Impact of discharge checklist on guideline-directed medical therapy and mid-term prognosis in heart failure
Article information
Abstract
Background/Aims
Despite the proven benefit of the guideline-directed medical therapy (GDMT), it remains underutilized in patients hospitalized with acute heart failure (HF). We aimed to evaluate the impact of the discharge checklist on GDMT installation and the prognosis of HF patients.
Methods
This study was a single-center, observational study that included all patients admitted for HF from March 2021 to February 2023. The data were retrieved from electronic medical records and discharge checklists. A comparison was conducted between the checklist group and the non-checklist group. The primary endpoint was a composite of all-cause mortality or readmission for HF within 6 months.
Results
The checklist was completed for 537 patients (checklist group) and not for 187 patients (non-checklist group). The proportion of patients to whom two or more components of GDMT were prescribed was significantly higher in the checklist group than in the non-checklist group (59.6% vs 42.2%, p < 0.001). The checklist group exhibited a significantly lower primary outcome compared to the non-checklist group (27.4% vs. 36.4%, HR 0.73, 95% CI 0.55–0.98, p = 0.036). The effect of the checklist was more prominent in HF with reduced ejection fraction (HR 0.51, 95% CI 0.34–0.77, p = 0.001) than in HF with mildly-reduced and preserved ejection fraction (HR 0.91, 95% CI 0.58–1.42, p = 0.676) (p for interaction = 0.06).
Conclusions
The implementation of the discharge checklist was associated with an improvement in GDMT prescription and an improved prognosis in patients with HF with reduced ejection fraction.
INTRODUCTION
Patients who are admitted to hospitals with acute heart failure (HF) have a grim prognosis, and they show a very high event rate within up to six months after discharge. According to data from the Korean Acute HF Registry (KorAHF), the mortality rate for acute HF during hospitalization is approximately 6%, while the mortality rate for acute HF within one year after discharge is reported to be around 18% [1]. Guideline-directed medical therapy (GDMT), which consists of renin-angiotensin system inhibitors (RASI) including angiotensin receptor/neprilysin inhibitor, beta-blockers, mineralocorticoid receptor antagonists (MRAs), and the recently added sodium-glucose cotransporter-2 inhibitors (SGLT2i), has been reported to improve the treatment outcomes of HF patients [2–5].
Despite its proven benefits, the implementation of GDMT in real-world practice remains unsatisfactory [6]. In the Asia-Pacific regions, including Korea, the GDMT prescription rate upon discharge has only been reported to be around 50% [7]. There are several factors that contribute to the suboptimal application of GDMT in HF patients. Patient non-adherence and intolerance to medications, which occur due to side effects or fragile patient condition, including low blood pressure and marginal renal function, hinder optimal GDMT application. However, physician inertia also represents a significant obstacle to optimal GDMT application. The reluctance or hesitance among physicians to initiate or intensify GDMT may stem from concerns about its potential side effects, perceived patient tolerance issues, or a preference for maintaining the status quo in treatment plans. One of the strategies that can be used in an attempt to overcome physician inertia is the fulfillment of a discharge checklist to review the discharge prescriptions being administered, thus offering a chance to initiate or intensify GDMT [8].
In a previous work, we reported on the beneficial effect of the discharge checklist in GDMT initiation before discharge [9]. The discharge checklist can be downloaded from the following URL: https://www.kshf.or.kr/uploaded/board/kspatientdata/_bb80f6b90c312d6310e270d11cf6f0731.pdf.
Surprisingly, the use of the discharge checklist was associated with a 55% lower risk of short-term death and rehospitalization within two months. However, since the previous study was small-scale and only had a follow-up period of two months, we aimed to confirm the beneficial effect in a larger-scaled, longer follow-up study. We also evaluated the impact of the discharge checklist according to the HF phenotypes: HF with reduced ejection fraction (HFrEF) and HF with mildly-reduced or preserved ejection fraction (HFmrEF and HFpEF).
METHODS
Study population
All patients who were hospitalized for HF in the cardiology wards of Seoul National University Hospital from March 2021 to February 2023 were included in this retrospective study. HF was diagnosed by cardiologists according to a standard definition by cardiologists [10,11]. Patients who were transferred to other departments or died during hospitalization were excluded from the present analyses. The protocols of the study were approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. H-2311-001-1481). This study adhered to the ethical guidelines of the 2013 Declaration of Helsinki. The requirement for informed consent from the IRB was exempted due to the retrospective nature of the study and the fact that the data were anonymized.
Clinical variables
The clinical data of the research subjects were collected retrospectively from the discharge checklist distributed by the Korean Society of HF and electronic medical records. The discharge checklist provided information such as discharge date, HF phenotype, HF etiology, comorbidities, type of medication at discharge, and aggravating factors. Other baseline characteristics, including age, sex, height, weight, body mass index (BMI), history of HF, and vital signs at admission were collected from the electronic medical records. Follow-up data, dose of prescribed medication, and vital signs at discharge were also collected from the electronic medical records. Participants were encouraged to complete the discharge checklist before discharge. In cases where some information was missing on the discharge checklist or the checklist was not completed, data were collected from the electronic medical record. Mortality data, which is not ascertainable solely from the electronic medical record, were requested from Statistics Korea.
Since GDMT includes various medications, we introduced the GDMT adequacy score as a single indicator to enable comparisons of patients’ GDMT prescription statuses. The criteria for the adequacy scores were modeled after the format used in a previous study conducted by our team [9]. Based on these criteria, we calculated each patient’s GDMT adequacy score using information obtained from the electronic medical record (Supplementary Table 1). Although SGLT2i are part of GDMT, they are not covered by insurance for patients without diabetes, thus making prescriptions of SGLT2i less common [12]. Therefore, SGLT2i were not considered when calculating the GDMT adequacy score [9]. Supplementary Table 1 presents the evaluation criteria use for the GDMT adequacy score, which were used in the previous reports [9].
Endpoints
The primary endpoint of the study was the composite outcome of all-cause mortality and readmission due to worsening of HF within 6 months. Secondary endpoints included all-cause mortality, readmission due to worsening of HF within 6 months, GDMT adequacy scores, and the number of GDMT prescriptions, with the data categorized by HF phenotype and discharge checklist fulfillment. GDMT was assessed at discharge.
Statistical analysis
The baseline characteristics were described using descriptive statistics. Continuous variables were described in terms of median and interquartile range, while categorical variables were expressed in terms of frequencies (n) and percentages (%). The choice between the Mann–Whitney U test or the unpaired Student’s t-test was made based on the nature of the continuous variables, while Fisher’s exact test or the chi-square test was used to compare proportions. The Cox proportional hazard model was used to identify the predictors of the primary endpoint. As a first step, a univariable Cox regression analysis was conducted to identify factors affecting primary outcome in patients with HF. Considering the number of subjects in this study, only some variables with a p value less than 0.05 were selected in the univariable Cox regression analysis. Next, to select the best-fitting variables for the multivariable Cox regression model, a bootstrap resampling procedure based on Cox regression analysis was used [13,14]. The most commonly occurring (more than 50% times) significant predictors were selected based on forced entry Cox regression using 1,000 bootstrap samples, and these variables were included in the final multivariable model. Survival analysis was conducted using Kaplan–Meier estimation, and Kaplan–Meier curves were used to illustrate the time-to-event distribution of the primary endpoint. The effect of discharge checklist on primary outcome was presented in terms of an adjusted hazard ratio (HR) and its 95% confidence interval (CI). Data analyses were carried out using the SPSS statistical software (version 26; IBM Corp, Armonk, NY, USA). Statistical significance was determined using a p value threshold of 0.05.
RESULTS
Baseline characteristics of patients based on discharge checklist completion status
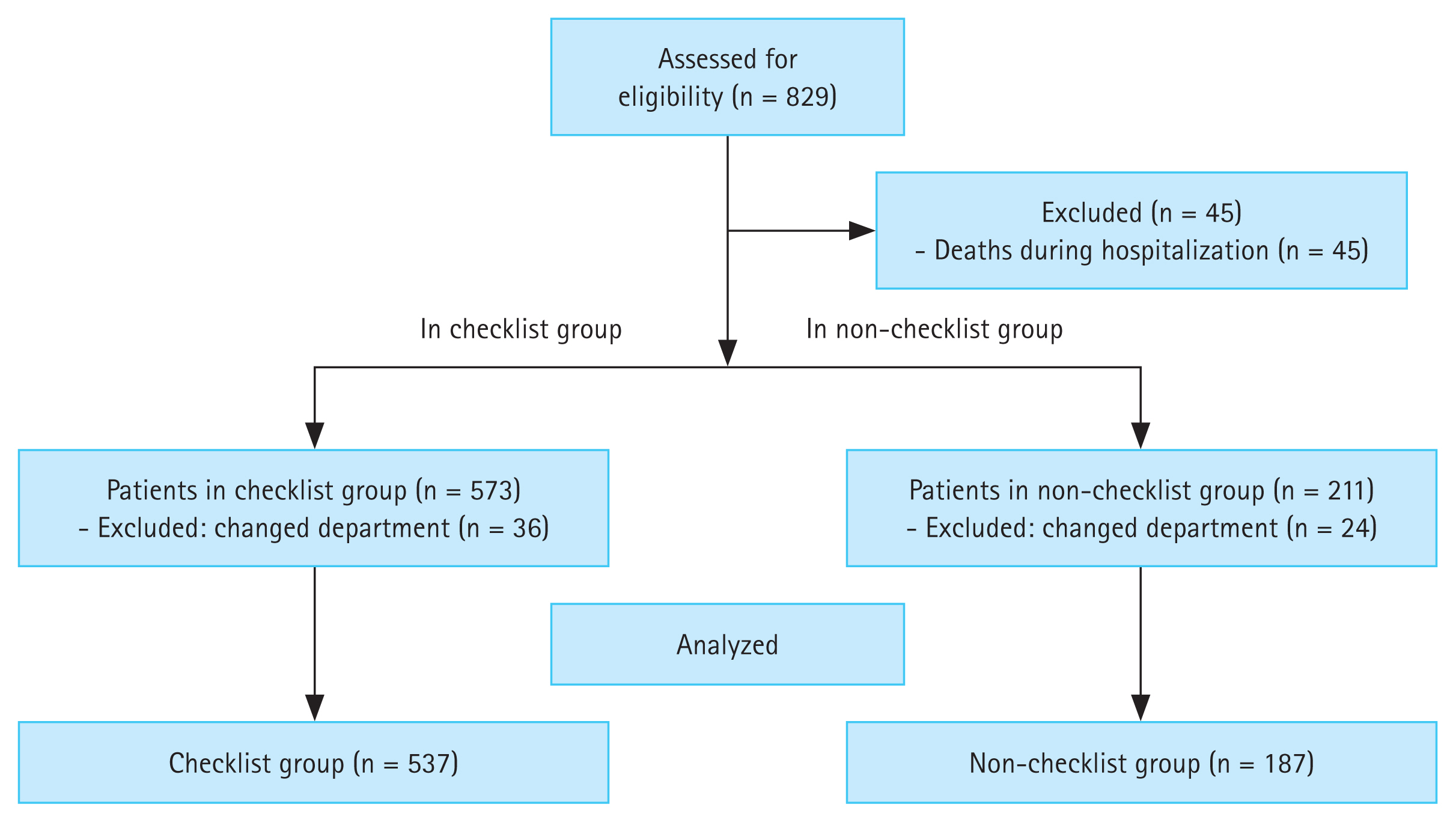
Of the 829 initially enrolled patients, 45 who died during hospitalization and 60 transferred to other departments were excluded from the study. Therefore, a total of 724 patients were included in the analysis, comprising 537 in the checklist group and 187 in the non-checklist group. Figure 1 shows a flowchart of the study.
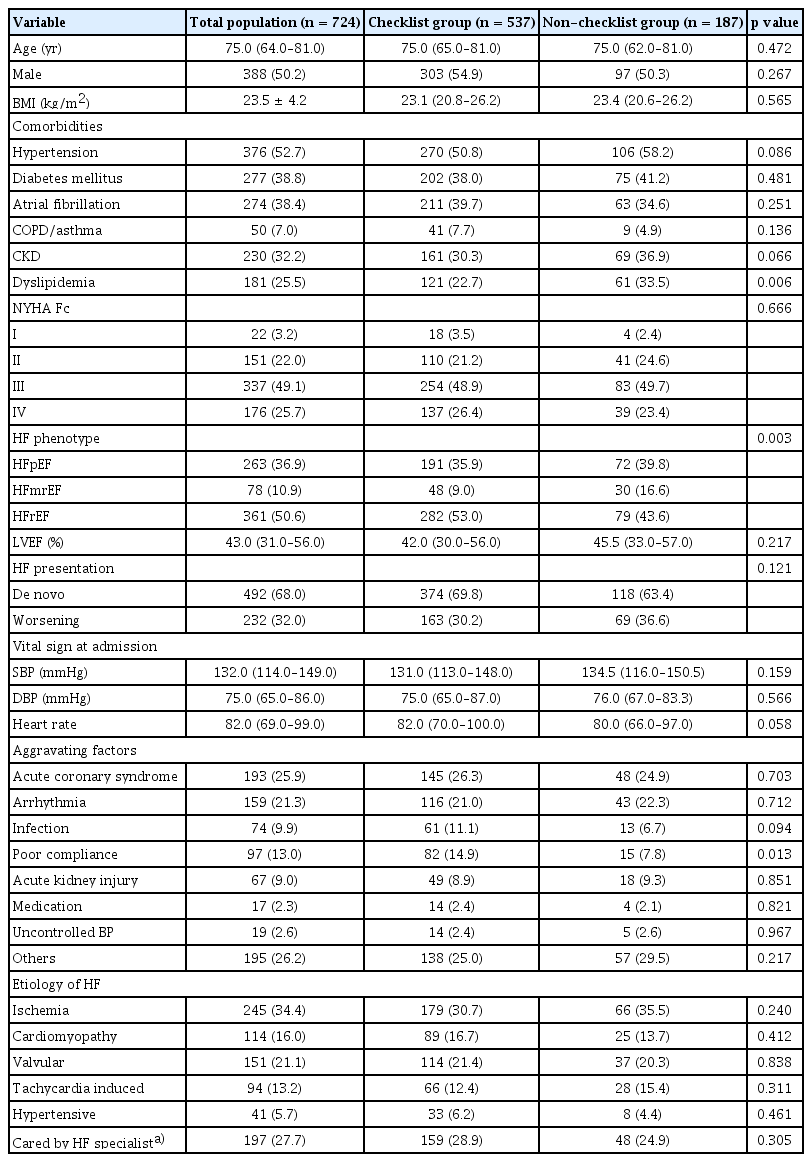
Table 1 presents the baseline characteristics of the patients. The median age of the patients with HF was 75 years old, and more than 50% of patients were male. There was no difference in the incidence of comorbidities between the checklist group and the non-checklist group, aside from dyslipidemia. Regarding the HF phenotype, the checklist group exhibited a significantly higher proportion of patients with HFrEF (53.0% vs. 43.6%), but there was no significant difference in left ventricular ejection fraction between two groups. The presentation of HF at admission in both groups was mainly de novo HF. The two groups showed similar median values of median systolic and diastolic blood pressure at admission, but median heart rate was numerically higher in the checklist group. The checklist group had a higher proportion of patients for whom poor compliance was an aggravating factor (14.9% vs. 7.8%, p = 0.013). The two groups were comparable in the other characteristics.
The effect of discharge checklist on clinical outcomes of HF patients
Figure 2 presents survival curves for the primary outcome based on checklist completion. In overall HF patients, the checklist group showed a significantly lower event rate compared to the non-checklist group (27.4% vs. 36.4%; HR 0.73, 95% CI 0.55–0.98, p = 0.036) (Fig. 2A). In HFrEF patients, the difference between the two groups was greater (29.3% vs. 48.7%; HR 0.51, 95% CI 0.34–0.77, p = 0.001) (Fig. 2B). On the other hand, in patients with HFpEF and HFmrEF, the checklist group showed a numerical decrease in the event rate compared to the non-checklist group, but there was no significant difference between the two groups (25.8% vs. 27.5%; HR 0.91, 95% CI 0.58–1.42, p = 0.676) (Fig. 2C) (p for interaction = 0.06 between discharge checklist and HF classification).
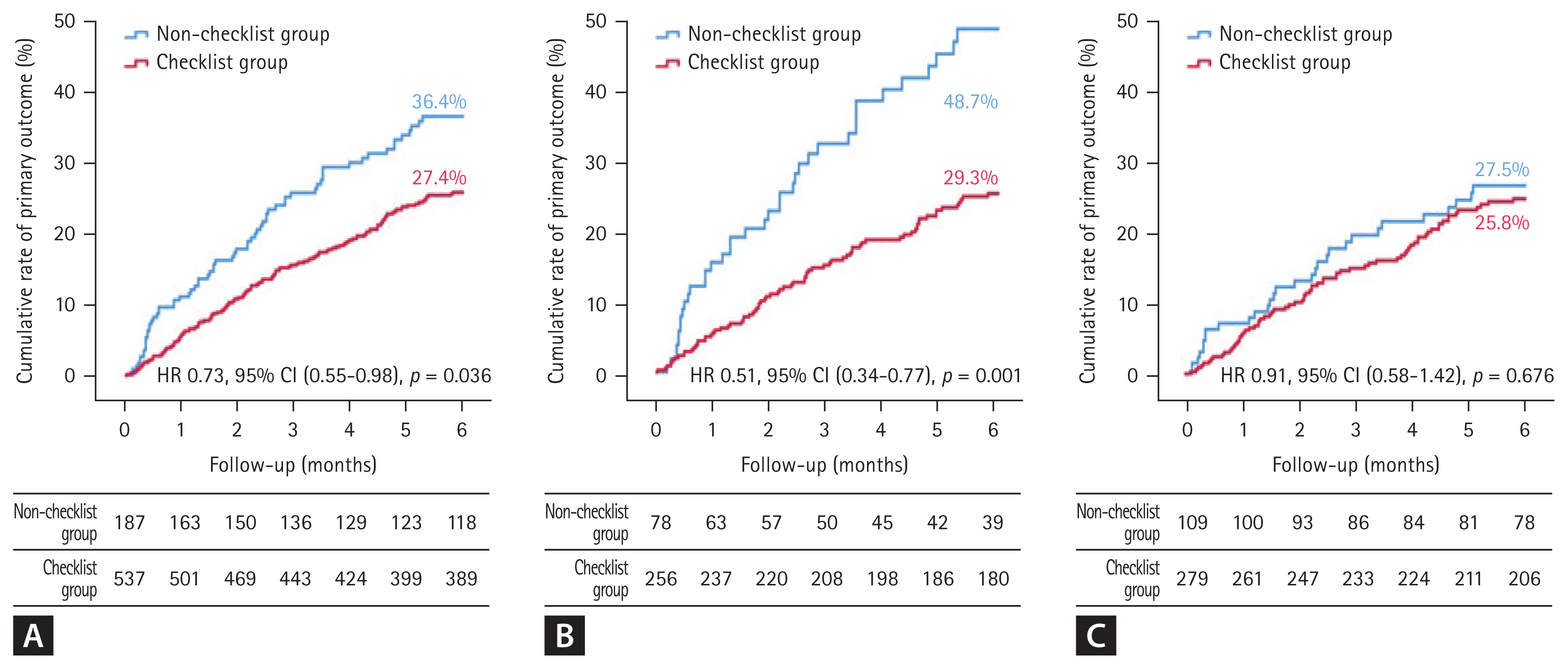
Kaplan–Meier curve for primary outcome in patients with HF based on the completion status of the discharge checklist. (A) Kaplan–Meier curve for the primary outcome of all HF patients, (B) for the primary outcome of HFrEF patients, and (C) for the primary outcome of HFpEF and HFmrEF patients. HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; HR, hazard ratio; CI, confidence interval.
When considering secondary outcomes (Supplementary Fig. 1), among HFrEF patients, the checklist group showed a significantly lower all-cause death rate (9.0% vs. 17.9%, p = 0.021) (Supplementary Fig. 1B) along with a numerically lower rehospitalization rate (23.4% vs. 33.3%, p = 0.051) (Supplementary Fig. 1E) when compared to the non-checklist group. However, there were no differences in either all-cause death or rehospitalization rate in patients with HFpEF and HFmrEF (Supplementary Fig. 1C, F). The difference in the two groups also continued to increase over time.
The systolic and diastolic blood pressure readings at the point of discharge were lower in the checklist group than they were in the non-checklist group (117.4 ± 17.9 mmHg vs. 121.2 ± 21.5 mmHg, p = 0.002, and 69.9 ± 10.2 mmHg vs. 72.0 ± 11.3 mmHg, p = 0.019). Further, more patients in the checklist group belonged to the higher score heart rate category (thus lower heart rate at discharge) even though the checklist group had a significantly higher baseline heart rate than the non-checklist group (Supplementary Table 2).
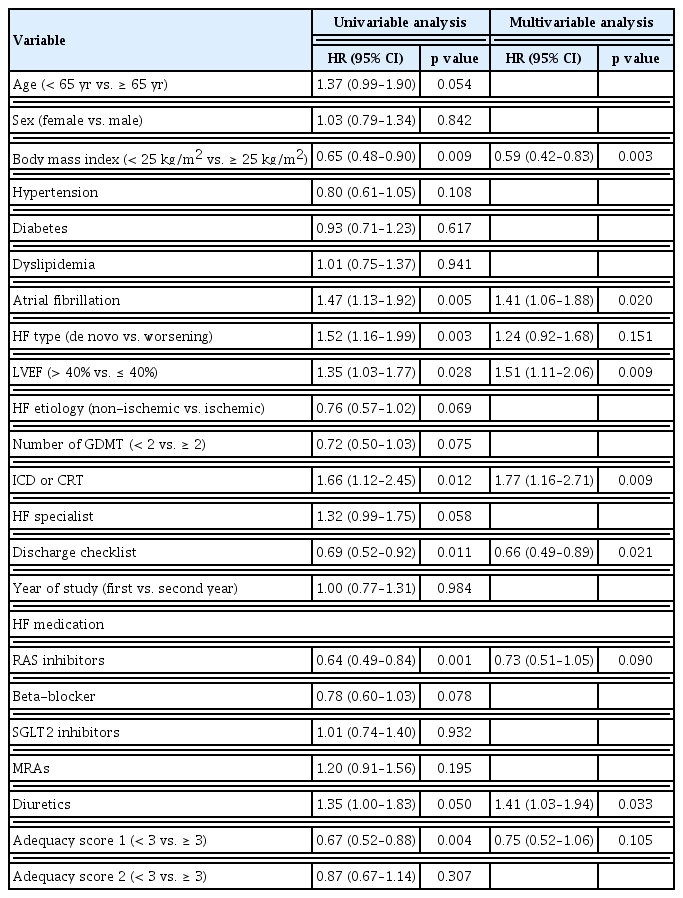
Lastly, univariable and multivariable Cox proportional hazards regression analyses were conducted to identify the independent predictors for primary outcome and to assess the impact of discharge checklist on primary outcome. In the univariable analysis, completion of the discharge checklist, high BMI, RASI therapy, and high adequacy score were all significantly associated with lower event rates, while the presence of atrial fibrillation, de novo/worsening of HF, HFrEF, and need for treatment with implantable cardioverter defibrillator/cardiac resynchronization therapy (ICD/CRT) and diuretics therapy were all significantly associated with higher event rates (Table 2). Based on forced entry Cox regression using 1,000 bootstrap samples, the most commonly occurring (more than 50% times) significant predictors are the completion of the discharge checklist (74.4% times), de novo/worsening of HF (68.5% times), diuretics therapy (65.8% times), and RASI therapy (55.8% times). These four variables were ultimately included in the multivariable Cox regression model (Supplementary Fig. 2).
In multivariable analysis, completion of the discharge checklist and RASI therapy were significantly associated with lower event rates, while de novo/worsening of HF remained significantly associated with higher event rates of primary outcome. Remarkably, the fulfillment of discharge checklist was associated with a 27% (95% CI 0.55–0.98, p = 0.036) reduction in the risk of 6-month all cause death or rehospitalization.
GDMT prescription based on the discharge checklist completion status
Figure 3 presents the prescription status of GDMT based on checklist completion. When comparing the types of GDMT medications prescribed, the checklist group received an average of 1.7 ± 0.9 classes of GDMT, which was significantly higher than that in the non-checklist group (1.3 ± 0.9, p < 0.001). The adequacy scores of type 1, with a maximum value of ten, were higher in the checklist group than they were in the non-checklist group (mean ± standard deviation) (3.7 ± 2.4 vs. 2.7 ± 2.2, p < 0.001). Meanwhile, the adequacy score of type 2, with a maximum value of nine, was also significantly higher in the checklist group than it was in the non-checklist group (3.0 ± 2.1 vs. 2.1 ± 2.0, p < 0.001) (Supplementary Table 2).
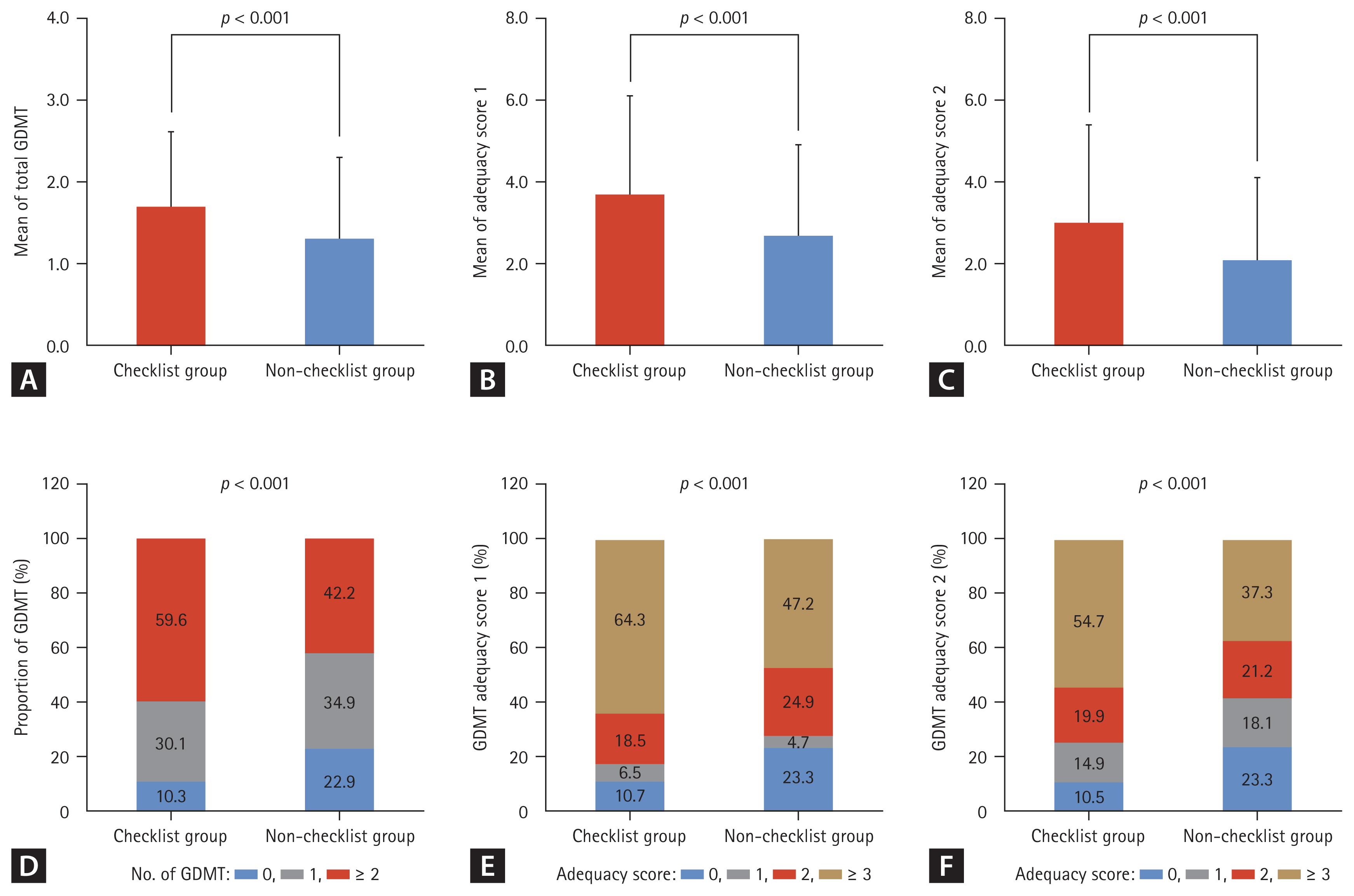
Effect of discharge checklist on GDMT prescription and adequacy score. (A) The mean of total GDMT, (B) the mean of adequacy score 1, (C) the mean of adequacy score 2, (D) the distribution of GDMT prescription, (E) the distribution of adequacy score 1, and (F) the distribution of adequacy score 2 in checklist and non-checklist groups. GDMT, guideline-directed medical therapy.
Impact of discharge checklist completion on GDMT prescription stratified by HF phenotypes
Although GDMT is established for HFrEF patients, there has been limited researching examining its efficacy in HFpEF and HFmrEF patients [15,16]. In this regard, we evaluated its impact in an analysis stratified by HF phenotypes. Because the number of hospitalized patients with HFmrEF and HFpEF was smaller than that of hospitalized patients with HFrEF, we merged patients with HFmrEF and HFpEF for further analysis. Figure 4 presents the prescription rates of the medications that are included in GDMT for HFrEF patients and HFpEF/HFmrEF patients. In HFrEF patients, the checklist group showed significantly higher prescription rates of RASI (74.5% vs. 59.0%, p = 0.010), beta-blockers (80.4% vs. 67.9%, p = 0.029), and SGLT2i (31.1% vs. 19.2%, p = 0.045) compared to the non-checklist group. There was also a trend toward higher prescription rates of angiotensin receptor-neprilysin inhibitor in the checklist group, although this difference did not reach statistical significance (p = 0.097). However, in HFpEF and HFmrEF patients, there were no significant differences in the rate of prescription of any medication.
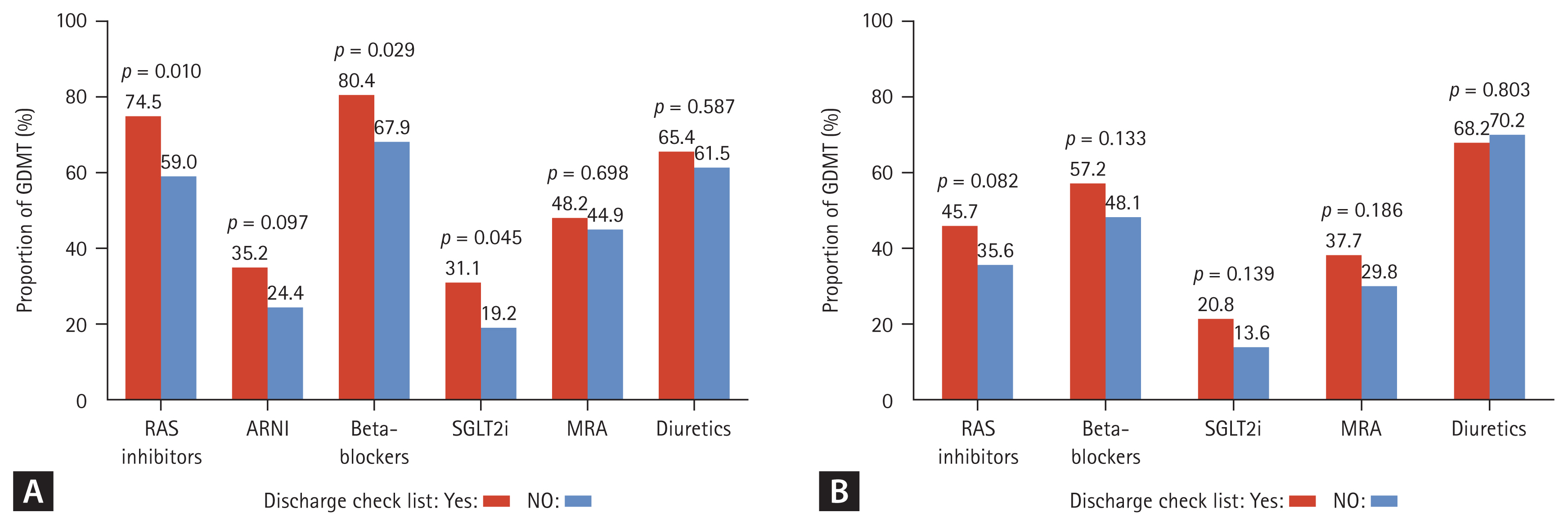
Effect of discharge checklist completion on GDMT prescription. (A) Prescription rates of medications included in GDMT for HFrEF patients, (B) for HFpEF and HFmrEF patients. GDMT, guideline-directed medical therapy; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mildly reduced ejection fraction; RAS, renin-angiotensin system; ARNI, angiotensin receptor-neprilysin inhibitor; SGLT2i, sodium-glucose cotransporter-2 inhibitors; MRA, mineralocorticoid receptor antagonist.
DISCUSSION
The current study evaluated the impact of a discharge checklist on the prescription of GDMT and clinical outcomes in patients who were hospitalized for acute HF. The checklist group exhibited a significantly lower composite outcome of all-cause mortality or HF readmission within 6 months, which could be attributable to the significantly higher GDMT prescription rate. This beneficial effect was shown to persist after adjusting for various clinical variables, suggesting that the completion of the discharge checklist contributes to improved outcomes in HF patients. In a previous paper we reported the results of a small, short-term observational study which suggested that discharge checklist usage might be an effective strategy for GDMT initiation during hospitalization and that it could also be beneficial in terms of short-term outcome in two months [9]. This study confirmed the findings of the previous study in a larger, longer follow-up study. The present study is also meaningful for the following reasons: First, completion of the discharge checklist had greater impacts on GDMT rate and clinical outcomes in patients with HF in HFrEF than it did in HFpEF/HFmrEF. Most of the main contents of the HF discharge checklist that have been published by the Korean Heart Failure Society focus on HFrEF management. This may explain why completing the discharge checklist had a greater impact on clinical outcomes in patients with HFrEF than it did in those with HFpEF/HFmrEF in our study. Approximately 50% of HF patients are reported to have HFpEF [17]. HFpEF/HFmrEF is also associated with considerable mortality [18]. Early intervention and optimization of the prevention and treatment of comorbidities could potentially prevent adverse events in patients with HFmrEF/HFpEF. Because HFmrEF/HFpEF has different characteristics from HFrEF, the contents of the discharge checklist should be constructed differently according to the HF classification. Second, the rate of completion of the discharge checklist was more than 50% in patients with HFrEF, while it was less than 50% in patients with HFpEF/HFmrEF in our study. Therefore, despite the reported effectiveness of SGLT2 inhibitors in patients with HFmrEF/HFpEF, the prescription rate of SGLT2 inhibitors was lower in patients with HFmrEF/HFpEF than it was in those with HFrEF. This suggests that physicians are either unaware of or not interested in the management of HFmrEF/HFpEF patients. Therefore, there is a need for further research and efforts to care for and effectively manage patients with HFmrEF/HFpEF. Third, although the adverse event rate was lower in the checklist group than it was in the non-checklist group, the rate of adverse events gradually increased over time in both groups (Fig. 2). This result suggests that the discharge checklist may have affected the initiation of GDMT, but that it did not affect the up-titration of GDMT. According to the STRONG-HF study, an intensive treatment strategy of rapid up-titration of GDMT after an acute HF admission reduced symptoms, improved quality of life, and reduced the risk of death or HF readmission [19]. Altogether, these findings indicate that it is important not only to initiate GDMT, but also to rapidly up-titrate GDMT until the patient can tolerate it. Considering this, although the completion of the discharge checklist impacts GDMT initiation, it may not affect the up-titration of GDMT or clinical inertia.
In this study, the discharge checklist was used solely for educational purposes. However, if the clinical significance of the discharge checklist were to be recognized, it is anticipated that the checklist could also come to be utilized by HF specialists to confirm patient discharges.
The results revealed the existence of a significant association between checklist completion and increased GDMT prescription, showcasing the checklist’s efficacy in optimizing medical therapy for HF patients. Although GDMT prescription has benefits for HF patients, research has shown a lack of adherence to GDMT prescriptions in the real world. According to the Acute Decompensated HF National Registry (ADHERE) in the United States, only 83%, 80%, and 33% of patients received prescriptions for RASI, beta-blockers, and MRA, respectively [20]. In the Asia-Pacific region, GDMT prescription rates were even lower. According to the Acute Decompensated HF Registry International-Asia Pacific (ADHERE-AP), only 63%, 41%, and 31% of patients were prescribed RASI, beta-blockers, and MRA, respectively [21]. The existence of a higher number of GDMT classes and higher adequacy scores in the checklist group underscores the checklist’s role in guiding physicians toward a more comprehensive prescription. This finding aligns with the results of previous studies emphasizing the underutilization of GDMT in real-world practice and highlights the checklist as a potential solution to address this gap. The checklist’s impact on blood pressure readings and heart rate categories suggests that it influences not only medication choice but also individualized treatment adjustments, which ultimately emphasizes its role in tailoring therapy to patient needs.
An analysis in which the results were stratified based on HF phenotypes revealed a substantial impact on HFrEF patients, with higher prescription rates of RASI, beta-blockers, and SGLT2i in the checklist group. This aligns with existing evidence supporting the efficacy of GDMT in HFrEF. Previous studies demonstrated the efficacy of four pillars for HFrEF therapy, and our previous study reported that discharge checklist usage is an effective strategy for GDMT initiation during hospitalization. The discharge checklist also improves adherence to GDMT in patients with HFrEF. Survival analysis demonstrated the existence of a significantly lower event rate in the checklist group, particularly in HFrEF patients. This suggests that the checklist’s impact extends beyond prescription rates, as it influences clinical outcomes such as all-cause mortality and HF readmission. However, the lack of a significant difference on clinical outcomes in HFpEF and HFmrEF patients underscores the complexity involved in managing these conditions and raises questions about the checklist’s effectiveness in improving outcomes in these specific populations. This may reflect the ongoing challenges in establishing evidence-based therapies for these HF subtypes while also highlighting the need for further research to identify effective treatment strategies for patients with preserved or mildly-reduced ejection fraction [15,16]. As effective therapies come to be established for patients with preserved or mildly-reduced ejection fraction, the utility of the discharge checklist is expected to increase for these individuals.
Survival analysis demonstrated a significantly lower event rate in the checklist group, particularly in HFrEF patients. This suggests that the checklist’s impact extends beyond prescription rates, in turn influencing clinical outcomes such as all-cause mortality and HF readmission. The lack of a significant difference in HFpEF and HFmrEF patients underscores the complexity of managing these conditions and raises questions about the checklist’s effectiveness in improving outcomes in these specific populations. The results of univariable and multivariable analyses further supported the checklist’s independent association with lower event rates, ultimately emphasizing its potential utility as a valuable tool in the management of HF patients.
This study set a hypothesis predicting that the completion of a discharge checklist would improve patient outcomes by ensuring better physician adherence to guidelines when prescribing medications. This hypothesis can be divided into two assumptions: first, that checklist completion increases the prescription rate of GDMT, and second, that elevated GDMT prescription rates lead to improved patient outcomes. Previous research has supported each of these assumptions. In a study evaluating the effectiveness of a discharge checklist in the care of patients with acute decompensated HF, it was concluded that the discharge checklist serves as a simple tool to ensure the prescription of GDMT [22]. Further, although not a discharge checklist specifically, another study evaluating the impact of checklists on medication prescriptions for HF patients found that the checklist group exhibited a higher prescription rate of RASI compared to the non-checklist group [8]. In the current study, patients in the checklist group also demonstrated a higher GDMT adequacy score compared to patients in the non-checklist group, thus aligning with the anticipated results.
There is substantial evidence supporting the idea that GDMT contributes to improved patient outcomes. According to the real-life SMYRNA study, the non-use or suboptimal use of GDMT is associated with increased rates of hospitalization and cardiovascular death in patients with HF [23]. Clinical trials evaluating the effects of GDMT in specific patient populations have also emphasized the importance of considering the prescription of medications included in GDMT for potential benefits in such populations [24,25].
Despite the compelling findings it has obtained, the present study has certain limitations that warrant consideration. First, its retrospective nature may introduce biases and limit the establishment of causation. For instance, clinicians might have been inclined to utilize the checklist more frequently for patients with more severe profiles, with the aim of ensuring thorough care at discharge. To mitigate bias, we conducted comparisons of baseline characteristics and performed subgroup analyses. Additionally, the study was conducted in a single-center, which potentially limited the generalizability of its results to broader populations. The reliance on retrospective data collection and the exclusion of patients who died or were transferred to other departments during hospitalization may also have introduced selection bias. Further, the study’s duration and follow-up period may not capture long-term effects, and caution should be taken when considering the checklist’s generalizability to different healthcare settings.
In conclusion, the findings of this study support the use of a discharge checklist as a valuable intervention to enhance GDMT prescription and improve clinical outcomes in patients hospitalized for HF, particularly those with reduced ejection fraction. However, this study’s limitations underscore the need for further research, including prospective, multicenter studies, to validate these findings and address potential biases. Future investigations should explore the effectiveness of the checklist in different HF phenotypes and its long-term impact on patient outcomes to optimize its implementation in clinical practice.
KEY MESSAGE
1. The implementation of the discharge checklist was associated with enhancements in the prescription of GDMT.
2. The checklist group showed a significantly lower rate of the primary outcome (composite of all-cause mortality and readmission due to worsening of HF within 6 months) compared to the non-checklist group (27.4% vs. 36.4%, p = 0.036).
3. The impact of the checklist was more significant in patients with HF with reduced ejection fraction, while there was no statistical difference observed in patients with HF with mildly-reduced or preserved ejection fraction.
Notes
CRedit authorship contributions
Won-Seok Lee: data curation, writing - original draft; Kyu-Sun Lee: data curation, formal analysis; Helsi Rismiati: conceptualization, methodology; Hae-Young Lee: conceptualization, methodology, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None