Efficacy of single-dose evolocumab injection in early-phase acute myocardial infarction: a retrospective single-center study
Article information
Abstract
Background/Aims
Achieving rapid reduction of low-density lipoprotein cholesterol (LDL-C) levels below 55 mg/dL in patients with acute myocardial infarction (AMI) can be challenging with statins alone. This single-center, retrospective study aimed to assess the impact of single-dose injection of evolocumab 140 mg on LDL-C levels during the peri-percutaneous coronary intervention (PCI) period in patients with AMI.
Methods
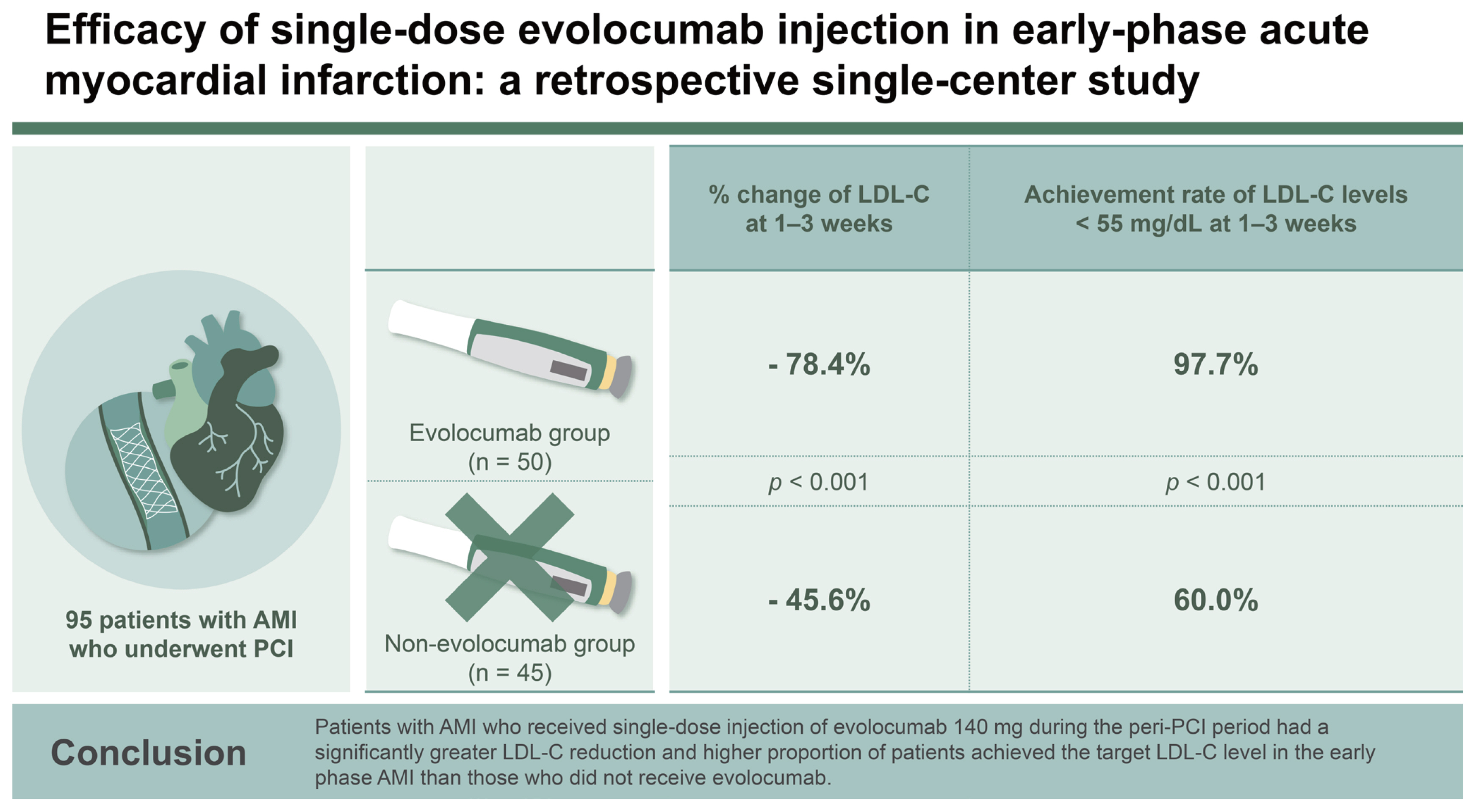
A total of 95 patients with AMI who underwent PCI were divided into the evolocumab (n = 50) and non-evolocumab (n = 45) groups.
Results
The percentage change of LDL-C level at 1–3 weeks from baseline was 78.4 ± 13.4% reduction in the evolocumab group versus 45.6 ± 22.6% in the non-evolocumab group, with a mean difference of −33.5% between the groups (95% CI: −42.6 to −24.5%; p < 0.001). The achievement rate of LDL-C levels below 55 mg/dL at 1–3 weeks was significantly higher in the evolocumab group than in the non-evolocumab group (97.7% vs. 60.0%, p < 0.001).
Conclusions
Patients with AMI who received single-dose injection of evolocumab 140 mg during the peri-PCI period had a significantly greater LDL-C reduction and higher proportion of patients achieved the target LDL-C level in the early phase AMI than those who did not receive evolocumab.
INTRODUCTION
Management of elevated low-density lipoprotein cholesterol (LDL-C) levels has been identified as a key strategy for patients with acute myocardial infarction (AMI), and is associated with a significant reduction in cardiovascular mortality [1,2]. Guidelines recommend the early initiation of high-intensity statins to rapidly achieve an LDL-C target level of < 55 mg/dL in this population, followed by the administration of ezetimibe or proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors if statin monotherapy proves ineffective [3,4]. Novel PCSK9 inhibitors, such as evolocumab and alirocumab, have recently gained attention for their potential to not only induce plaque regression but also promote plaque stabilization [5–8]. Moreover, early administration of PCSK9 inhibitors has been shown to rapidly reduce LDL-C levels in high-risk patients. However, limited data are available on the effect of PCSK9 inhibitors in patients with AMI who have undergone percutaneous coronary intervention (PCI), particularly in the early phase of AMI [7,8]. Therefore, this study aimed to evaluate the efficacy and safety of evolocumab single-dose injection during the peri-PCI period in patients with AMI.
METHODS
This retrospective study was conducted at Yongin Severance Hospital and considered 111 patients with AMI who received treatment between February 2022 to November 2022. A total of 95 patients who underwent PCI were included in the final analysis, after excluding four patients who underwent coronary artery bypass grafting, three who received medical treatment, three who died within 1 week of AMI event, and six for whom LDL-C level data was unavailable at the time of admission. The enrolled patients were divided into two groups: the evolocumab group (n = 50), those who were subcutaneously injected with single-dose evolocumab 140 mg within 24 hours before or after the PCI, regardless of the initial LDL-C level, and the non-evolocumab group (n = 45), those who were not injected with evolocumab (Supplementary Fig. 1). In both groups, the changes in the lowest LDL-C level between 1–3 weeks and 3–8 weeks were investigated. In the evolocumab group, the lowest LDL-C levels among 1–3 days and 4–6 days were additionally investigated. Regarding efficacy outcomes, the primary endpoint was the percent change of LDL-C level at 1–3 weeks from the baseline level. The secondary endpoint was the achievement rate of LDL-C level < 55 mg/dL at 1–3 weeks. As safety endpoints, changes in aspartate aminotransferase (AST)/alanine aminotransferase (ALT) and high-sensitivity C-reactive protein (hs-CRP) levels were investigated. Regarding clinical outcomes, death, myocardial infarction, stroke, bleeding, and symptoms of myalgia or fatigue over 6 months were also investigated.
The study protocol was approved by the Institutional Review Board (IRB) of Yongin Severance Hospital (approval number: 9-2023-0124), and the study received an exemption from the requirement of informed consent owing to its retrospective nature. All experiments were performed in accordance with the Declaration of Helsinki.
Statistical analysis
All data are reported as means ± standard deviations for continuous variables and as frequencies (percentages) for categorical variables. We compared the two groups by conducting independent-samples t-tests and chi-square tests for continuous and categorical variables, respectively. The primary outcomes were analyzed using a linear mixed model with repeated measures, with baseline values, treatment group, time points, and interaction between the treatment group and time points as fixed effects, and patients as a random effect. Statistical significance was set at p < 0.05. All statistical analyses were performed with R software (version 4.3.0; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
We compared the baseline characteristics and outcomes of the two groups (Table 1). The average age of all the patients was 61.4 ± 13.1 years, males accounted for 84.2% of the total cohort, and 35.8% of patients showed ST elevation of myocardial infarction. Comparing the baseline demographic and clinical characteristics of two groups, the evolocumab group had a younger mean age of 57.9 ± 11.2 years compared to that of the non-evolocumab group, which was 65.3 ± 14.1 years (p = 0.006). The evolocumab group demonstrated a slightly higher BMI as opposed to the non-evolocumab group (25.8 ± 3.4 kg/m2 vs. 24.1 ± 3.0 kg/m2, p = 0.011). The proportion of patients with drug-eluting stents and the total number of stents per patient did not significantly differ between the two groups, nor did the use of medication upon discharge. Most patients in both groups were discharged on aspirin and P2Y12 inhibitors, predominantly prasugrel. The intensity of statin therapy at discharge was not significantly different between the two groups, with high-dose statins being used in above 60% of cases in each. Additionally, ezetimibe was used by all patients.
Baseline laboratory test results are summarized in Table 2. The total, triglyceride, LDL-C, and non-high-density lipoprotein cholesterol levels were significantly higher in the evolocumab group. The groups did not significantly differ in terms of hemoglobin A1c level, glucose level, hemoglobin, platelet count, AST, ALT, or hs-CRP. The creatinine level was higher in the non-evolocumab group, which resulted in a lower estimated glomerular filtration rate. The level of peak troponin T after PCI between the two groups was not statistically significant.
The median interval between hospital arrival and single-dose injection of evolocumab 140 mg was 3.0 hours (interquartile range: 2.0–5.0 hours). There were no statistically significant differences in the median day between 1–3 weeks and 3–8 weeks when the LDL-C level was measured from PCI. The initial LDL-C levels were higher in the evolocumab group than in the non-evolocumab group (144.4 ± 53.6 mg/dL vs. 100.4 ± 37.8 mg/dL, p < 0.001). In the evolocumab group, average LDL-C level was 72.7 mg/dL (95% confidence interval [CI]: 72.1–98.7 mg/dL) after 1–3 days and the 53.7 mg/dL (95% CI: 37.8–65.1 mg/dL) after 4–6 days (Fig. 1). The LDL-C level at 1–3 weeks was significantly lower in the evolocumab group than in the non-evolocumab group (27.4 ± 14.1 mg/dL vs. 48.7 ± 15.4 mg/dL, p = 0.003). The absolute change in decline at 1–3 weeks from baseline was −112.4 ± 49.9 mg/dL in the evolocumab group and −50.0 ± 33.4 mg/dL in the non-evolocumab group. Regarding the change in LDL-C level from baseline, significant reductions were observed at 1–3 and 3–8 weeks in both groups (both p < 0.001) (Fig. 1, Table 3). The percentage change in the LDL-C level at 1–3 weeks after baseline was −78.4 ± 13.4% in the evolocumab group and −45.6 ± 22.6% in the non-evolocumab group, with a mean difference of −33.5% between the groups (95% CI: −42.6 to −24.5%; p < 0.001; Fig. 2, Table 3). The achievement rate of LDL-C level < 55 mg/dL at 1–3 weeks was higher in the evolocumab group than in the non-evolocumab group (97.7% vs. 60.0%, p < 0.001), although the rate of achieving LDL-C levels < 70 mg/dL did not differ between the groups at 1–3 weeks (Table 3). As for the LDL-C level at 3–8 weeks, the absolute LDL-C levels (58.2 ± 23.3 mg/dL vs. 49.3 ± 20.9 mg/dL, p = 0.172) did not differ between two groups, but absolute change from baseline (−86.1 ± 47.6 mg/dL vs. −51.1 ± 36.6, p < 0.001) and % change from baseline (−55.8 ± 21.4% vs. −46.5 ± 25.1%, p = 0.033) were higher in evolocumab group. The rates of reaching LDL-C levels below 55 mg/dL (48.0% vs. 73.3%, p = 0.004) were higher in the non-evolocumab group, but that below 70 mg/dL (80.0% vs. 84.4%, p = 0.473) did not differ between the groups (Fig. 1, 2, Table 3).
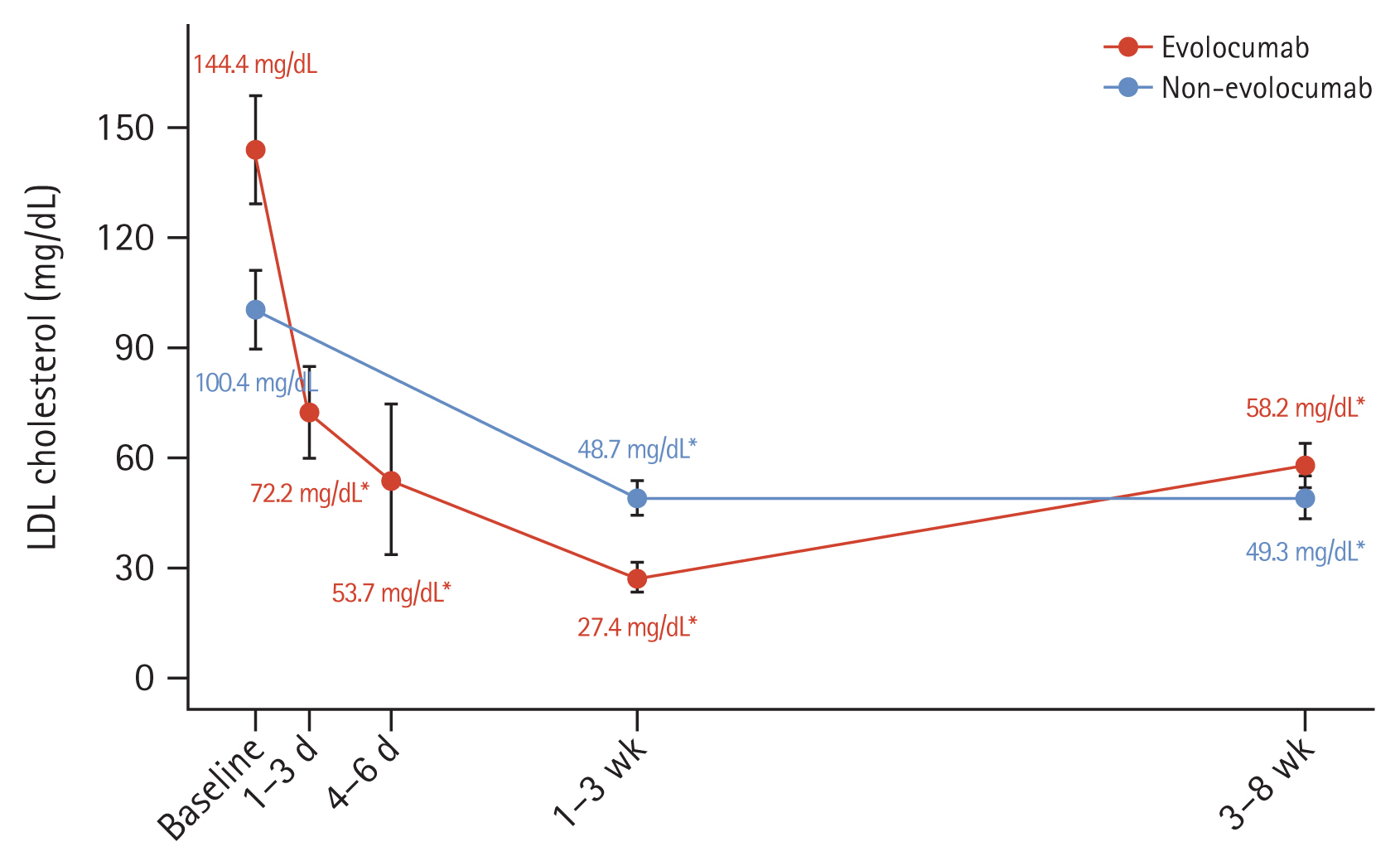
LDL-C levels over time. LDL-C mean values in the two study groups are shown; error bars indicate 95% confidence intervals. LDL-C, low-density lipoprotein cholesterol. The p value was calculated using paired t-test. *p < 0.001 for LDL-C change from baseline.

Percentage reduction in LDL-C level. Mean percentage changes in LDL-C level from baseline to 1–3 and 3–8 weeks in both groups are shown; error bars indicate 95% confidence intervals. LDL-C, low-density lipoprotein cholesterol. The p value was calculated using independent samples t-tests.
In terms of safety outcomes, there was no significant difference in AST/ALT abnormality, defined as more than three times the upper limit of the normal range, or in hs-CRP levels at 1–3 weeks and 3–8 weeks between the two groups, respectively (Supplementary Table 1). Regarding the clinical outcomes, the event of death, myocardial infarction, stroke, and bleeding at 6 months did not significantly differ between the groups. Symptoms of myalgia and fatigue were also similar in both the groups (Supplementary Table 2).
DISCUSSION
This study provided unique insights into LDL-C changes in AMI patients who underwent PCI, specifically in terms of the impact of single-dose evolocumab 140 mg combined with moderate- or high-intensity statins/ezetimibe in the early phase of AMI. Despite the baseline LDL-C level being higher in the evolocumab group than in the non-evolocumab group, the percentage reduction was higher in the evolocumab group at 1–3 weeks than in the non-evolocumab group (−78.4% vs. −45.6%, p < 0.001), and 97.7% of patients in the evolocumab group had an LDL-C level below 55 mg/dL at 1–3 weeks.
The current European and American guidelines both recommend a stepwise approach for the treatment of patients who experienced AMI, starting with the initiation of high-intensity statin therapy, followed by the addition of ezetimibe, and considering PCSK9 inhibitor treatment if the target LDL-C level is not reached [3,4]. As a result, although many early clinical events may occur after AMI, especially in patients with higher LDL-C levels, PCSK9 inhibitors are added only several months after AMI events in real-world practice. Furthermore, the proportion of patients with AMI taking PCSK9 inhibitors reportedly accounts for less than 1% of patients with an LDL-C level above 55 mg/dL in clinical practice [9]. However, an in vivo study has revealed that PCSK9 levels increase in the early stages of myocardial ischemia. If the PCSK9 level is inhibited during myocardial ischemia, size of the myocardial infarction, post-infarction inflammation, and cardiac dysfunction after ischemia/reperfusion injury are reduced [10]. Clinical data suggest that the inhibition of PCSK9 not only plays a role in reducing LDL-C levels but also improves endothelial function in the early stages of AMI [11]. Furthermore, rapid administration of PCSK9 inhibitors for very low LDL-C levels has been proven to promote plaque stabilization and regression through studies using coronary intravascular modalities [5,6]. Additionally, patients with the lowest LDL-C levels achieved via PCSK9 inhibitors within 4 weeks of AMI exhibit better long-term clinical outcomes [12]. Patients who have had an AMI have a higher risk of repeat cardiovascular events during this initial period. Despite issues of cost and accessibility, PCSK9 inhibitors have been used and shown in the early stages in pilot studies to address this risk [7,8]. Those studies revealed that PCSK9 inhibitors used alongside high-intensity statins rapidly lowered LDL-C levels to within the target level. In those studies, however, the percentage of the ezetimibe usage proportions were less than 5%. Ezetimibe has already been spotlighted as overcoming the limitations of high-dose statins and enhancing clinical outcomes [13]. The addition of ezetimibe alone to statins results in further reduction of 15–22% in the LDL-C level [14]. Moreover, in the 3-year follow-up period, moderate-intensity statins with ezetimibe had no inferiority in clinical outcomes as regards lowering LDL-C level and improved the compliance rate as side effects were reduced compared with the use of high-intensity statins alone [15].
In this study, both moderate- (33.7% of cases) and high- (66.3% of cases) intensity statins were used; interestingly, all the patients initially received ezetimibe after AMI events. Despite a single injection of evolocumab 140 mg during the peri-PCI period, the LDL-C level was reduced by 78.4%, and the rate of achieving an LDL-C level below 55 mg/dL was 97.7% in the early period after AMI, whereas the percentage reduction in LDL-C level decreased to only 48.0% at 3–8 weeks after AMI. Choi et al. [16] reported the single injection of evolocumab 140 mg reduced LDL-C level from the baseline to 2 weeks by 58.5% in Korean patients with angina who underwent PCI. In the non-evolocumab group, who used only moderate- or high-dose stains and ezetimibe, the target achievement at both time points was approximately 60–70%. In our retrospective analysis, the addition of evolocumab to moderate- or high-intensity statins and ezetimibe was associated with a potent and rapid reduction in the LDL-C level in the early stages after AMI. Therefore, this triple combination therapy in the very early stages after AMI might promote the improvement of clinical outcomes via the stabilization of vulnerable plaques in the future, as previously demonstrated via intravascular imaging modalities [5,6]. Although data on the long-term benefits of PCSK9 inhibitors immediately after an AMI are limited, starting PCSK9 inhibitors and ezetimibe alongside non-high-intensity statins in the early stages after AMI for patients treated with PCI may enhance patient compliance and long-term outcomes.
Our study had some limitations. First, owing to its retrospective nature and limited sample size, the patients prescribed evolocumab tended to have a higher initial LDL-C level than those not prescribed evolocumab. Thus, we used a linear mixed model to adjust for this confounding factor. Second, in the evolocumab group, after the lowest LDL-C level in the first 1–3 weeks, a rebound phenomenon can be seen in 3–8 weeks. This could be seen as a limitation of single injection, and due to cost and insurance restrictions. Therefore, further research should be considered to confirm the impact of rebound phenomenon. Third, despite the notable LDL-C level decrease following the single injection of evolocumab 140 mg in AMI patients undergoing PCI, this study does not sufficiently predict the long-term benefits of the clinical outcomes, possibly owing to the small sample size and short-term follow up. Therefore, long-term outcomes of early PCSK9 inhibitor use by patients who have had an AMI should be studied in a large-scale randomized controlled trial.
In conclusion, in patients who underwent AMI with PCI, single-dose injection of evolocumab 140 mg during the peri-PCI showed a significantly greater LDL-C reduction, and a higher proportion of patients achieved an LDL-C target level below 55 mg/dL at 1–3 weeks than those who did not receive evolocumab.
KEY MESSAGE
1. The LDL-C level at 1–3 weeks was 78.4% ± 13.4% lower than baseline in the evolocumab group versus 45.6 ± 22.6% in the non-evolocumab group, with a statistically significant mean difference of −33.5% between the groups.
2. The achievement rate of a LDL-C level below 55 mg/dL at 1–3 weeks was significantly higher in the evolocumab group (97.7%) than that in the non-evolocumab group (60.0%).
Acknowledgments
The authors thank all the staff working in the cardiac catheterization laboratory at the Yongin Severance Hospital for their commitment to this study.
Notes
CRedit authorship contributions
Yongcheol Kim: conceptualization, methodology, resources, investigation, formal analysis, writing - original draft, writing - review & editing, visualization; Ji Woong Roh: conceptualization, methodology, investigation, data curation, formal analysis, writing - original draft, writing - review & editing; Oh-Hyun Lee: investigation, validation, writing - review & editing; Seok-Jae Heo: resources, formal analysis, validation, software, visualization, project administration; Eui Im: validation, software, supervision, project administration; Deok-Kyu Cho: conceptualization, investigation, writing - review & editing, supervision, project administration; Byeong-Keuk Kim: conceptualization, methodology, investigation, writing - review & editing, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None
Data availability statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.