Fatty liver index and development of lung cancer: a nationwide cohort study
Article information
Abstract
Background/Aims
This study aimed to evaluate the impact of steatotic liver disease severity on the cumulative incidence of lung cancer utilizing data from the Korea National Health Insurance Service (NHIS).
Methods
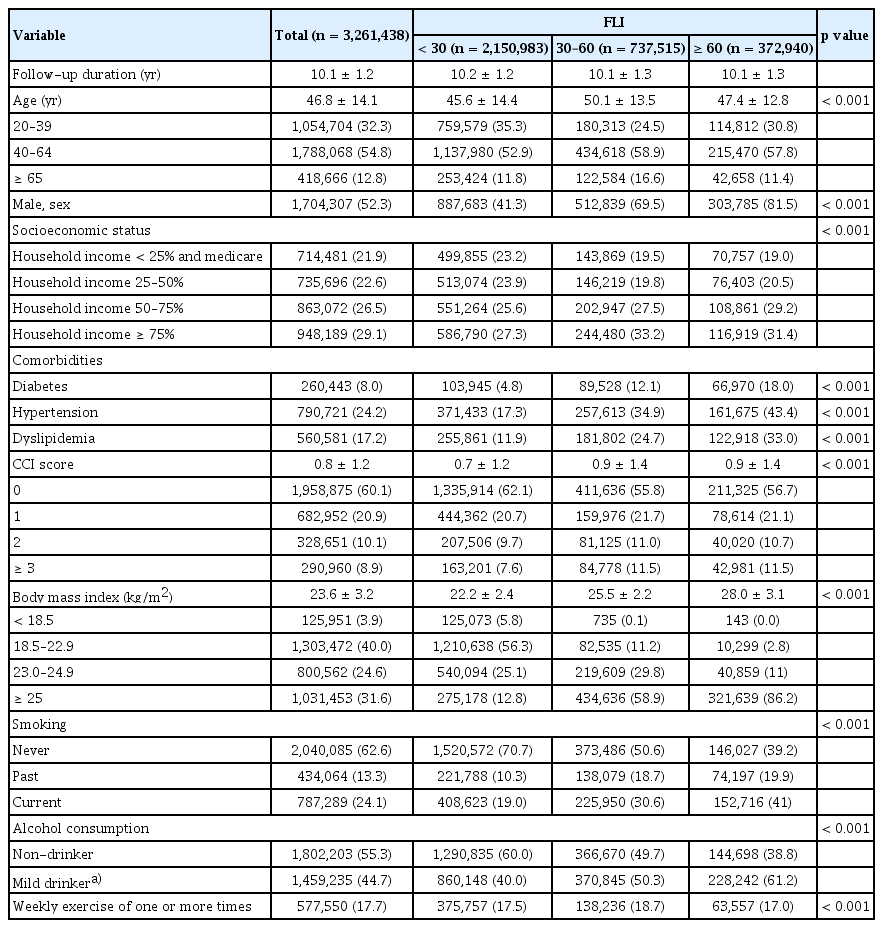
This study examined the risk of lung cancer in the general population in conjunction with the incidence of steatotic liver disease. The study population consisted of 3,261,438 individuals aged 20 years or older who underwent a general health examination in 2009.
Results
Individuals with fatty liver index (FLI) of 30–59 exhibited a 1.08-fold increased risk of lung cancer (95% CI: 1.04–1.11), while FLI ≥ 60 was associated with a 1.22-fold elevated risk of lung cancer (95% CI: 1.17–1.28) compared to those with FLI < 30. The risk varied with smoking status; in current smokers, the adjusted HR for the FLI 30–59 group was 1.05 (95% CI: 1.00–1.10), while that in the FLI ≥ 60 group was 1.11 (95% CI: 1.04–1.18). In never- or past-smokers, the adjusted HR for the FLI 30–59 group was 1.10, and that for the FLI ≥ 60 group was 1.31. Subgroup analysis revealed an incidence rate of 1.06 per 1,000 person-years in the consistently high FLI group compared to 1.15 in those with improved FLI. Improving FLI over time was associated with a 0.93-fold decrease in lung cancer risk.
Conclusions
Our study demonstrated a correlational relationship between lung cancer incidence and the severity of steatotic liver disease as measured by FLI.
INTRODUCTION
Steatotic liver disease viewed as a hepatic manifestation of metabolic dysfunction, has been reported to have an association with hepatocellular carcinoma (HCC). Steatotic liver disease prevalence, which is expected to increase, is approximately 30% in Korea and 30–50% in Western countries [1]; clinical interest in diseases associated with steatotic liver disease is increasing. The carcinogenetic correlation with steatotic liver disease does not apply only to primary liver cancer. Excessive adipose tissue promotes the production of proinflammatory cytokines and suppresses cell death, creating an environment favorable to tumor development [2]. A meta-analysis of observational cohort studies showed an increased risk of extrahepatic cancers, e. g., lung, breast, gynecological, and urinary system cancers, in individuals with steatotic liver disease, suggesting that the carcinogenic correlation extends beyond HCC and includes extrahepatic solid cancers [3,4]. A study utilizing the Swedish National Patient Registry revealed that both the incidence of HCC and the risk for certain non-HCC solid cancer subtypes were significantly higher in the steatotic liver disease group compared to controls. However, the number of patients included in the extrahepatic subtype groups was small [5].
Lung cancer, the leading cause of cancer-related deaths worldwide, is primarily associated with environmental and lifestyle factors [6]. The most significant risk factors for its development include tobacco use, exposure to secondhand smoke, air pollution, and occupational or household exposure to carcinogenic materials [7–10]. Despite the established links to these risk factors, the role of metabolic conditions, such as metabolic syndrome and fatty liver disease, in lung cancer development is an emerging area of interest. Recent studies suggest a potential association between metabolic dysfunctions and lung cancer risk [11,12]. Although the impact may not be as pronounced as that of smoking or air pollution, understanding this relationship is crucial because fatty liver disease represents a modifiable risk factor that could be targeted through lifestyle changes.
Therefore, while the primary causes of lung cancer have been identified, the implications of metabolic syndrome and fatty liver disease on lung cancer development is a significant task. To this end, we conducted a study comparing the cumulative incidence of lung cancer in cases of steatotic liver disease with that in the general population using data from the Korea National Health Insurance Service (NHIS). We also examined the pertinent risk factors of steatotic liver disease on lung cancer occurrence.
METHODS
Study design and population
This study examined the risk of lung cancer in steatotic liver disease cases in the general population. A cohort was established using nationwide claims data from the Korea NHIS [13]. Korea’s universal health coverage system has provided healthcare coverage to the entire population since 1989. The claims data include a comprehensive overview of healthcare history, including demographic data; socioeconomic status determined by monthly household income; diagnoses following the International Classification of Diseases, 10th Revision (ICD-10) guidelines; and detailed treatment and prescription data. Additionally, the NHIS has been offering general health screenings once every two years to all Koreans aged 20 years or older. Participant rates in this examination program have ranged from 56.1–67.7% during the recent decade [14]. The general health examination comprises body measurements, blood pressure assessment, blood tests, and a collection of questionnaires to acquire behavior information.
The study population consisted of adult individuals aged 20 years or older who underwent a general health examination in 2009 (n = 4,234,412). Heavy alcohol consumers (n = 354,376), those who had viral hepatitis infection (n = 4,045,888) or liver cirrhosis (n=14,835), those who had a history of any malignancies (n = 50,128), those had insufficient medical records (n = 140,677), and those diagnosed with lung cancer within the first year of enrollment (n = 8,370) were excluded from the analysis. After exclusions, our study included 3,261,438 individuals. Participants were followed from the index date until death, emigration, or the conclusion of the study in December 2020.
Our study was approved by the Institutional Review Board of Soongsil University (IRB No. SSU-202007-HR-236-01) and was conducted in compliance with both the Declarations of Helsinki and Istanbul. Informed consent was not required as only de-identified data were utilized.
Data collection
We gathered data on a number of variables, including age; gender; socioeconomic status; comorbidities; body measurements of height, weight, and waist circumference; blood tests for γ-glutamyl transpeptidase and triglycerides; smoking and alcohol consumption habits; and physical activity levels from the NHIS claims data. Comorbidities claimed within one year before the enrollment were evaluated. Based on this information, we calculated the overall comorbidity burden using the Charlson Comorbidity Index (CCI).
We employed the fatty liver index (FLI) to identify steatotic liver disease. The FLI was calculated using the formula [15]:
Among various clinical approaches, the FLI has been validated as a reliable diagnostic tool for detecting fatty liver disease with a number of associated etiologies without imaging techniques [15–17]. Participants with FLI scores less than 30 were classified as low risk for steatotic liver disease; those scoring between 30 and 59 were placed in the category of indeterminate risk, and individuals with FLI scores of 60 or higher were considered to be at high risk for steatotic liver disease.
Study outcomes
The primary outcome was development of lung cancer during the study period. Lung cancer was defined as a claim with the ICD-10 code C34 in conjunction with cancer-specific rare and incurable disease (RID) codes, specifically V193. Cancer patients registered under the RID system are responsible for 5% of the total medical expenses incurred for both outpatient and inpatient care. This necessitates stringent documentation of biochemical, histological, and clinical evidence so the accuracy of RIDs in Korea is reliable at greater than 90% [18]. The secondary aim of the study was determination of the change in lung cancer risk in conjunction with decreases in risk of steatotic liver disease in patients initially at an intermediate or high risk of steatotic liver disease (FLI ≥ 30).
Statistical analysis
For baseline characteristics, categorical variables are presented as numbers and percentages, and continuous variables are presented as means ± standard deviations. Continuous variables were analyzed using the ANOVA test, and categorical variables were assessed using the chi-square test. We calculated the incidence rate of lung cancer per 1,000 person-years (PYs). Utilizing the Kaplan–Meier method, cumulative incidence curves for the risk of lung cancer were generated based on the FLI. Covariates adjusted in the model were age; sex; socioeconomic status; presence of diabetes, hypertension, or dyslipidemia; body mass index; smoking status; alcohol consumption; and weekly exercise level. Subgroup analysis based on smoking status was conducted to evaluate the impact of steatotic liver disease on the risk of lung cancer according to smoking status. Additionally, we investigated the change in lung cancer risk in individuals who underwent physical examinations in two consecutive years and experienced a decrease in steatotic liver disease from an intermediate or high risk (FLI ≥ 30) status. p values < 0.05 were deemed to indicate statistical significance. All statistical operations were conducted using SAS Enterprise Guide Software Version 9.4 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Baseline characteristics of the entire cohort
The baseline characteristics of the study population are shown in Table 1. The mean follow-up duration was 10.1 ± 1.2 years. Of the 3,261,438 participants, 2,150,983 (66.0%) were classified low risk of steatotic liver disease with an FLI < 30; 737,515 (22.6%) were in the intermediate risk group with an FLI of 30–59; and 372,940 (11.4%) were categorized as high risk with an FLI ≥ 60. Individuals with FLI < 30, at low risk of steatotic liver disease, tended to be younger and were predominantly female. Additionally, this group exhibited fewer metabolic dysfunction-related comorbidities such as hypertension, diabetes mellitus, and dyslipidemia and had the lowest CCI score of 0.7 ± 1.2 vs. 0.9 ± 1.4 vs. 0.9 ± 1.4; p < 0.001 in the low-, intermediate-, and high-risk groups, respectively. The low-risk group had the highest proportion of individuals classified as underweight or within the normal weight range. Moreover, the prevalence of never-smokers (70.7% vs. 50.6% vs. 39.2%; p < 0.001) and non-drinkers (60.0% vs. 49.7% vs. 38.8%; p < 0.001) was also highest among the FLI < 30 group.
Lung cancer incidence and FLI
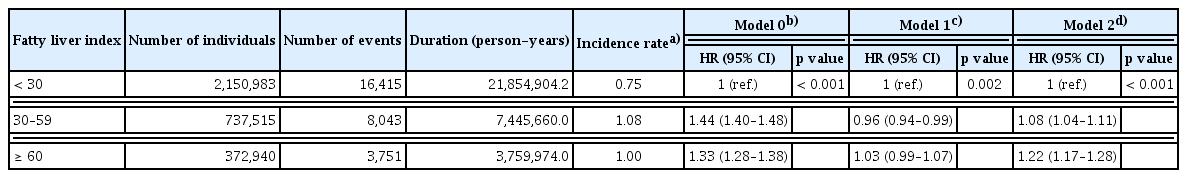
Throughout the observation period, 28,209 patients were diagnosed with lung cancer. Among these subjects, there were 16,415 cases of lung cancer in individuals with FLI < 30; the annual incidence was 0.75 per 1,000 PYs. There were 8,043 cases of lung cancer in the intermediate-risk, FLI 30–59 group, with an annual incidence of 1.08 per 1,000 PYs. Additionally, there were 3,751 cases in the high-risk, FLI ≥ 60 group, with an annual incidence of 1.00 per 1,000 PYs. The cumulative incidence rates of lung cancer for FLI < 30, FLI 30–59, and FLI ≥ 60 rates at one year were 0.05%, 0.07%, and 0.06%, respectively. These rates at five years were 0.30%, 0.42%, and 0.38%, respectively; and those at 10 years were 0.74%, 1.06%, and 0.99% (p < 0.001). These data are illustrated in Figure 1. Individuals with FLI < 30 demonstrated a significantly lower risk of lung cancer compared to the other groups; the crude hazard ratios (HRs) were 1.44 (95% confidence interval [CI]: 1.40–1.48) in the FLI 30–59 group and 1.33 in the FLI ≥ 60 group (95% CI: 1.28–1.38) before adjustment. Following adjustment for multiple variables, individuals with FLI 30–59 exhibited a 1.08-fold increased risk of lung cancer (95% CI: 1.04–1.11), and subjects with FLI ≥ 60 had a 1.22-fold elevated risk of lung cancer (95% CI: 1.17–1.28) compared to those with FLI < 30 (Table 2).
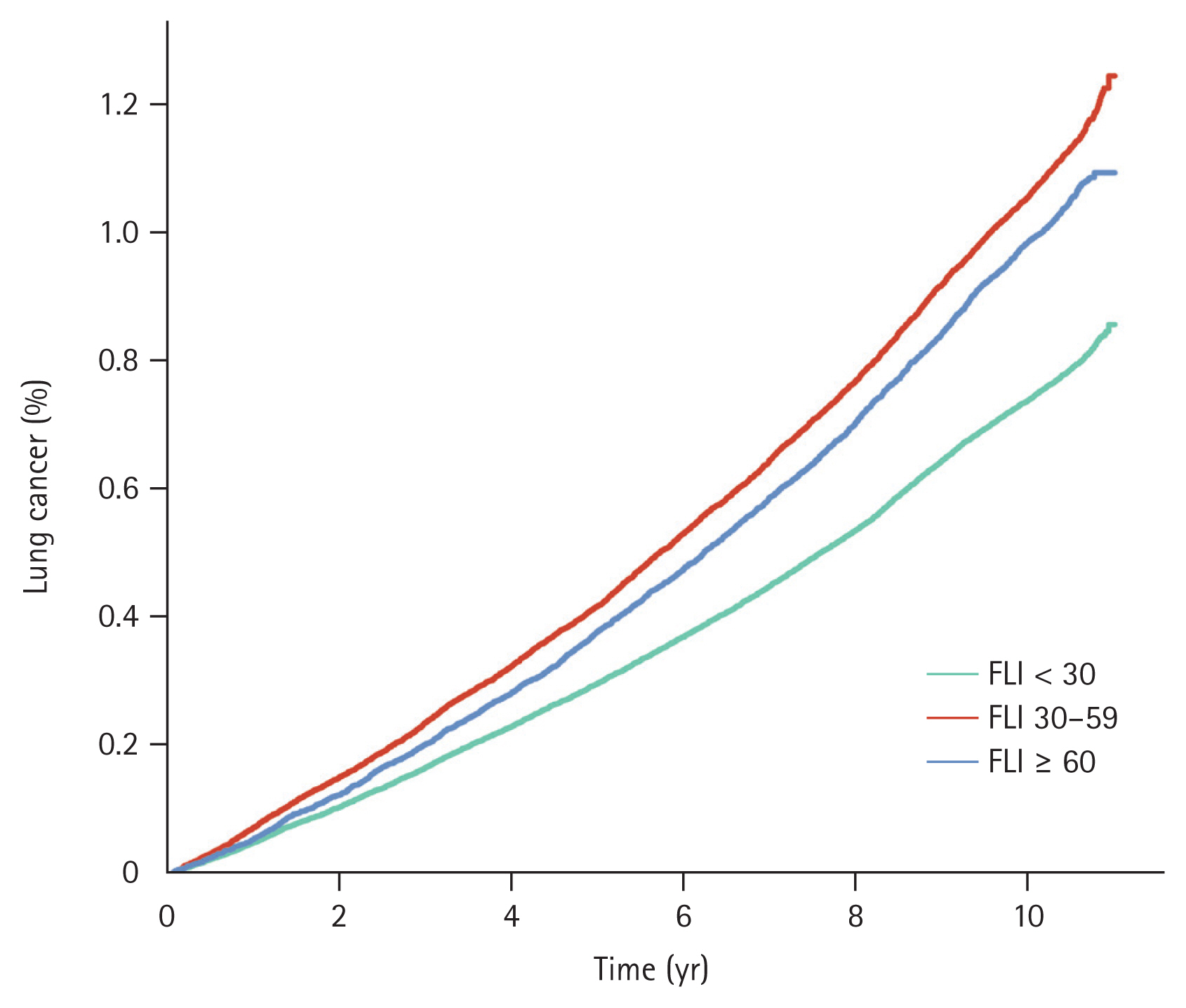
Event frequencies and Kaplan–Meier estimates for lung cancer according to metabolic disease-associated fatty liver disease risk. FLI, fatty liver index.
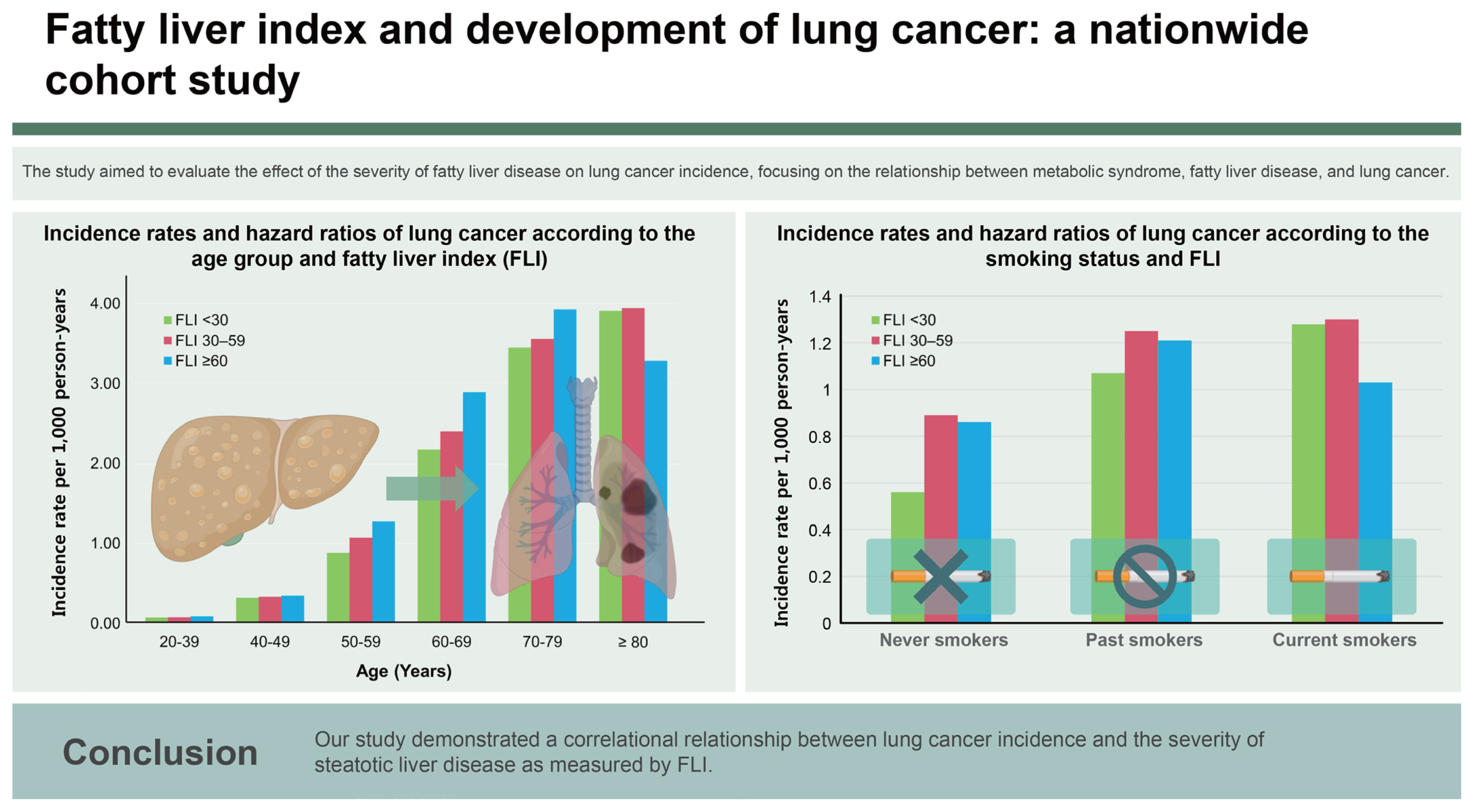
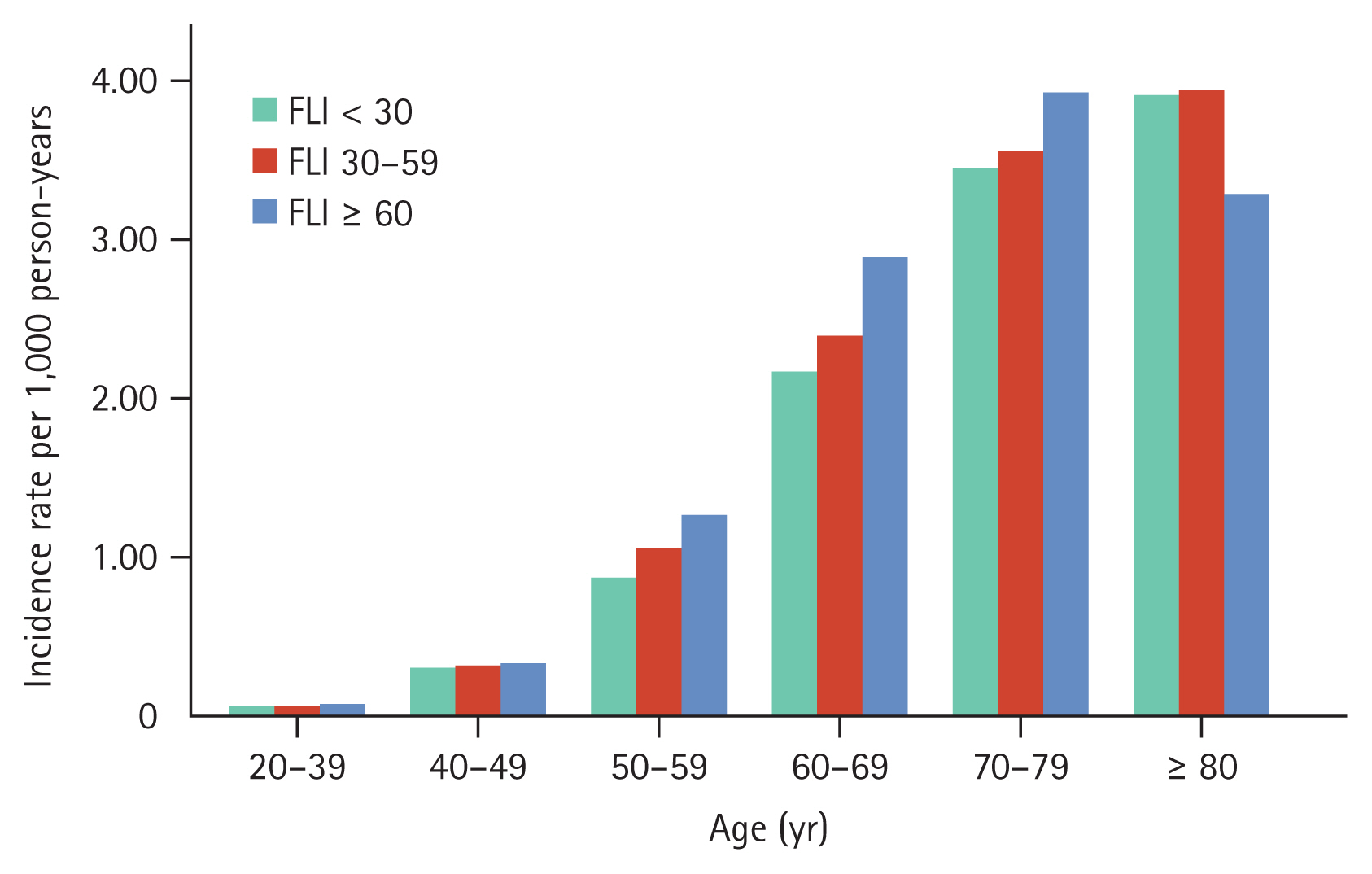
Lung cancer incidence rates by steatotic liver disease across age groups
Lung cancer incidence rates increased with increasing age. The incidence rates for lung cancer across age groups were 0.08 for ages 20–39 years, 0.32 per 1,000 PYs for ages 40–49 years, 0.98 per 1,000 PYs for ages 50–59 years, 2.32 per 1,000 PYs for ages 60–69 years, 3.52 per 1,000 PYs for ages 70–79 years, and 3.88 per 1,000 PYs for ages 80 years and above. In each age group, the incidence rate of lung cancer increased in conjunction with FLI increases except for those aged 80 years or above (Fig. 2). The adjusted HRs for lung cancer among individuals aged 50–59 were 1.02 (95% CI: 0.96–1.08) for FLI 30–59 and 1.18 (95% CI: 1.10–1.27) for FLI ≥ 60. Similarly, in the 60–69 age group, the adjusted HRs were 1.12 (95% CI: 1.07–1.17) and 1.37 (95% CI: 1.29–1.47), respectively. For those aged 70–79, the adjusted HRs were 1.19 (95% CI: 1.13–1.26) and 1.40 (95% CI: 1.29–1.53), respectively; and in individuals aged 80 years or older, the adjusted HRs were 1.21 (95% CI: 1.04–1.41) and 1.13 (95% CI: 0.85–1.51; Supplementary Table 1).
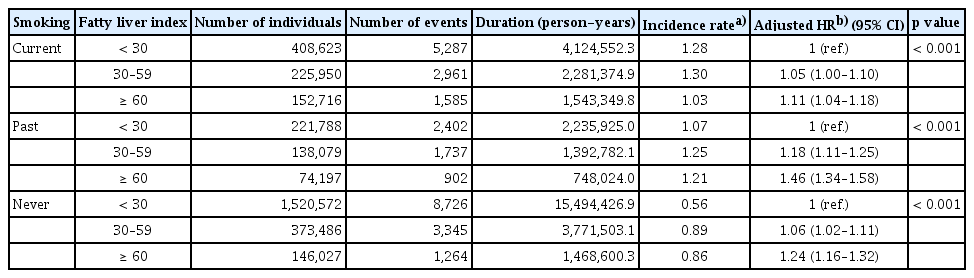
Subgroup analysis: lung cancer risk by steatotic liver disease in smoking subgroups
We further stratified the study population into current smokers (n = 787,289), past smokers (n = 434,064), and never-smokers (n = 2,040,085). Regardless of smoking status, as FLI increased, the risk of lung cancer also increased. In current smokers, the adjusted HR for the FLI 30–59 group was 1.05 (95% CI: 1.00–1.10), while that of the FLI ≥ 60 group was 1.11 (95% CI: 1.04–1.18). Similarly, in past smokers, the adjusted HR for the FLI 30–59 group was 1.18 (95% CI: 1.11–1.25), while that of the FLI ≥ 60 group was 1.46 (95% CI: 1.34–1.58).
For the never-smoker group, the adjusted HR for lung cancer increased in those with FLI 30–59 (1.06, 95% CI: 1.02–1.11) and those with FLI ≥ 60 (1.24, 95% CI: 1.16–1.32; Table 3).
Subgroup analysis: change in lung cancer risk with steatotic liver disease risk variation
Of the 1,110,455 individuals with an FLI ≥ 30 at enrollment, 665,801 underwent a general health examination two years later. We compared individuals with consistently intermediate or high FLI (n = 519,598) to those whose FLI improved from ≥ 30 to < 30 (n = 146,203). The baseline characteristics are summarized in Supplementary Table 2. Individuals who experienced an improvement in FLI showed a lower prevalence of comorbidities of hypertension, diabetes mellitus, and dyslipidemia compared to their counterparts. During the observational period of the subgroup analysis, the incidence rate was 1.07 per 1,000 PYs in the consistently FLI ≥ 30 group and was 1.14 per 1,000 PYs in the improved FLI group. Utilizing multivariable Cox regression analysis, the risk of lung cancer decreased by 0.93-fold (95% CI: 0.88–0.99) in the groups with improved FLI (Table 4).
DISCUSSION
Our study showed that steatotic liver disease, manifested as high FLI, was associated with increased lung cancer development in various subgroups. steatotic liver disease was also an independent risk factor of lung cancer regardless of smoking status. Moreover, resolution of steatotic liver disease exhibited a possible protective influence against lung cancer among individuals with the condition.
Studies on the association of steatotic liver disease and lung cancer are relatively rare. The few studies on an association between lung cancer development and metabolic syndrome had inconsistent results. A meta-analysis that included five cohort studies with a total of 188,970 subjects did not find a meaningful association between metabolic syndrome and lung cancer (HR 0.95, 95% CI: 0.81–1.10) [19]. A large Korean study that used a national insurance database of subjects showed a significant association between metabolic syndrome and lung cancer. Metabolic syndrome positively correlated with an increased risk of lung cancer in men (HR 1.15, 95% CI: 1.12–1.18) [12]. A recently published UK study including 331,877 participants with a median follow-up duration of 10.9 years showed an increased risk of lung cancer with metabolic syndrome (HR 1.21, 95% CI: 1.09–1.33); these data support our results [11].
In the present study, individuals in the low-risk steatotic liver disease category (FLI < 30) were younger, predominantly female, and showed lower prevalence of metabolic dysfunction-related comorbidities such as hypertension, diabetes mellitus, and dyslipidemia. This group also demonstrated the lowest CCI scores, suggesting a generally healthier baseline status compared to intermediate- and high-risk groups. These findings are consistent with those of previous studies on steatotic liver disease defined by FLI [20,21]. After adjusting for multiple variables, the persistence of increased risk of lung cancer (1.08-fold for the FLI 30–59 group and 1.22-fold for the FLI ≥ 60 group) compared to those with FLI < 30 supports the hypothesis that the relationship between FLI and lung cancer risk is not merely coincidental. The mechanism underlying the association between metabolic disorders and carcinogenesis is complex and has not been characterized fully. Possible pathways involve chronic inflammation caused by deregulation of cytokine production, insulin resistance, and overexpression of estrogen; these may be linked to the carcinogenesis process [22]. Lipid accumulation in hepatocytes and an associated lipotoxicity create a dynamic proinflammatory environment that promotes eventual fibrogenesis and subsequent carcinogenesis [23]. As for the potential impact of steatotic liver disease on lung cancer risk, we assume that mechanisms differ based on effects of liver diseases as the process of liver fibrogenesis does not directly lead to the development of lung cancer. We believe that the broader systemic inflammation associated with steatotic liver disease, driven by lipid accumulation, may increase the risk of lung cancer through pro-inflammatory pathways that support a tumor-friendly environment [24]. To further clarify the potential molecular and pathological connections between lung cancer and steatotic liver disease, extensive epidemiological data on the interplay between these two disease entities is needed. Furthermore, additional studies are needed to explore not only the isolated impact of steatotic liver disease on lung cancer risk, but also its synergistic effect when combined with known risk factors such as tobacco use and air pollution. Lipotoxicity associated with steatotic liver disease may act as a catalyst for these risk factors.
In current smokers, the adjusted HRs for lung cancer increased modestly but significantly as FLI increased. This is consistent with findings on metabolic syndrome and lung cancer risk; the risk of lung cancer resulting from metabolic syndrome was elevated mainly among patients who used tobacco [11]. Conversely, in the never- or past-smokers subgroup, the impact of FLI on lung cancer risk was more pronounced. These findings suggest that, in the absence of active smoking, metabolic dysfunction plays a more significant role in lung cancer risk, potentially acting through mechanisms independent of those associated with tobacco exposure. Furthermore, this finding supports interventions targeting metabolic health regardless of individual smoking status.
We separately analyzed data from individuals who initially presented with intermediate or high steatotic liver disease risk (FLI ≥ 30) and underwent a general health examination two years later to evaluate the potential impact of longitudinal change in FLI on lung cancer risk. Notably, those who achieved an improvement in their FLI demonstrated a lower prevalence of comorbid conditions of hypertension, diabetes mellitus, and dyslipidemia and were more likely to be never-smokers, non-drinkers, and 65 years or older. In the crude analysis, contrary to expectations, the incidence rate of lung cancer was slightly higher in the group that improved their FLI compared to those with consistent FLI ≥ 60. There is a possibility that this longitudinal analysis did not capture the beneficial effect of intentionally lowering the FLI index. Future studies need to determine if changes in the FLI result from lifestyle modifications [25–27] or from the development of new systemic conditions such as chronic kidney diseases or autoimmune diseases that may affect body composition or BMI. These conditions ultimately influence the FLI [28–31]. Furthermore, considering that the proportion of elderly patients was higher in the group that showed improved FLI, an age effect on cancer risk cannot be ignored [32]. In the multivariable analysis that included factors such as age and exercise frequency, however, the results showed that FLI improvement was associated with a 0.93-fold decrease in lung cancer. This significant reduction in multivariate analysis suggests a consistent potential beneficial effect from improvement in FLI. Interestingly, weight reduction, the first-line treatment for steatotic liver disease, tended to lower cancer risks in previous studies. Luo et al. [33] showed that postmenopausal women with weight loss significantly lowered the risk of endometrial cancer compared to those with stable weight (HR 0.71, 95% CI: 0.54–0.95). Also, Chlebowski et al. [34] reported that postmenopausal women who experienced weight loss had a decreased risk of breast cancer compared to those who maintained a stable weight (HR 0.88, 95% CI: 0.78–0.98). Moreover, rigorous lifestyle interventions among overweight or obese individuals and those with type 2 diabetes reduced the risk of obesity-related cancer by 0.84 times compared to the diabetes support and education group in a randomized clinical trial [35]. To accurately determine if lifestyle modifications that improve the FLI can reduce cancer risk, a well-designed, prospective, matched comparison study is necessary.
Our study that investigated the link between steatotic liver disease and lung cancer risk had some limitations. First, due to the innate limitation of claims data, we were not able to gather detailed medical information. Therefore, we could not assess the lung cancer stage or pathologic types and severity of other comorbidities. Also, steatotic liver disease and lung cancer share common risk factors such as chronic inflammation, insulin resistance, and oxidative stress, which may potentially be managed with medication. It highlights the need for further research to explore the mechanisms underlying the relationship between steatotic liver disease and lung cancer. Second, the incidence of lung cancer may be underestimated since it did not consider individuals with lung cancer who did not seek medical attention. Third, even if we defined steatotic liver disease by FLI, the lack of imaging techniques remains a limitation of our study. We defined change of hepatic steatosis with 2-year period variation, it might have an innate limitation of fully reflect individual variations of hepatic steatosis overtime. Last, detailed tobacco exposure or concurrent chronic lung disease information was not acquired. Despite these limitations, our study has strengths in its basis on a national population database with sufficient observation time.
In conclusion, our study utilizing nationwide population data demonstrated that the presence and severity of steatotic liver disease as assessed by the FLI have an incremental relationship with lung cancer risk. Future research that includes more detailed information on comorbidities and lifestyle modifications will further clarify this association.
KEY MESSAGE
1. In the analysis included over three million individuals who underwent a general health examination, higher FLI scores were associated with an increased risk of lung cancer, with variations in risk observed based on smoking status.
2. For the never-smoker group, the risk of lung cancer increased with higher FLI scores, with significant risk elevation observed in those with FLI 30–59 and even more so in those with FLI ≥ 60.
3. Individuals with consistently high FLI levels had a higher incidence of lung cancer compared to those who improved their FLI over time.
Notes
CRedit authorship contributions
Jihye Lim: conceptualization, methodology, resources, data curation, formal analysis, software, writing - original draft, visualization, funding acquisition; Bongseong Kim: methodology, resources, data curation, formal analysis, validation, software, visualization; Kyungdo Han: methodology, resources, investigation, data curation, formal analysis, validation, software, supervision, project administration, funding acquisition; Jeong Uk Lim: conceptualization, methodology, resources, investigation, validation, writing - original draft, writing - review & editing, supervision, project administration, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This research was supported by the grant of the Institute of Clinical Medicine Research in Yeouido St. Mary’s Hospital, The Catholic University of Korea.
Data availability
Data used in this study are maintained by the Korea National Health Insurance Service (NHIS, https://nhiss.nhis.or.kr) and are available upon request to the NHIS.