG abnormal findings in proximal sensory or motor nerves. In addition to central sensitization, SFN may represent another pathological component of fibromyalgia. Additionally, serum serotonin levels (which are usually decreased in fibromyalgia patients) were correlated with mESC. Abnormal findings of central sensitization (lower serotonin levels) may be associated with the occurrence of SFN in fibromyalgia patients, and evaluating serum serotonin levels may help discriminate fibromyalgia patients with a higher probability of SFN. Furthermore, SUDOCAN is a non-invasive method that could be used to predict the TCA treatment responses of fibromyalgia patients.
KEY MESSAGE
1. ESC values are decreased in fibromyalgia patients.
2. SFN is detected in about 70% of fibromyalgia patients.
3. ESC values of fibromyalgia patients are significantly correlated with disease duration, and ESC values may predict responses to TCA therapy.
Notes
CRedit authorship contributions
Hong Ki Min: investigation, formal analysis, validation, writing - original draft, writing - review & editing, visualization; Sun Im: conceptualization, resources, data curation, validation; Geun-Young Park: investigation, validation, software; Su-Jin Moon: conceptualization, methodology, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, visualization
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1A2B5B01002253).