Association between specific IgE to staphylococcal enterotoxin B and the eosinophilic phenotype of asthma
Article information
Abstract
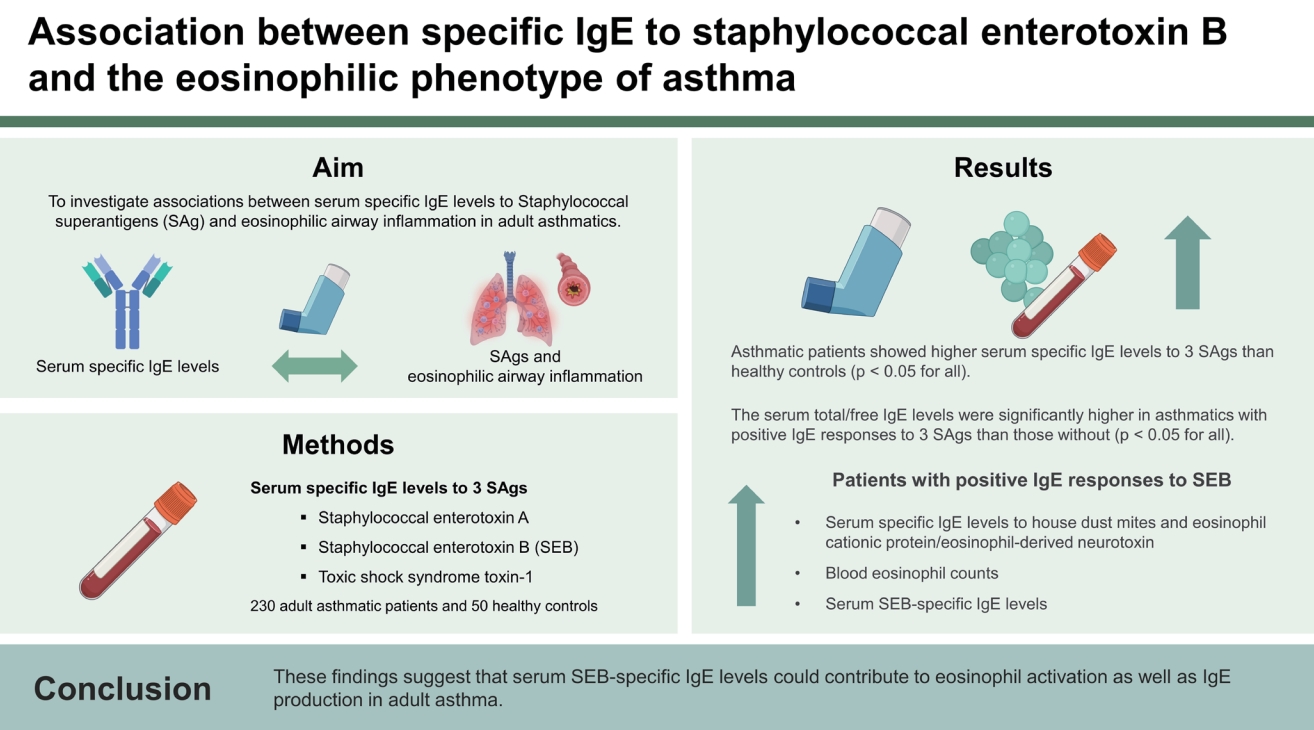
Background/Aims
Sensitization to staphylococcal superantigens (SAgs) could contribute to asthma severity. However, its relevance with eosinophilic phenotype has not yet been clarified. This study aimed to investigate associations between serum specific IgE levels to SAg and eosinophilic airway inflammation in adult asthmatics.
Methods
The serum specific IgE levels to 3 SAgs, including staphylococcal enterotoxin A (SEA) and B (SEB), and toxic shock syndrome toxin-1 (TSST-1) were measured by ImmunoCAP in 230 adult asthmatic patients and 50 healthy controls (HCs). Clinical characteristics and laboratory parameters, including serum total/free IgE, and 2 eosinophil-activation markers, eosinophil cationic protein (ECP), and eosinophil-derived neurotoxin (EDN), were analyzed according to blood eosinophil counts (BEC; 150 cells/μL) and serum specific IgE levels to 3 SAgs (0.35 kU/L).
Results
Asthmatic patients showed higher serum specific IgE levels to 3 SAgs than HCs (p < 0.05 for all). The serum total/clinfree IgE levels were significantly higher in asthmatics with positive IgE responses to 3 SAgs than those without (p < 0.05 for all). There were no significant differences in clinical parameters including age, asthma severity, comorbidities, or smoking according to IgE responses to 3 SAgs. Patients with positive IgE responses to SEB (not to SEA/TSST-1) had higher serum specific IgE levels to house dust mites and ECP/EDN as well as higher BEC with positive correlations between serum SEB-specific IgE levels and BEC/ECP/EDN (p < 0.05 for all).
Conclusions
These findings suggest that serum SEB-specific IgE levels could contribute to eosinophil activation as well as IgE production in adult asthma.
INTRODUCTION
Asthma is characterized by chronic airway inflammation with increasing prevalence and economic burdens, impairing the quality of life in patients [1,2]. Due to its heterogeneity, there have been constant attempts to establish asthma phenotypes or endotypes for understanding underlying pathogenic mechanisms [3]. For decades, asthma has commonly classified into type 2 (T2)-high and T2-low phenotypes based on immunologic mechanisms [4], where eosinophils are major effector cells responsible for driving T2 airway inflammation and remodeling by releasing interleukin (IL)-4, IL-5, and IL-13 and granule proteins, such as eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (EDN), via interactions among diverse immune cells including T helper (Th) 2 cells, group 2 innate lymphoid cells (ILC2), B cells, and mast cells [5].
Staphylococcus aureus is a commensal gram-positive bacterium mainly found in human skin tissues and upper respiratory tract [6,7]. However, S. aureus can be pathogenic as it releases several virulent factors, such as staphylococcal enterotoxins (SEs) and toxic shock syndrome toxin-1 (TSST-1), which also act as superantigens (SAgs), contributing to IgE-mediated responses in patients with atopic dermatitis and upper airway disease such as chronic rhinosinusitis and nasal polyps [8-10]. Regardless of antigen specificity, SAgs can directly activate T cells by linking their T cell receptors to class II major histocompatibility complex molecules on antigen-presenting cells, resulting in explosive release of T2 cytokines (IL-4, IL-5, and IL-13), which enhance eosinophilic inflammation and IgE production [11,12]. Recently, several studies have shown that SE sensitization is one of the environmental risk factors responsible for the development of asthma [13]. In particular, SE sensitization is associated with lower lung functions, higher blood/sputum eosinophil counts, and serum ECP levels [14-16], suggesting the role of IgE-sensitization to SE in eosinophilic phenotypes or disease severity [17].
Despite increasing evidence for the pathogenic role of staphylococcal SAgs in asthma, their relevance with eosinophilic airway inflammation has not yet been fully understood. Therefore, we evaluated clinical relevance of IgE-sensitization to 3 staphylococcal SAgs (SEA, SEB, and TSST-1) in association with eosinophilic inflammatory parameters in adult asthmatics in clinical practice.
METHODS
Patient recruitment
In this study, 230 adult asthmatic patients (over 20 years old) and 50 adult healthy controls (HCs) were enrolled. All the study subjects submitted written informed consent. The study was approved by the Institutional Review Board of Ajou University Hospital (AJIRB-GEN-SMP-13-108). The sera were collected at initial diagnosis and kept in a frozen state at -70°C until ELISA or immunoCAP assays were performed.
Evaluation of clinical parameters
In this study, asthmatic patients were diagnosed by the allergy specialists based on clinical histories and according to the recent GINA guideline [18]. Asthmatics who had used systemic steroids were excluded from this study. Atopy was defined as at least one positive result in skin prick tests for common inhaled allergens (Bencard, Bradford, UK), including 2 house dust mites (Dermatophagoides pteronyssinus [Dp] and Dermatophagoides farinae [Df]). The presence of chronic rhinosinusitis and nasal polyp was evaluated by clinical symptoms, radiologic findings (paranasal sinus X-rays and/or CT scans), and/or rhinoscopy. The diagnosis of atopic dermatitis and chronic urticaria were assessed by the allergists. Severe asthma was defined based on the guidelines of the European Respiratory Society and American Thoracic Society [19]. Clinical and immunologic parameters, including blood/sputum eosinophil counts, were assessed at the initial diagnosis. The degree of airway obstruction was examined using spirometry and airway hyperresponsiveness by methacholine bronchial challenge tests. The concentrations of serum total IgE as well as SEA-, SEB-, and TSST-1-specific IgE were measured using the ImmunoCAP system (ThermoFisher Scientific, Waltham, CA, USA). SAg-specific IgE levels > 0.35 kU/L were considered positive. The serum free IgE levels were measured by ELISA as previously described [20]. The serum levels of ECP and EDN were measured by ELISA (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s recommendations.
Statistical analysis
All the statistical analyses were performed using IBM SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA). All data are presented as mean ± standard deviation. Group comparisons were made by Student’s t-test and chi-square test. To analyze correlations between 2 variables, Spearman’s rank correlation was conducted. p values of < 0.05 were considered statistically significant. GraphPad Prism 8.0 software (GraphPad Inc., San Diego, CA, USA) was used to create graphs.
RESULTS
Serum specific IgE levels to SEA, SEB, and TSST-1 in adult asthmatics
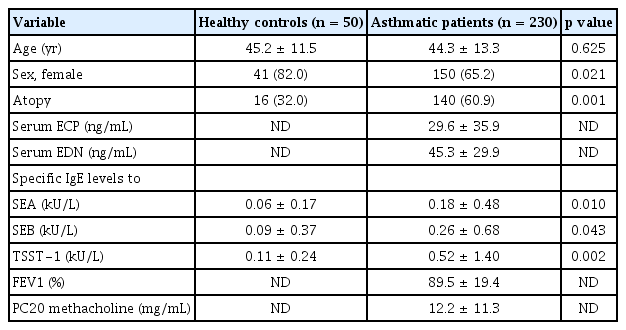
The clinical characteristics of the study subjects are summarized in Table 1. Atopy prevalence and male ratio as well as serum SEA-, SEB, and TSST-1-specific IgE levels were significantly higher in asthmatic patients than in HCs (p < 0.05 for all) as shown in Table 1.
Comparisons between SAg-specific IgE levels according to blood eosinophil counts
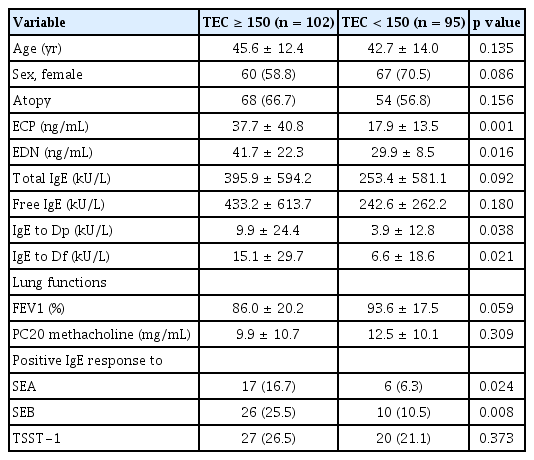
When asthmatic patients were divided into the high-eosinophil (≥ 150 cells/μL) and low-eosinophil groups (< 150 cells/μL), significantly higher serum levels of ECP, EDN, and IgE specific to Dp and Df were noted in the high-eosinophil group than in the low-eosinophil group (p < 0.05 for all) as depicted in Table 2. However, these 2 groups were not statistically different in terms of age, sex, atopy rate, immunologic parameters (such as serum total and free IgE levels), or FEV1 (%) and PC20 values. Nevertheless, both serum SEA- and SEB-specific IgE levels were significantly higher in the high-eosinophil group than in the low-eosinophil group (p = 0.009 and p = 0.004, respectively). Furthermore, IgE-sensitization rates to SEA and SEB were greater in the high-eosinophil group than in the low-eosinophil group (p = 0.024 and p = 0.008, respectively; Table 3), while no differences were noted in serum TSST-1-specific IgE levels or sensitization rates to TSST-1 between the 2 groups.
Associations between SAg-specific IgE levels and eosinophilic inflammatory markers
The study further classified asthmatic patients to the SAg-positive and SAg-negative groups (> 0.35 kU/L and ≤ 0.35 kU/L, respectively) according to positivity for IgE responses to 3 SAgs. As a result, there were no significant differences in clinical characteristics between the 2 groups, except sex proportion and prevalence of atopy or atopic dermatitis. The proportion of male patients was significantly higher in the SEB-positive and TSST-1-positive groups than in corresponding counterparts (p = 0.001 and p = 0.026, respectively). Moreover, the presence of atopy was higher in the TSST-1-positive group than in the TSST-1-negative group while that of atopic dermatitis was lower in the SEA-positive group than in the SEA-negative group (p = 0.001 and p = 0.036, respectively) as described in Table 4. When laboratory parameters were compared according to the positivity of IgE responses to 3 SAgs, the serum levels of total and free IgE as well as Dp-specific IgE were higher in the SAg-positive groups than in the SAg-negative groups (p < 0.05 for all). Although the serum Df- and Dp-specific IgE levels were higher in the SEB-positive group than in the SEB-negative group (p = 0.020 and p = 0.029), no differences were found between the SEA-positive and SEA-negative groups or between the TSST-1-positive and TSST-1-negative groups. Moreover, peripheral eosinophil counts and serum ECP and EDN levels were significantly higher in the SEB-positive group than in the SEB-negative group (p < 0.05 for all), albeit not significantly different between the SEA-positive and SEA-negative groups, and between the TSST-1-positive and TSST1-negative groups. In addition, serum SEB-specific IgE levels were positively correlated with blood eosinophil counts as well as with serum ECP and EDN levels in asthmatic patients (r = 0.254, p < 0.001; r = 0.150, p = 0.037; and r = 0.251, p = 0.035, respectively) (Fig. 1), while no significant correlations were noted in SEA- or TSST1-sensitized patients.
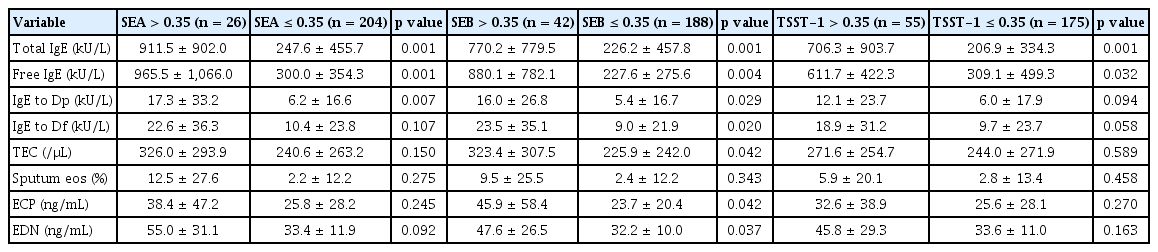
Comparison of laboratory parameters according to serum specific IgE levels to SAgs in asthmatic patients
DISCUSSION
SAgs can activate various immune cells, such as T cells, B cells, mast cells, eosinophils, and epithelial cells, resulting in massive production of cytokines and granule proteins as well as polyclonal IgE formation, contributing to asthma pathogenesis [21]. Among them, SEB, a major enterotoxin of S. aureus, amplifies T2 immune responses by inducing the production of T2 cytokines including IL-4, IL-5, and IL-13, leading to blood and airway eosinophilia [22,23]. Previous studies have demonstrated that IgE-sensitization to SEs could increase IgE production and blood/sputum eosinophil counts in association with severe asthma and late-onset elderly asthma [14,16]. In this study, significantly higher prevalence rates of serum specific IgE to 3 kinds of staphylococcal SAgs (SEA, SEB, and TSST-1) were associated with increased serum total/free IgE levels in adult asthmatics. Furthermore, significant correlations were noted between serum specific IgE levels to SEB (not to SEA or TSST-1) and higher blood eosinophils as well as higher serum ECP and EDN levels in adult asthmatics. These findings demonstrate that, among 3 staphylococcal SAgs, sensitization to SEB could be mainly involved in T2/eosinophilic inflammation, presenting the eosinophilic phenotype of asthma.
SAgs are known to induce B cell expansion and activation in response to T2 cytokines, promoting class switching to IgE and polyclonal IgE production [24]. Several studies have shown that bronchial exposure to SAg elevates serum total IgE and allergen-specific IgE in an allergic asthma mouse model sensitized by ovalbumin (OVA) [23,25]. Moreover, sensitization to SEs, not to aeroallergens, such as grass pollen or house dust mites, was closely related to higher total serum IgE and disease severity characterized by frequent asthma exacerbations and lung function decline [26,27]. The present study demonstrated that asthmatic patients with positive IgE responses to staphylococcal SAgs had higher serum total/free IgE levels, suggesting that IgE sensitization to SAgs could promote IgE production in B cells. Increased IgE activates diverse immune cells including mast cells, basophils, and dendritic cells by binding FcεRⅠ on their surfaces, which enhances T2/eosinophilic responses [28]. Our previous study reported that high serum free IgE levels could be a more reliable marker than serum total IgE levels for predicting T2 inflammation along with other traditional T2 markers, such as blood eosinophilia, and for monitoring therapeutic responses after omalizumab treatment in asthmatic patients [20]. Therefore, sensitization to 3 staphylococcal SAgs could contribute to polyclonal B cell activation, leading to increased serum total/free IgE levels, where anti-IgE antibody may be a therapeutic strategy in SAg-sensitized asthmatics.
There is accumulating evidence that sensitization to staphylococcal SAgs increases the risk of severe asthma by inducing persistent Th2/eosinophilic inflammation in asthmatic airways [13]. Severe asthma is characterized by persistent blood eosinophilia and uncontrolled symptoms, such as frequent asthma exacerbations and progressive lung function decline, even on medication with high-dose inhaled corticosteroids with an additional controller and/or systemic corticosteroid [19]. In addition, the levels of serum ECP and EDN are useful biomarkers for eosinophil activation and degranulation, contributing to asthma severity in adult asthmatics [29,30]. Furthermore, blood eosinophil counts are easily measurable indicators for predicting disease severity, exacerbation frequency, and impaired lung function [31,32], while no associations were found in recent studies [33,34]. In the present study, positive IgE responses to staphylococcal SAgs were not associated with asthma severity, lung function parameters, or asthma comorbidities. However, IgE specific to SEB (not to SEA or TSST-1) was significantly associated with blood eosinophilia and increased eosinophil activation/degranulation, indicating that IgE sensitization to SEB may contribute to eosinophilic inflammation in asthmatic airways. Previous in vivo studies have shown that bronchial exposure to SEB (not to SEA or TSST-1) induced allergic airway inflammation with increased eosinophil counts in bronchoalveolar lavage fluid as well as serum total IgE and OVA-specific IgE in OVA-sensitized mice [25]. Moreover, several in vitro studies have demonstrated SAg induced the formation of eosinophil extracellular eosinophilic traps in defected airway epithelium, enhancing T2/eosinophilic responses via ILC2 activation, which is the major cause of persistent airway inflammation and steroid resistance in eosinophilic asthma [35-37]. However, some studies have reported no association between IgE sensitization to SAgs and asthma severity or disease control [38-41]. This controversy may be attributed to variations in positive cutoff values (> 0.35 or > 0.1 kU/L) or study populations. Thus, further investigations are needed to elucidate pathogenic mechanisms of how specific IgE to SEB (not to SEA or TSST-1) contributes to eosinophilic inflammation in asthmatic airways. Taken together, the present study suggests that high serum specific IgE to SEB may be a potential biomarker for T2/eosinophilic inflammation in asthma. Further studies using standardized cutoff values are needed to confirm our results.
There are some limitations in this study. First, the number of subjects enrolled in the study was not large enough to represent adult asthmatics having various phenotypes. Furthermore, there were missing values in some variables as some patients did not fully perform all the laboratory tests at initial visit. However, we evaluated IgE-sensitization to 3 kinds of major SAgs in the sera of 230 adult asthmatics and analyzed their associations with various clinical/immunologic/inflammatory parameters. Secondly, this is a cross-sectional study, not a longitudinal outcome study tracking the endotypes of asthma. Further investigations are needed to evaluate the role of specific IgE to these 3 SAgs in the long-term outcome model of adult asthma that includes therapeutic responses.
In conclusion, we suggest that IgE-sensitization to 3 staphylococcal SAgs could increase serum IgE levels regardless of atopy status, and that IgE-sensitization to SEB may enhance eosinophilic inflammation in adult asthmatics.
KEY MESSAGE
1. IgE-sensitization to 3 staphylococcal SAgs increases serum IgE levels via stimulating B cells regardless of atopic status.
2. IgE-sensitization to SEB enhances eosinophilic inflammation in adult asthma.
3. IgE is a major therapeutic target for controlling eosinophilic airway inflammation.
Notes
CRedit authorship contributions
Soyoon Sim: investigation, writing - original draft, visualization; Youngwoo Choi: investigation, writing - review & editing; Eun-Mi Yang: data curation, formal analysis; Hae-Sim Park: conceptualization, writing - review & editing, supervision, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This research was supported by the Korean Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI) grant funded by the Ministry of Health and Welfare, Republic of Korea (HR16C0001).