Patterns of hormonal changes in hypophysitis by immune checkpoint inhibitor
Article information
Abstract
Background/Aims
Immune checkpoint inhibitors (ICIs) can induce immune-related adverse events, including endocrine dysfunctions, which can have serious consequences on patient health and quality of life. The clinical course and characteristics of immune-related hypophysitis (irH) are not well established. This study aimed to analyze the clinical course and characteristics of irH.
Methods
This single-center, retrospective study analyzed data from electronic medical records of Asan Medical Center, spanning January 2017 through June 2021. It included adult patients with solid tumors who underwent thyroid and adrenal function tests, along with gonadotropin and/or growth hormone evaluations, following the initiation of ICI treatment within the same period. The study explored the clinical characteristics of ICI-treated patients with and without irH, the incidence of irH, the time to irH onset, and the associated hormonal changes.
Results
Twenty-one patients were included in this analysis. Clinical characteristics did not differ significantly between the irH (n = 13) and non-irH (n = 8) groups. Deficiency rates in the irH group were 23.1% for thyroid-stimulating hormone (n = 3), 76.9% for adrenocorticotropic hormone (n = 10), 61.5% for gonadotropin (n = 8), and 15.4% for growth hormone (n = 2). The overall incidence was 0.9 per person-year, with 6-month and 1-year cumulative incidences of 38.8% and 57.1%, respectively. The median time from ICI initiation to irH diagnosis was 7.7 months. Time to levothyroxine replacement was shorter in the irH group.
Conclusions
The findings provide evidence that could facilitate the prediction of ICI-induced irH based on clinical course and characteristics.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) constitute a revolutionary, highly effective cancer treatment that has precipitated a paradigm shift in oncological treatment. ICIs target cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed cell death-ligand 1 (PD-L1) by preventing ligand–receptor binding, thereby disrupting tumor cell signaling, which facilitates T-cell recognition and the subsequent destruction of cancer cells [1]. ICI use has been growing exponentially, and in 2019, approximately 40% of cancer patients in the United States were treated with ICIs [2]. ICI-induced endocrine adverse events have diverse manifestations, including hypothyroidism, adrenal insufficiency, hypogonadism, hypophysitis, and diabetes, which can have severe consequences for patients’ health and quality of life, including the need for lifelong hormone replacement therapy [3,4].
Hypophysitis caused by ICIs is known as immune-related hypophysitis (irH) [5,6]. IrH usually develops within the first 2 to 3 months following ICI treatment initiation although its occurrence even after 19 months has been reported [6,7]. Symptoms of irH can vary but usually include headache, fatigue, nausea, vomiting, and symptoms of hormone deficiencies [7,8]. The precise incidence of irH is not well established, but is approximately 11% to 14% [9]. The prevalence of irH is reported to be between 6.4% and 11.7% in association with combined ipilimumab and nivolumab therapy, compared with between 3.2% and 17.4% with ipilimumab as a single agent [7,8,10-14]. In contrast, irH associated with anti-PD-1 and anti-PD-L1 inhibitors has rarely been reported (< 0.1–3%) [14,15]. Furthermore, few studies have investigated the incidence of irHs in South Korea. Few studies have investigated, in detail, the primary hormonal axis affected in irH patients, the duration of immunochemotherapy before irH onset, its incidence, and the pattern of hormonal changes throughout ICI treatment.
This study aimed to analyze the clinical characteristics of patients with solid metastatic cancers undergoing immunochemotherapy who exhibited endocrine dysfunction. We compared and analyzed the clinical characteristics of patients with and without irH, focusing on those treated with ICIs and monitored via pituitary hormone axis testing. Additionally, we examined the incidence of irH, the duration of ICI treatment until the onset of irH, and the pattern of hormonal changes during the course of ICI treatment.
Materials and Methods
Data source and study sample
This single-center retrospective study analyzed electronic records data from patients managed at Asan Medical Center between January 1, 2017, and June 30, 2021. All adult patients (aged ≥ 20 yr) with solid cancer and endocrine immune-related adverse events (irAEs) who started immunotherapy were screened for inclusion in the analysis. The types of ICI used at our hospital included an anti-CTLA-4 inhibitor (ipilimumab), anti-PD-1 inhibitors (nivolumab and pembrolizumab), and anti-PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab). Patients diagnosed with any solid cancer (for example—lung cancer, esophageal/gastric cancer, liver cancer, biliary tract cancer, or kidney cancer) but not hematological malignancies were eligible.
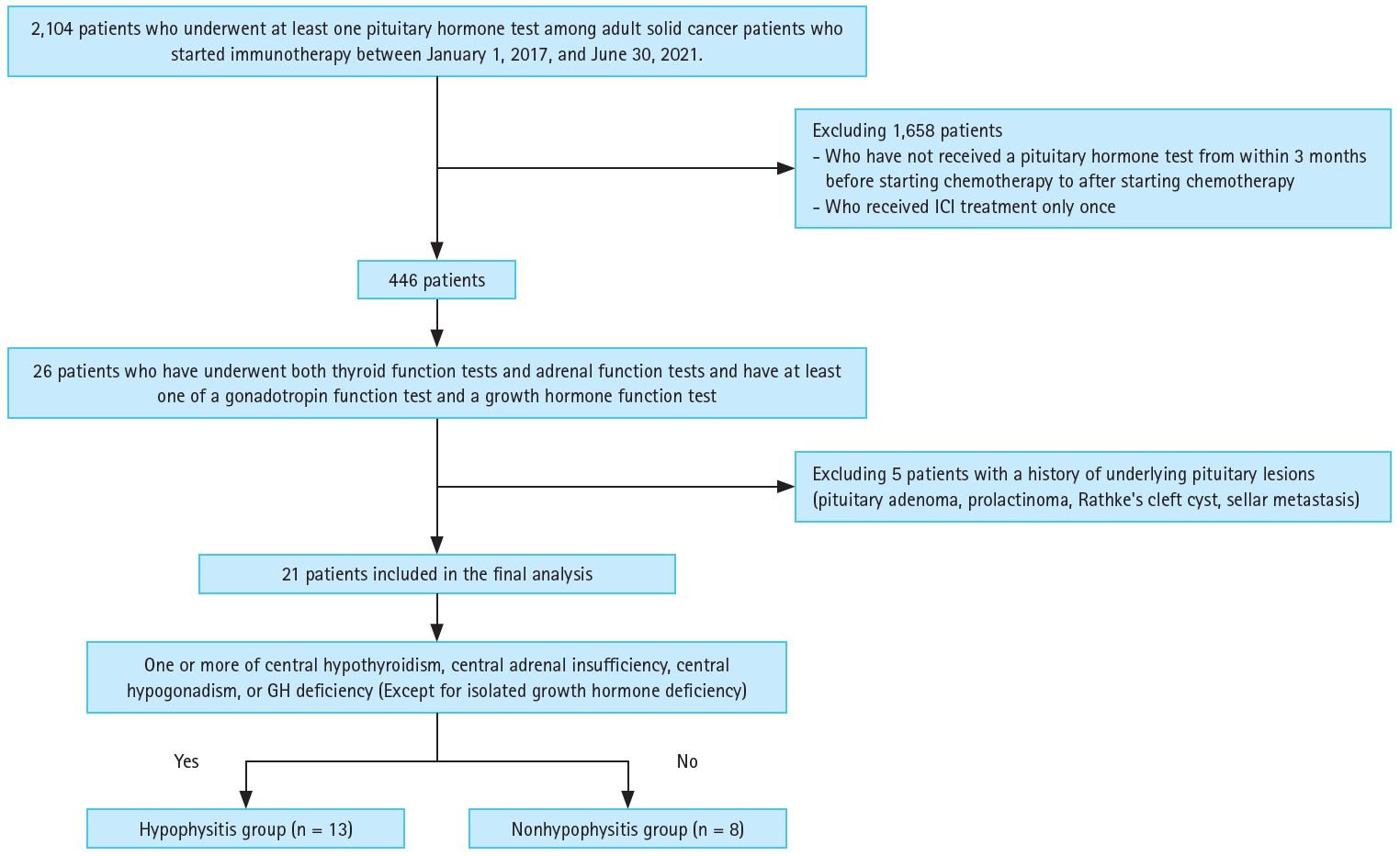
Patients included in the final analysis were those with available data on thyroid and adrenal function tests, as well as on gonadotropin or growth hormone function, following the commencement of ICI therapy. We excluded patients who did not undergo any hormone tests after the initiation of chemotherapy, those who received only one dose of immunotherapy, and patients with underlying pituitary disease. Finally, 21 patients were included in the analysis (Fig. 1). Patients who used a tyrosine kinase inhibitor were excluded only from the thyroid function analysis. Moreover, patients who received glucocorticoid treatment for other reasons–such as immune-related hepatitis, drug-induced pneumonitis, or post-radiotherapy brain edema–before starting ICI therapy were excluded from the adrenal function analysis. We excluded patients whose gonadal dysfunction occurred more than 18 months after beginning ICI therapy, as well as those among whom central hypogonadism could not be determined due to menopause.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Asan Medical Center (IRB No. 2021-0965). The requirement for informed consent was waived by the IRB because de-identified data were used in the analyses. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cohort studies.
Clinical and laboratory measurements
Patient data were collected through a review of electronic medical records. Body mass index was calculated as weight in kilograms divided by height in square meters. Blood pressure was measured using an automatic manometer with a cuff placed around the patient’s right upper arm after resting for 5 minutes. Blood samples were drawn into vacuum tubes from antecubital veins and analyzed in Asan Medical Center’s accredited central laboratory. Laboratory serum measurements included fasting plasma glucose, total cholesterol, and C-reactive protein (CRP) levels. Blood samples for the measurement of fasting plasma glucose and total cholesterol levels were collected after overnight fasting (after 8 h of fasting for plasma glucose and after 12 h of fasting for total cholesterol). Fasting plasma glucose and CRP were measured using enzymatic colorimetric and immunoturbidimetric methods, respectively, on a Toshiba 200FR autoanalyzer (Toshiba Medical System Co. Ltd., Tokyo, Japan) [16]. Fasting total cholesterol was measured using enzymatic colorimetric methods on a Toshiba 200FR Neo analyzer [16]. The reference range for each hormone test is listed in Supplementary Table 1.
Diabetes mellitus was defined according to the following criteria: (1) at least one claim per year for the prescription of antidiabetic medication; (2) fasting glucose level ≥ 126 mg/dL; or (3) HbA1c (glycated hemoglobin) ≥ 6.5%. Hypertension was defined as the presence of at least one claim per year for the prescription of antihypertensive medication or a systolic/diastolic blood pressure measurement ≥ 140/90 mmHg. Hypercholesterolemia was defined as a total cholesterol level ≥ 240 mg/dL or treatment with lipid-lowering medication [17].
Definition of irH and non-hypophysitis endocrine irAEs
The definition of hypopituitarism was adapted from the Endocrine Society Clinical Practice Guidelines and the description in the relevant position statement published by the Korean Endocrine Society [8]. Based on biochemical evaluation of the hypothalamic-pituitary axis, hypopituitarism is defined as the presence of one or more of the following hormonal deficiencies: central hypothyroidism, central adrenal insufficiency, central hypogonadism, or growth hormone deficiency [8,18]. Although there is some variation in the details between studies, hypophysitis is generally defined as the presence of at least one of the following: evidence of hypopituitarism as assessed by biochemical testing, clinical symptoms (fatigue, headache, anorexia, nausea, or vomiting), hyponatremia that may be seen in hypophysitis, or pituitary imaging findings suggestive of hypophysitis [7,19-21]. Owing to the retrospective nature of this study, clinical manifestations and imaging findings were nonspecific and unclear as diagnostic criteria. Therefore, irH was defined as a deficiency of one or more hormones following ICI treatment [22]. Patients were classified into the irH group if they exhibited any of the following conditions: central hypothyroidism, central adrenal insufficiency, central hypogonadism, or growth hormone deficiency.
Central hypothyroidism is diagnosed when thyroid-stimulating hormone (TSH) levels are low, normal, or slightly elevated (TSH < 10 mU/L) and free thyroxine (fT4) levels are below the laboratory reference range [18,23]. Primary hypothyroidism was defined as TSH > 10 mU/L or TSH above the upper limit of normal and fT4 below the lower limit of normal [22]. Distinctions were made between immune-related hypothyroidism, thyroiditis, and central hypothyroidism. Immune-related hypothyroidism was characterized by a TSH level > 10 mU/L immediately after ICI administration. Immune-related thyroiditis was indicated by initial hyperthyroidism after ICI administration, followed sequentially by hypothyroidism.
Central adrenal insufficiency was defined as a morning cortisol level < 3 μg/dL between 8:00 and 9:00 AM or a peak cortisol level < 18 μg/dL (< 500 nmol/L, on radioimmunoassays) at 60 minutes on a corticotropin stimulation test when the adrenocorticotropic hormone (ACTH) level did not exceed the normal range [18]. The distinction between central hypothyroidism and sick thyroid syndrome, along with the distinction between central adrenal insufficiency and adrenal insufficiency caused by other factors (opioids or megestrol acetate), was determined by the existence of coexisting pituitary hormone deficiencies, such as central hypogonadism and growth hormone deficiency [7]. Growth hormone deficiency was defined as an age-specific serum insulin-like growth factor 1 level lower than the lower limit of normal [24,25].
Central hypogonadism was defined as low levels of sex hormones (testosterone in men and estradiol in women) and low (or inappropriately normal) gonadotropin (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) levels in the absence of androgen deprivation therapy or hormone replacement therapy. In postmenopausal women, the absence of elevated serum FSH and LH levels indicates gonadotropin dysfunction [18].
Non-hypophysitis endocrine irAEs were defined in patients who did not meet the criteria for irH but exhibited endocrine abnormalities [10].
Outcome variables
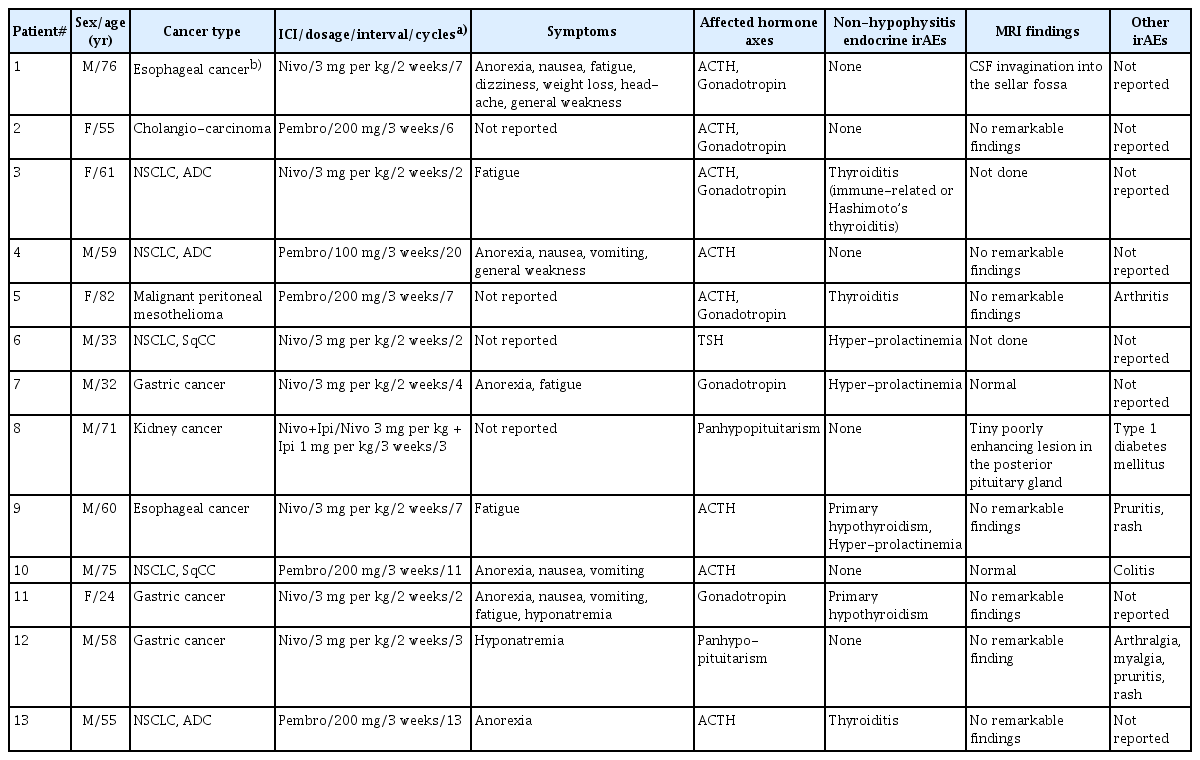
Thirteen patients in the irH group were analyzed in terms of the type of cancer diagnosed, type of ICI therapy, dosage, treatment intervals, cycles, symptoms, affected hormonal axes, and associated non-hypophysitis endocrine irAEs, and magnetic resonance imaging (MRI) findings. Among all participants who were included in the analysis, baseline characteristics were analyzed by stratifying participants into an irH group and a non-irH group. For each group, age, sex, body mass index, fasting glucose, total cholesterol, CRP, and underlying diseases (diabetes mellitus, hypertension, or hyperlipidemia) were investigated. Additionally, the type of cancer diagnosed in patients with irH, the type of ICI used, and the dosing cycle of ICI were investigated.
Within the irH group, the proportions of each type of hormonal deficiency were analyzed across the affected hormonal axes. Furthermore, the cumulative incidence and time to onset of irH were investigated. Twenty-one participants underwent hormone level quantification at baseline prior to ICI treatment initiation, at the onset of endocrine irAEs (including irH), or at the end of follow-up. Notable trends in TSH and fT4 levels were observed from the start of ICI therapy to irH onset, with comparisons between the irH and non-irH groups.
Statistical analysis
Descriptive statistics were used to analyze the patients’ baseline characteristics, cancer types, ICI use, and the frequency of hormonal axis deficiencies. The analysis encompassed all patients from the initiation of chemotherapy, spanning from January 1, 2017, to June 30, 2021. We compared the clinical characteristics of patients treated with ICI, with and without irH. For baseline characteristics, continuous variables are presented as median (interquartile range), and categorical variables are presented as frequency (proportion). Demographic and biochemical characteristics according to irH status were compared using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables.
Cumulative bar graphs and Venn diagrams were used to depict the proportions of affected hormonal axes in patients with irH. The Kaplan–Meier method was used to analyze the cumulative incidence and time to onset of irH. We evaluated the trend changes in TSH and fT4 levels in relation to time as a line graph by setting the start date of ICI administration for each patient as zero and the duration from this time point to the date of irH onset in each patient on the time axis.
Statistical analyses were performed using SPSS Statistics for Windows, version 28.0 (IBM Corp., Armonk, NY, USA) and R, version 4.3.1 Foundation for Statistical Computing, Vienna, Austria. A p value < 0.05 was considered significant.
Results
Baseline characteristics of patients in the irH and non-irH groups
A total of 2,104 adult patients with solid tumors who received immunochemotherapy during the study period underwent at least one pituitary hormone test. After applying the exclusion criteria and removing those without the necessary hormone test results, 21 patients were included in the final analysis. Of the 2,104 patients screened, 896 received nivolumab (an anti-PD-1 inhibitor) alone, 733 received pembrolizumab, 416 received atezolizumab (anti-PD-L1 inhibitor), 18 received ipilimumab (anti-CTLA4 inhibitor) alone, 46 received nivolumab plus ipilimumab combination therapy, 44 received durvalumab (anti-PD-L1 inhibitor), and 1 received pembrolizumab and ipilimumab combination therapy. Twenty patients received multiple ICI agents sequentially: 10 with atezolizumab, one with ipilimumab, 12 with nivolumab, three with nivolumab plus ipilimumab, and 15 pembrolizumab.
Of the 21 patients included in the analysis, 13 had hormone test results suggesting the possibility of irH, and eight did not show any evidence of hypophysitis. Thus, irH was diagnosed in 13 of the 21 patients (61.9%) in our study. Table 1 lists the characteristics of all patients with irH. In the irH group, 11 patients exhibited at least one manifestation of hypophysitis, such as anorexia, nausea, vomiting, fatigue, dizziness, weight loss, headache, or general weakness. Two patients exhibited hyponatremia, which is a potential indicator of hypophysitis. On MRI, two patients had abnormal pituitary gland findings (Supplementary Fig. 1).
The median ages in the irH and non-irH groups were 59.0 and 57.0 years, respectively. The irH group included nine males (69.2%), while the non-irH group included five males (62.5%). Of the irH patients, 12 (92.3%) were treated with anti-PD-1 inhibitors: nivolumab in seven patients (53.8%) and pembrolizumab in five patients (38.5%). One patient (7.7%) received nivolumab and ipilimumab combination therapy, and none received atezolizumab. In the non-irH group, seven patients (87.5%) used anti-PD-1 inhibitors (n = 4, 50% for nivolumab and n = 3, 37.5% for pembrolizumab). None of the patients used a combination therapy of nivolumab and ipilimumab, and only one patient (12.5%) used atezolizumab.
Hormonal deficiencies in irH patients: analysis of affected axes, cumulative incidence, and diagnostic timing
Table 2 lists the laboratory hormone test values at the initiation of ICI therapy and at the time of irH diagnosis for all subjects, assessing deficiencies across each hormonal axis based on the reference ranges.
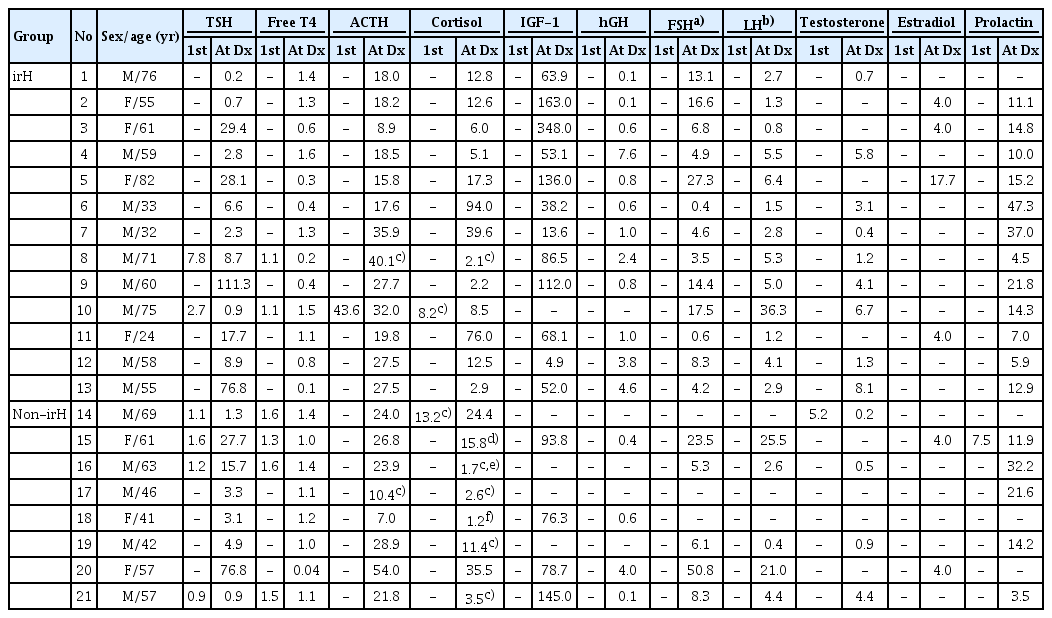
Hormone laboratory values at baseline and at the onset of endocrine irAEs (including irH) or the end of follow-up
Table 3 shows the proportion of patients who were deficient in each hormone type. Among the 13 patients with irH, three had central hypothyroidism, whereas five had primary hypothyroidism. ACTH levels and morning cortisol or peak cortisol levels at 60 minutes on a corticotropin stimulation test were measured in all irH patients to evaluate the function of the ACTH axis. Among these, 10 (76.9%) had central adrenal insufficiency. No patients with primary adrenal insufficiency were identified. Eight patients (61.5%) had central hypogonadism, and two (15.4%) had growth hormone deficiency. Panhypopituitarism was observed in two patients (15.4%).

Proportions of hormonal deficiencies by axis (TSH, ACTH, gonadotropin, and GH) among patients with hypophysitis (irH group)
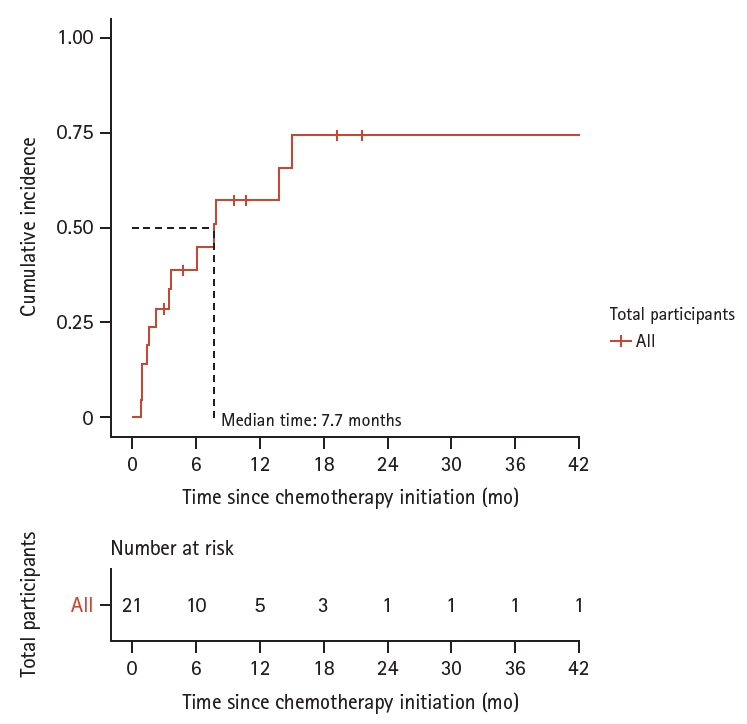
During a follow-up period of 15.2 person-years, 13 patients developed irH. The overall incidence of irH was approximately 0.9 per person-year. Kaplan–Meier analysis indicated that the cumulative incidence of irH was 38.8% at 6 months and 57.1% at 1 year. The median time from the first ICI dose to the diagnosis of irH was 7.7 months (Fig. 2).
TSH axis trends from immunotherapy start to hypothyroidism onset
Trends in TSH and fT4 values from ICI initiation to hypothyroidism onset were reviewed in participants whose TSH and fT4 levels were measured. One patient in the non-irH group had overt hypothyroidism associated with sunitinib administration before ICI treatment initiation and a history of levothyroxine treatment; therefore, his thyroid function test results were excluded from the analysis.
Hormonal abnormalities appeared earlier in the irH group than in the non-irH group. In the irH group, eight of the 13 patients received levothyroxine replacement. For these eight patients, the median duration for levothyroxine replacement initiation after ICI commencement was 2.7 months. Specifically, the median time to begin levothyroxine replacement for central hypothyroidism was 3.8 months among these patients. Of the seven patients in the non-irH group, two received levothyroxine replacement for primary hypothyroidism, and the median time until replacement initiation was 7.1 months (Fig. 3). The cumulative incidence of levothyroxine replacement events was higher in the irH group than in the non-irH group, but the difference was not statistically significant (p = 0.01) (Supplementary Fig. 2).

Changes in TSH and free T4 levels and timing of levothyroxine replacement according to the immune checkpoint inhibitor administration period (irH group [A] vs. non-irH group [B]). TSH, thyroid-stimulating hormone; T4, thyroxine; irH, immune-related hypophysitis; non-irH, non-immune-related hypophysitis.
Discussion
This study examined the clinical characteristics, incidence, and duration of hypophysitis, as well as changes in hormone axes according to the time of ICI administration in patients with solid cancers who were treated with ICIs. Diagnostic criteria for irH classification vary across studies. The clinical manifestations of hypophysitis are nonspecific and are difficult to distinguish from cancer symptoms. Therefore, in this study, irH was defined based on the most objective standard laboratory hormone values. In this study, most irH patients received anti-PD-1 inhibitors. This study presents valuable data on the clinical characteristics, cancer-related characteristics, and clinical course of patients treated with ICIs, particularly anti-PD-1 inhibitors.
The estimated prevalence of irH is 9.1%, which contrasts with the occurrence of idiopathic autoimmune hypophysitis at a rate of one in 9 million individuals [4,26]. The reported incidence of irH ranges from 0.4% to 17.2% [10,27-29], with variations likely arising from study design differences. Most reported irH is caused by anti-CTLA-4 antibodies, with anti-PD-1 antibody–induced irH being less common (< 1.0%) [27,30,31]. In one study, the cumulative incidence of irH at 52 weeks for patients receiving anti-PD-1/PD-L1 inhibitor plus anti-CTLA-4 inhibitor therapy was 16%, compared with 8% for anti-PD-1/PD-L1 monotherapy and 7% for anti-CTLA-4 inhibitor therapy [32]. The prevalence and cumulative incidence of irH in this study were higher than those in other studies because relatively few patients underwent the entire panel of pituitary hormone tests after ICI administration. In most cases, only thyroid or adrenal function tests were performed, and these patients were not included in the study because there were insufficient data to diagnose or exclude irH.
It is known that irH usually develops within 6 months of ICI therapy [30]. In the present study, the median time to irH onset was 7.7 months. Patients treated with anti-CTLA-4 inhibitors typically have a median time to onset of 8 to 12 weeks, whereas those treated with anti-PD-1/PD-L1 inhibitors have a median time to onset of 24 to 26 weeks after treatment initiation [9]. The timing of irH onset in this study aligned with the latter, likely attributable to the prevalent use of anti-PD-1/PD-L1 inhibitors.
The mechanisms of hypophysitis development are thought to vary based on whether they are induced by anti-CTLA-4 or anti-PD-1/PD-L1 inhibitors. Anti-CTLA-4 inhibitor–induced hypophysitis causes lymphocytic inflammation and complement deposition in the pituitary gland, and circulating pituitary autoantibodies and CTLA-4 expression in anterior pituitary cells have been detected. This suggests that type II hypersensitivity is related to off-target effects of anti-CTLA-4 antibodies in the pituitary gland [5]. In contrast, in anti-PD-1 antibody–associated hypophysitis, the pituitary gland shows evidence of type IV hypersensitivity mediated by cytotoxic T lymphocytes. Recently, it has been reported that anti-corticotroph antibodies that recognize proopiomelanocortin (POMC) are detected in 10% of hypophysitis cases associated with anti-PD-1/anti-PD-L1 antibodies [33]. In the tumors of these patients, ectopic expression of POMC in the form of a paraneoplastic syndrome induces autoimmunity, suggesting that ICIs enhance the autoimmune response to corticotropes [9]. This supports the finding that most irH patients in this study had ACTH insufficiency.
ACTH and TSH insufficiencies are the predominant pituitary hormone abnormalities associated with irH [10,29]. The incidences of ACTH deficiency, TSH deficiency, and central hypogonadism are 6.1%, 7.6%, and 7.5%, respectively, while the incidence of GH deficiency remains indeterminate [4,30]. In this study, deficiencies were most frequently observed in the ACTH axis, followed by the gonadal, TSH, and growth hormone axes. This finding is consistent with evidence in the existing literature that central adrenal insufficiency accounts for 83% of irH [34]. Nonetheless, the actual prevalence of TSH deficiency may be lower than that indicated by the results of this study. This is because only patients who underwent three or more types of pituitary hormone testing to detect secondary hypothyroidism were included in the irH group, and most patients receiving immunotherapy underwent only thyroid function tests.
Comparisons between the irH and non-irH groups revealed that patients in the irH group experienced more rapid hormonal changes in the TSH axis and started levothyroxine treatment sooner. Notably, even among irH patients without TSH deficiency, those with primary hypothyroidism initiated hormonal changes and levothyroxine treatment earlier than their non-irH counterparts. Typically, ICI-related TSH deficiency occurs at a median of 8 to 12 weeks after ICI initiation [8,15,35]. This study revealed that TSH deficiency with irH appears earlier than isolated hypothyroidism (regardless of whether TSH deficiency is immune related) without irH. In the non-irH group, hypothyroidism may have been isolated from immune-related hypothyroidism or from underlying Hashimoto’s thyroiditis. As the pathophysiology that explains these phenomena is unknown, further complementary studies are needed.
This study had several limitations. First, owing to the study’s retrospective design, data collection on symptom assessment was limited in terms of detail and accuracy by chart review alone. Additionally, MRI findings were not used to diagnose irH in this study because pituitary hypertrophy, a typical finding of hypophysitis, was not evident in the irH group. This likely occurs because pituitary hypertrophy on MRI, a common indicator of irH, is more frequently associated with anti-CTLA-4 inhibitor use, whereas the majority of irH patients in this study were treated with anti-PD-1 inhibitors [28,36]. Moreover, cocktail tests were not routinely performed in patients receiving immunotherapy. Lastly, many patients from the total population of cancer patients treated with ICIs were not included in this study, as they did not undergo regular pituitary hormone testing after starting ICI therapy. Given the relatively small sample size, it was challenging to determine statistical significance by comparing irH cases and non-cases. There was potential for selection bias, as hormonal evaluations were conducted on only a subset of patients treated with ICIs, either for screening endocrine irAEs or based on clinical indications. Prospective studies requiring periodic hormone tests after ICI therapy initiation are essential for a comprehensive assessment of irH prevalence.
In conclusion, the results of this study provide additional evidence consistent with existing studies on the clinical characteristics and course of irH according to ICI type, frequency of occurrence, and time to onset. It is important to note that the exact incidence of endocrine adverse events can vary by several factors, including the type and dose of ICI used, the patient’s underlying medical conditions, and the presence of other risk factors. Additionally, the incidence of these events may change as more patients receive ICIs and more data become available. Nevertheless, endocrine adverse events are a significant concern for patients receiving ICIs and must be monitored and managed carefully. Future studies should include prospective routine hormone testing both before and after ICI administration, expansion to multiple centers, and the prospective collection of data for analysis.
KEY MESSAGE
1. Most patients with irH in this study were treated with anti-PD-1 inhibitors, with ACTH deficiency being the most common hormonal insufficiency observed.
2. The timeline of irH development in the irH group was similar to that previously reported for the onset of irH in association with anti-PD-1/PD-L1 inhibitors, and hypothyroidism manifested earlier in the irH group than in the non-irH group.
3. This study’s findings provide evidence that can be used to predict the clinical course and outcomes of patients with irH based on the type of ICI, frequency of occurrence, and time to irH onset.
Acknowledgements
We would like to thank the Scientific Publications Team of Asan Medical Center for providing language editing and proofreading support.
Notes
CRedit authorship contributions
Hyunji Sang: conceptualization, methodology, investigation, formal analysis, writing - original draft, visualization; Yun Kyung Cho: writing - review & editing, supervision; Sang-hyeok Go: investigation; Hwa Jung Kim: formal analysis; Eun Hee Koh: writing - review & editing, supervision, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
None