Clinical course of asymptomatic duodenal subepithelial lesions
Article information
Abstract
Background/Aims
There is limited knowledge regarding the management of duodenal subepithelial lesions (SELs) owing to a lack of understanding of their natural course. This study aimed to assess the natural course of asymptomatic duodenal SELs and provide management recommendations.
Methods
Patients diagnosed with duodenal SELs and followed up for a minimum of 6 months were retrospectively investigated.
Results
Among the 443,533 patients who underwent esophagogastroduodenoscopy between 2008 and 2020, duodenal SELs were identified in 0.39% (1,713 patients). Among them, 396 duodenal SELs were monitored for a median period of 72.5 months (interquartile range, 37.7–111.3 mo). Of them, 16 SELs (4.0%) showed substantial changes in size or morphology at a median follow-up of 35.1 months (interquartile range, 21.7–51.4 mo). Of these SELs with substantial changes, tissues of two SELs were acquired using endoscopic ultrasound-guided fine needle aspiration biopsy: one was a lipoma and the other was non-diagnostic. Three SELs were surgically or endoscopically removed; two were diagnosed as gastrointestinal stromal tumors, and one was a lipoma. An initial size of 20 mm or larger was associated with substantial changes during follow-up (p = 0.016).
Conclusions
While the majority of duodenal SELs may not exhibit substantial interval changes, regular follow-up with endoscopy may be necessary for cases with an initial size of 20 mm or larger, considering a possibility of malignancy.
INTRODUCTION
Esophagogastroduodenoscopy (EGD) is commonly used to evaluate gastrointestinal (GI) symptoms and to screen for cancer. During EGD, subepithelial lesions (SELs), previously known as submucosal tumors, are often encountered incidentally by endoscopists. SEL originate as a lesion, including tumor, bulge, or impression from the area underneath the epithelium of the GI tract, including the muscularis mucosae, submucosa, and muscularis propria. SEL can range from benign lesions, such as lipomas, leiomyomas, and duplication cysts, to malignant tumors, including gastrointestinal stromal tumors (GISTs) and neuroendocrine tumors.
SELs are found in 1 in every 300 endoscopies [1–3], with a higher frequency in Korea and Japan, where EGD is included in cancer screening [4]. The stomach is the most commonly involved organ, followed by the esophagus [5,6], whereas the duodenum is the least involved organ in the upper GI tract [5,7].
Recent advancements in endoscopic procedures have rendered endoscopic resection (ER) a potential treatment option for upper GI SELs [8–10]. However, ER for duodenal lesions is challenging because of the anatomical characteristics of the duodenum, including a relatively thin muscle layer and narrow luminal diameter, which increases the risk of perforation and makes endoscopic maneuverability difficult. Surgical resection of duodenal SELs is also technically challenging because of their close proximity to major systemic and splanchnic vessels, the pancreas, and biliary organs, as well as their retroperitoneal location. Therefore, careful consideration is necessary when selecting duodenal SELs for endoscopic or surgical resections.
While several management plans and guidelines have been proposed for gastric SELs, limited data and information are available for the management of duodenal SELs, owing to a lack of knowledge about their natural course. This study aimed to evaluate the natural course of duodenal SELs and suggest appropriate management strategies.
METHODS
Study populations
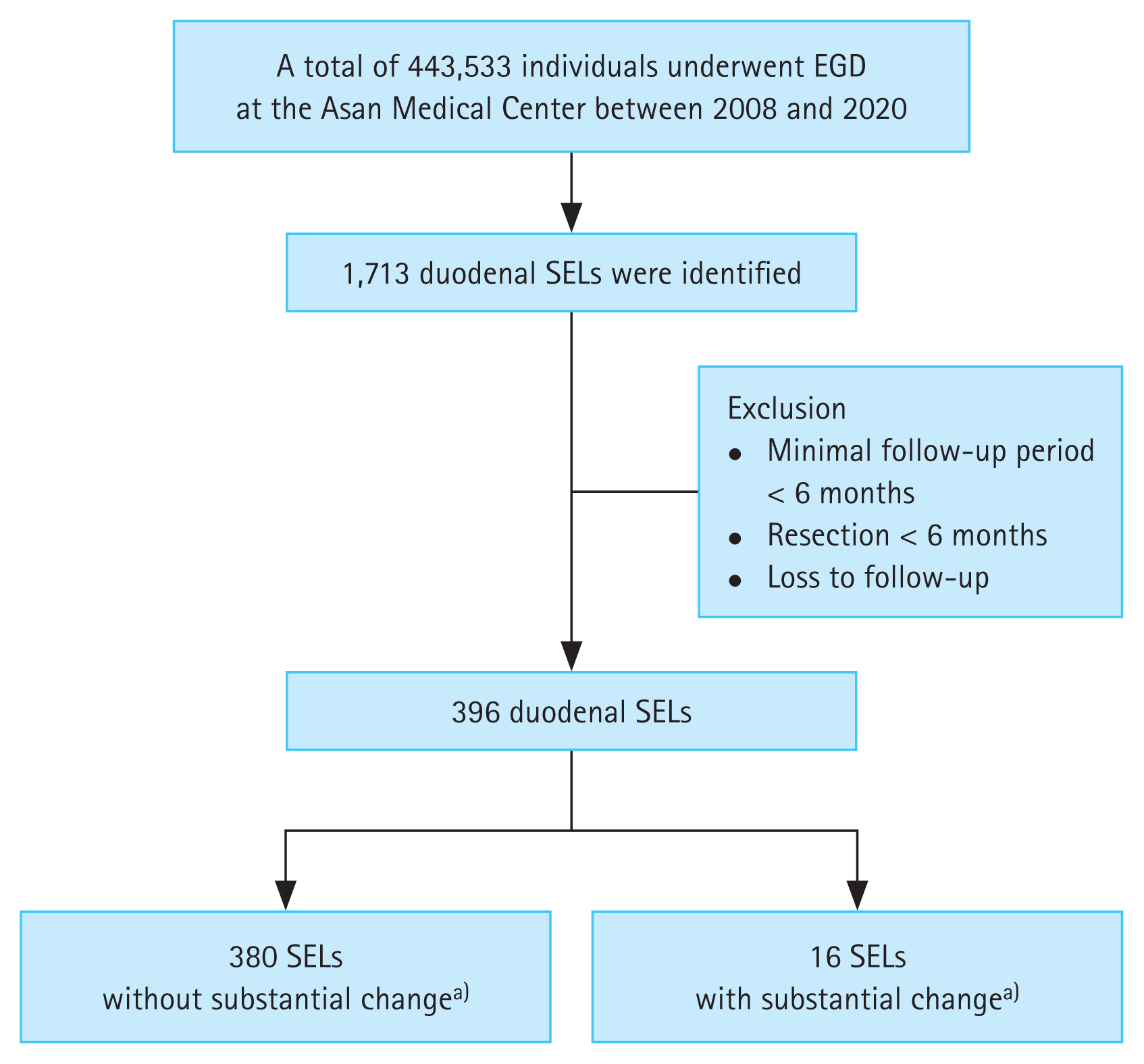
We identified new cases of duodenal SELs diagnosed during 443,533 EGDs between January 2008 and December 2020 by conducting a computerized search of the Asan Biomedical Research Environment, a de-identified clinical data warehouse of the Asan Medical Center, using the terms “duodenal subepithelial tumor,” “duodenal subepithelial lesion,” and “duodenal submucosal tumor.” Patients who were lost to follow-up or who underwent surgery or ER within 6 months were excluded. Finally, 396 cases with duodenal SELs with a minimal follow-up period of 6 months were enrolled (Fig. 1). None of the enrolled duodenal SELs had surface ulcerations at initial diagnosis. The baseline characteristics of the enrolled patients were collected from their medical records.
Endoscopy
SELs were defined as bulges or masses covered with normal-appearing mucosa, as observed during endoscopy. All EGDs were performed by board-certified endoscopists, with or without trainees. The characteristics of the SELs, including size, location, and presence of surface ulceration, were evaluated during the initial diagnosis. Among the enrolled 396 duodenal SELs, 70 were further evaluated at the physician’s discretion using endoscopic ultrasound (EUS) to determine the layer of origin and echogenicity pattern. EUS was performed by expert endoscopists (Kim DH, Na HK, Ahn JY, Lee JH, Choi KD, and Song HJ).
Follow-up
A total of 396 patients underwent follow-up EGD or EUS, with a minimum follow-up period of 6 months after the initial diagnosis. Patients without substantial changes in tumor size, echogenicity, or morphology underwent periodic EGD or EUS surveillance every 12 to 24 months. Substantial changes during follow-up were defined as follows: a size increment ≥ 25% in the longest diameter; surface ulceration; and echogenicity changes suggestive of malignancy, including irregular border, echogenic foci, cystic spaces, and heterogeneity [4]. EUS-guided fine needle aspiration biopsy (EUS-FNAB), ER, or surgery were recommended for patients who show substantial changes during follow-up.
Statistical analysis
Categorical variables were analyzed using Fisher’s exact test or the chi-squared test, as indicated, and continuous variables were analyzed using Mann–Whitney U test or Student’s t-test, as indicated. Univariate Cox regression analysis was conducted to identify factors associated with substantial changes in SELs. All statistical analyses were performed using R 4.1.1 (R foundation for Statistical Computing, Vienna, Austria). Two-sided p values < 0.05 were considered as statistically significant.
Ethics statement
Owing to the retrospective nature of the study, the need for informed consent was waived. This study was approved by the Institutional Review Board of the Asan Medical Center (approval number 2021-0388).
RESULTS
Baseline characteristics of study population
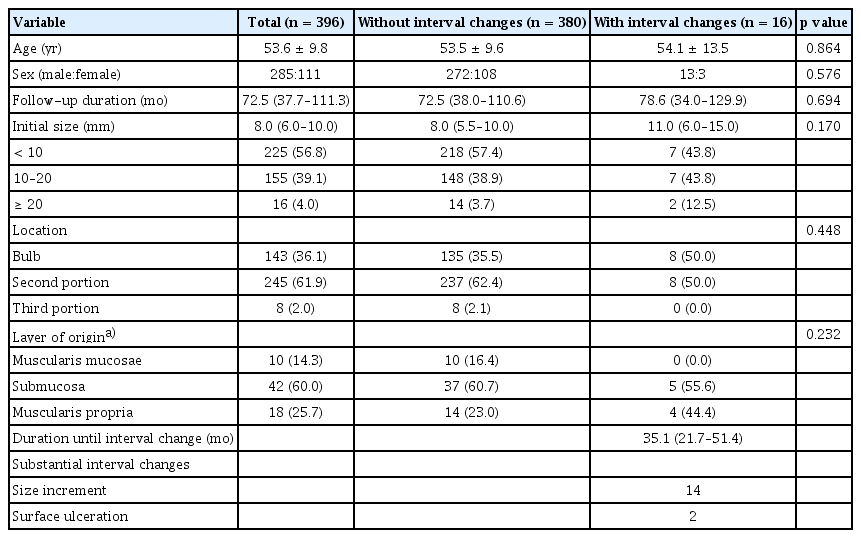
Table 1 presents the baseline characteristics of 396 patients with asymptomatic duodenal SELs. The mean age was 53.6 years, and 285 of 396 patients were males. The median and interquartile range (IQR) of the follow-up duration were 72.5 months and 37.7–111.3 months (range, 6.1–223.4 mo), respectively. The initial size of SELs was < 10 mm in 225 cases (56.8%), 10–20 mm in 155 cases (39.1%), and ≥ 20 mm in 16 (4.0%) cases. The median initial size was 8.0 mm (IQR, 6.0–10.0 mm). The most common location was the second portion (61.9%), followed by the bulb (36.1%) and the third portion (2.0%). Among the 396 SELs, 70 (17.7%) were evaluated using EUS (Supplementary Table 1, Supplementary Fig. 1), and the most common layer of origin was the submucosal layer (60.0%). There were no significant differences in baseline characteristics between SELs with and without interval changes. Additionally, no statistically significant differences were observed in substantial changes according to the layer of origin, even when compared in pairs (Supplementary Table 2).
Clinical course of asymptomatic duodenal SELs
Among the 443,533 patients who underwent EGD between January 2008 and December 2020 (Fig. 1), duodenal SELs were identified in 0.39% (1,713 patients).
Of the 396 duodenal SELs with a minimum follow-up period of 6 months, 16 (4.0%) showed substantial changes during the follow-up period. Fourteen SELs had size increments, and two SELs showed surface ulceration at a median follow-up duration of 35.1 months (IQR, 21.7–51.4 mo; range, 10.6–133.6 mo).
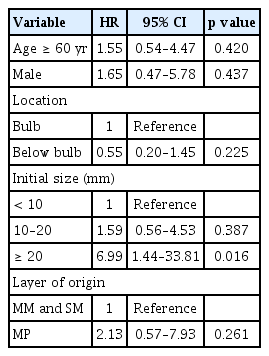
Age, sex, location, and the layer of origin were not statistically significant factors associated with substantial changes in SELs (Table 2), while an initial size ≥ 20 mm was a significant factor (p = 0.016).
Duodenal SELs with substantial interval changes
Figure 2 shows the flowchart of the 396 duodenal SELs during the follow-up period. Of the 225 SELs with an initial size of < 10 mm, 7 increased in size, but none underwent further evaluation for pathological confirmation due to patient refusal, loss to follow-up, or technical difficulties. Among the 155 SELs with an initial size of 10–20 mm, 7 exhibited interval changes (six increased in size and one showed ulcerative changes). EUS-FNAB was performed in one patient, but the sample was non-diagnostic owing to an insufficient amount of specimen acquired. Endoscopic and surgical resections were performed in two patients, resulting in a diagnosis of lipoma and GIST. Among the 16 SELs with an initial size ≥ 20 mm, two presented interval changes (size increment and ulceration): one was identified as a lipoma using EUS-FNAB, while the other was surgically resected and revealed as a GIST.
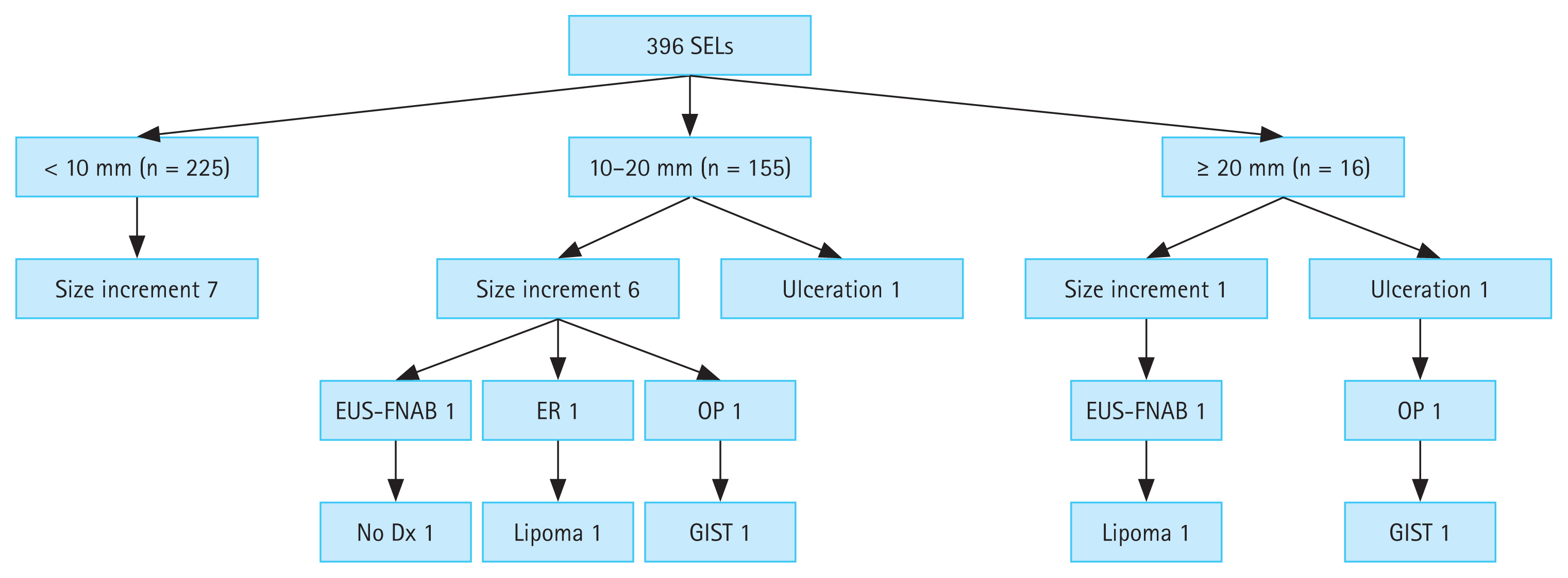
Flowchart of duodenal SELs during follow-up. SEL, subepithelial lesion; EUS-FNAB, endoscopic ultrasound-guided fine needle aspiration biopsy; ER, endoscopic resection; OP, operation; Dx, diagnosis; GIST, gastrointestinal stromal tumor.
Table 3 documents the characteristics of the 16 SELs showing substantial interval changes during the follow-up period. Figure 3 shows representative endoscopic images of duodenal SELs with substantial interval changes during the follow-up.
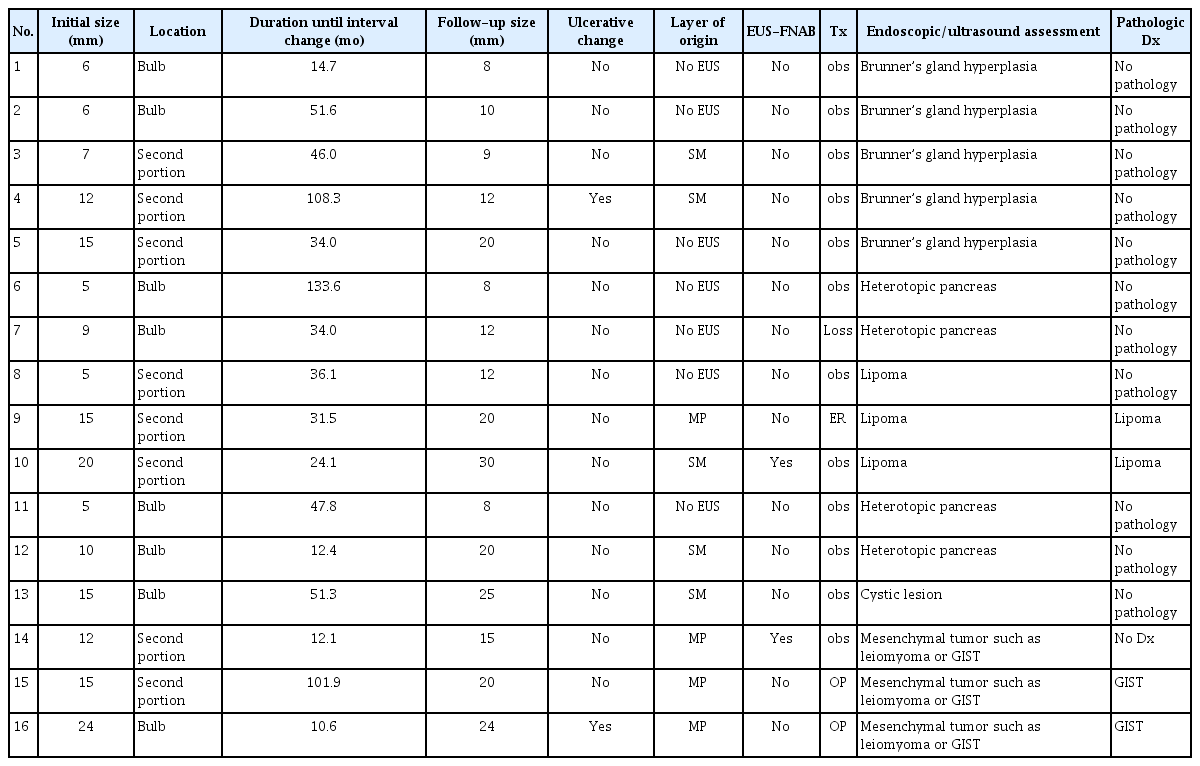
Characteristics of 16 duodenal subepithelial lesions showing substantial changes during the follow-up
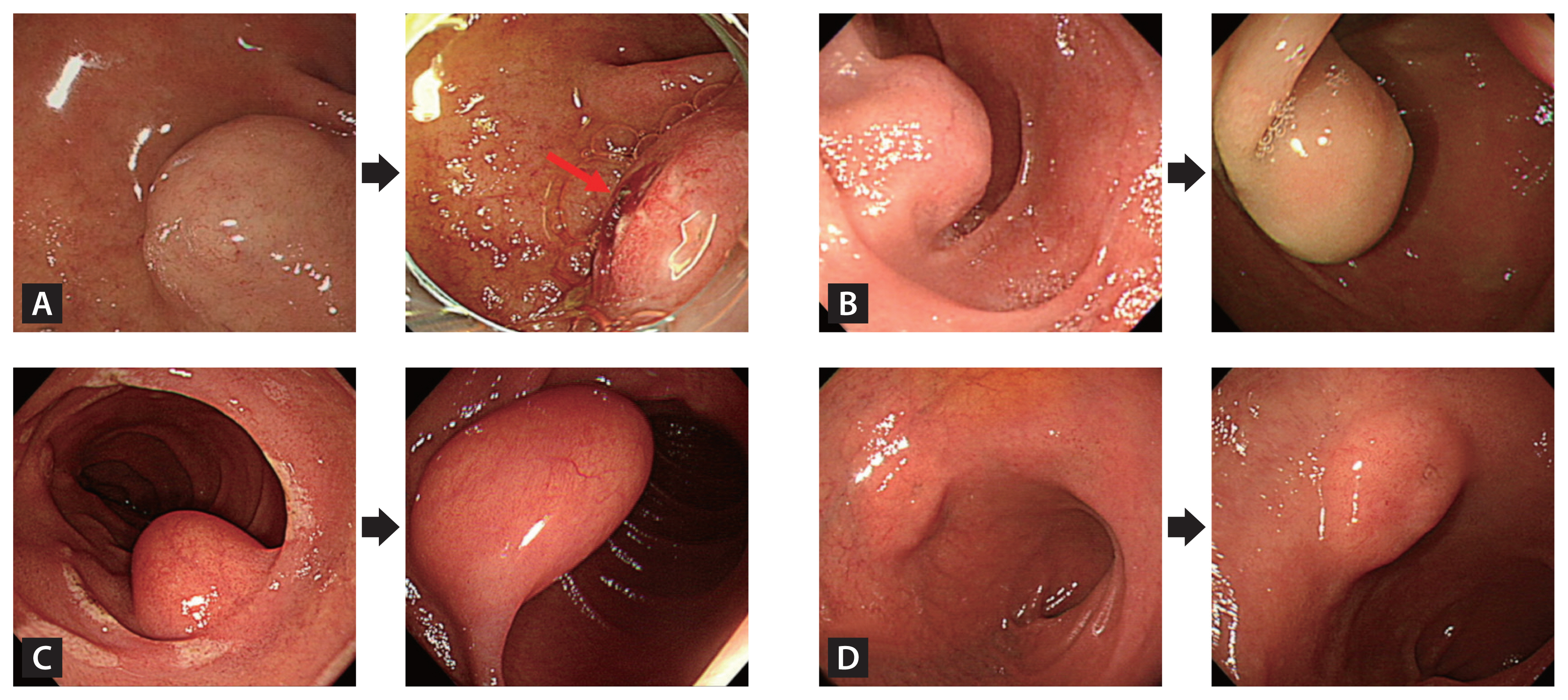
Endoscopic images of duodenal SELs exhibiting substantial interval changes during the follow-up period. (A) The duodenal SEL developed an ulceration (red arrow) at 10.6 months, underwent surgical resection, and was confirmed as a gastrointestinal stromal tumor. (B) The lesion presented size increment at 101.9 months, was surgically resected, and was identified as a gastrointestinal stromal tumor. (C) The lesion showed size increment at 31.5 months, was removed endoscopically, and was confirmed as a lipoma. (D) The lesion exhibited size increment at 12.4 months. An endoscopic ultrasound evaluation indicated a heterotopic pancreas, and it was monitored regularly for 55.2 months without substantial interval changes. SEL, subepithelial lesion.
DISCUSSION
Duodenal SELs are usually discovered incidentally during routine EGD and most are small (usually less than 2 cm) and asymptomatic. However, patients with SELs can present with GI bleeding, abdominal pain, or obstruction, for which resection is recommended [2,3,5]. Nevertheless, there are limited data and knowledge about the natural course of asymptomatic duodenal SELs. The European Society of Gastrointestinal Endoscopy stated that there is insufficient evidence to guide recommendations for duodenal SELs and suggested that obtaining a definitive diagnosis is necessary for making further decisions [5]. However, it is difficult to diagnose all asymptomatic duodenal SELs identified during routine EGD in a clinical setting.
In our study, duodenal SELs were identified in 0.39% of the cases (1,713/443,533). Among 396 asymptomatic duodenal SELs with a minimal follow-up period of 6 months, the majority (96.0%) did not exhibit substantial changes in size, morphology, or echogenicity during a median follow-up duration of 72.5 months (IQR, 37.7–111.3 mo). On the other hand, 16 (4.0%) SELs showed substantial changes at a median period of 35.1 months (IQR, 21.7–51.4 mo): 14 showed size increments and 2 showed surface ulceration. Among these cases, pathological confirmation was obtained for five SELs using EUS-FNAB, ER, or surgery, and only two (0.5%) were diagnosed as GISTs.
Several studies investigated the natural course of upper GI SELs. Gill et al. [11] found that 86.3% (44/51) of upper GI SELs, including two duodenal SELs, demonstrated changes in size and/or echogenicity at a mean period of 29.7 months, whereas none of the duodenal SELs showed changes. Bruno et al. [12] reported that 89.4% (42/47) of upper GI SELs, including four duodenal SELs, did not show changes in size and echogenicity. Lim et al. [7] observed that 96.8% (244/252) of upper GI SELs, including 18 duodenal lesions, showed no changes over a mean duration of 59.1 months. Similarly, Song et al. [13] reported that 96.4% (920/954) of upper GI SELs showed no interval changes during a median period of 47.3 months, and 96.2% (126/131) of duodenal SELs did not exhibit size increments during follow-up, which is consistent with the findings of our study. Kim et al. [14] reported that 85.6% (575/672) of the upper GI SELs did not show changes at a mean period of 68 months, and 90.0% (81/90) of the duodenal SELs did not show any changes. Unfortunately, these studies predominantly concentrated on gastric SELs and had a limited scope in addressing the natural course of duodenal SELs.
EUS is a useful tool for the differential diagnosis of SELs by evaluating the layer of origin, size, and echogenic characteristics [3,15,16]. EUS has demonstrated a sensitivity of 92% for distinguishing SELs from extrinsic compressions [16]. However, differentiating between benign and malignant SELs using EUS alone poses challenges due to its relatively lower sensitivity (64%) and specificity (80%) [2,17]. Furthermore, the interpretation of EUS findings can vary between operators, and there may be poor interobserver agreement, particularly when assessing echogenic features suggestive of malignancy (echogenic foci, cystic spaces, irregular borders, or heterogeneity).
Therefore, pathological confirmation is often necessary for a definite diagnosis of SELs [3,4]. Because SELs are located beneath the epithelium, mucosal biopsies using standard biopsy forceps often fail to acquire tissues [18]. EUS can facilitate tissue acquisition through EUS-FNAB, which is a widely used method for sampling lesions in the GI tract [3,19]. EUS-FNAB allows for the sampling of all GI tract lesions and has reported accuracy rates of 80–90% [20–25]. However, EUS-FNAB for duodenal SELs can be challenging because of difficulties in maneuvering an angulated scope-tip position in the duodenum [2]. Additionally, the diagnostic yield is poor for duodenal SELs compared to that for gastric lesions [26,27]. Thus, we believe that it is important to predict the malignant potential of SEL on the basis of endoscopic and EUS findings. In our study, an initial size ≥ 20 mm was a significant factor associated with substantial changes in SELs (p = 0.016), which aligns with previous reports and guidelines.
To date, our study is the largest to focus solely on duodenal SELs rather than on gastric lesions, as previous studies have predominantly concentrated on gastric SELs. In addition, factors such as age, sex, location, and the layer of origin were not statistically significant in relation to substantial changes in SELs (Table 2), whereas an initial size ≥ 20 mm was a significant factor. Of the 379 duodenal SELs, only two (0.5%) were identified as GISTs. This finding suggests that regular endoscopic follow-ups may be an appropriate strategy for the management of duodenal SELs.
Neuroendocrine tumors are a rare type of neuroendocrine cancer that occur less frequently in the duodenum than in other parts of the digestive tract. Duodenal neuroendocrine tumors are usually small [28], with mean sizes ranging from 7 to 15 mm [29]. Due to their malignant potential, several guidelines advocate resection, regardless of their small size [5,30]. Therefore, even when encountering small duodenal SELs (< 20 mm), careful examination is important to exclude the possibility of neuroendocrine tumors. Neuroendocrine tumors are typically characterized by rounded lesions with a yellowish or reddish color that differ from the surrounding mucosa and are generally diagnosed using endoscopic mucosal forceps biopsy [5].
Our study had several limitations. First, this was a single-center retrospective observational study, which might have limited the generalizability of our results. Second, multiple endoscopists performed EGD and EUS to evaluate SELs. To minimize inter-observer variations, the EGD and EUS images were reviewed by a single investigator. Third, EUS, which can provide valuable information for SEL assessment, was not performed in all cases. Fourth, not all 16 SELs with substantial changes underwent pathological confirmation; tissue acquisition was attempted in only five cases, four of which were diagnosed pathologically as two GISTs and two lipomas. Some patients refused further evaluation or were lost to follow-up. In other cases, technical difficulties were encountered owing to the acute angulation position of EUS or small lesion size. Finally, owing to the retrospective design of this study, certain endoscopic characteristics of SELs, including consistency and mobility, could not be evaluated using EGD images, and therefore, were not available.
In conclusion, the majority of duodenal SELs did not exhibit substantial interval changes during long-term follow-up. Nevertheless, regular endoscopic follow-up is recommended for cases with an initial size of 20 mm or larger, considering a possibility of malignancy.
KEY MESSAGE
1. Duodenal subepithelial tumors were identified in 0.39% of the cases (1,713/443,533). Among the 396 asymptomatic subepithelial tumors with a minimal follow-up period of 6 months, only 4.0% (16 cases) showed substantial changes.
2. Univariate Cox regression analysis demonstrated that an initial size ≥ 20 mm was a significant factor associated with substantial changes (p = 0.016).
3. While the majority of duodenal SETs may not exhibit substantial interval changes, regular follow-up with EGD may be necessary for cases with an initial size of 20 mm or larger, considering a possibility of malignancy.
Notes
CRedit authorship contributions
Seokin Kang: data curation, formal analysis, writing - original draft, writing - review & editing; Kwangbeom Park: data curation, formal analysis, writing - original draft, writing - review & editing; Do Hoon Kim: conceptualization, methodology, writing - original draft, writing - review & editing, supervision, project administration; Yuri Kim: data curation, formal analysis; Hee Kyong Na: methodology; Jeong Hoon Lee: methodology; Ji Yong Ahn: data curation; Kee Wook Jung: data curation; Kee Don Choi: data curation; Ho June Song: data curation; Gin Hyug Lee: data curation; Hwoon-Yong Jung: conceptualization, supervision
Conflicts of interest
The authors disclose no conflicts.
Funding
None