Drug retention of biologic and targeted synthetic disease-modifying antirheumatic drugs in Korean patients with seropositive rheumatoid arthritis
Article information
Abstract
Background/Aims
The aim of this study was to compare the short- and long-term retention rates of biologic and targeted synthetic disease-modifying anti-rheumatic drugs (b/tsDMARDs) in Korean patients with seropositive rheumatoid arthritis.
Methods
This study was conducted with 1,538 treatment courses of 1,063 patients, including adalimumab (n = 332), etanercept (n = 369), infliximab (n = 146), abatacept (n = 152), tocilizumab (n = 299), tofacitinib (n = 136), and baricitinib (n = 104), in patients with seropositive rheumatoid arthritis who started b/tsDMARD treatment between 2008 and 2020 at Seoul St. Mary’s Hospital. Discontinuation 1 and 3 years after the first prescription of each drug was investigated. Kaplan– Meier estimates of time to discontinuation were calculated to compare the difference in drug retention rate for each drug. Patient-level predictors of drug discontinuation were evaluated using a Cox proportional hazards model.
Results
The overall 1-year drug retention rate was from 60.1% for adalimumab to 90.0% for tofacitinib in the b/tsDMARD-naïve group, and from 55.2% for infliximab to 84.8% for tofacitinib in the b/tsDMARD-experienced group. The 3-year drug retention rate was from 36.9% for infliximab to 86.5% for tofacitinib in the b/tsDMARD-naïve group, and from 31.0% for infliximab to 65.4% for tocilizumab in the b/tsDMARD-experienced group. Drug discontinuation appeared to be affected by specific types of b/tsDMARDs.
Conclusions
Tocilizumab and tofacitinib are less commonly discontinued compared to tumor necrosis factor-α inhibitors at 1 and 3 years. Specifically, tofacitinib in the b/tsDMARD-naïve group and tocilizumab in the b/tsDMARD-experienced group showed the highest 3-year retention rates.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammatory polyarthritis. Biological disease-modifying anti-rheumatic drugs (bDMARDs) have been used for the treatment of RA for decades, with accumulating evidence for their effectiveness and safety [1,2]. Targeted synthetic DMARDs (tsDMARDs), represented by Janus kinase inhibitors (JAKi), are emerging as equivalent treatments to bDMARDs [3,4].
Multiple bDMARDs have been approved and prescribed in Korea, including etanercept (ETN), which was approved in 2004, adalimumab (ADA) in 2006, infliximab (IFX) in 2007, abatacept (ABT) in 2010, and tocilizumab (TCZ) in 2013. JAKi has recently been added as a main treatment strategy for RA and functions by inhibiting the JAK-STAT pathway [5-7]. Following bDMARDs, tofacitinib (TOF) has been used in Korea since 2015, while baricitinib (BAR), a specific JAK1/2 inhibitor, was approved in Korea in 2019. The number of officially approved and available b/tsDMARDs for the treatment of RA has increased. In real-world practice in Korea, JAKis are mainly considered in patients who have failed multiple bDMARDs for several reasons, including insurance issues, which is a major difference from randomized controlled trials (RCTs). For this reason, real-world data studies on the effectiveness of b/tsDMARDs are necessary, in addition to RCTs.
The drug retention rate can be a reliable parameter for overall treatment effectiveness in observational studies and is influenced by any one or a combination of drug efficacy, drug safety events, patient compliance, number of alternative treatment options available, and characteristics of the treated population [8,9]. Several cohort-based studies have compared the effectiveness of each drug using retention rates. Compared to tumor necrosis factor inhibitors (TNFi), higher drug retention rates of non-TNFi, such as rituximab, TCZ, and ABT, were confirmed in patients with RA who showed an inadequate response to TNFi within the Swiss RA cohort [10]. The drug retention rate of TOF did not differ significantly between bDMARD-naive and -experienced patients in the nationwide Korean College of Rheumatology Biologics registry [11].
The rheumatologist’s choice of b/tsDMARD may be affected by various factors, including patients’ demographic characteristics, severity of RA, concomitant use of conventional synthetic DMARDs (csDMARDs) or corticosteroids, and previous history of treatment with b/tsDMARDs. In a recent study from the BIOBADASER III registry, patients with RA with younger age, more comorbidities, and treatment with TNFi had a greater risk of b/tsDMARD discontinuation [12]. Based on nationwide data in Japan, the 18-month retention rate of bDMARDs was higher than that in Western countries, and significant differences in retention rates were revealed between the subgroups according to age, comorbidities, type of bDMARDs, and previous history of bDMARD use [13].
However, clinical data for patients with RA treated with JAKi, including BAR, in real-world clinical practice are rarely reported in Asia, including Korea. Therefore, the aim of this single-center, retrospective study was to clarify the retention rates and reasons for discontinuation of b/tsDMARDs in a real-world setting of Korean patients with seropositive RA, with a large number of treatment courses (TCs; n = 1,538, total patients = 1,063). Additionally, we attempted to confirm the difference in drug retention rate by subgroup according to the follow-up period (1 or 3 yr) and previous history of b/tsDMARD use (naïve or experienced). Finally, we analyzed the predictive factors for discontinuation of b/tsDMARDs.
METHODS
Patient selection and data collection
This study was conducted on patients with seropositive RA aged ≥ 18 years who were treated with b/tsDMARDs between 2008 and 2020 at Seoul St. Mary’s Hospital. These patients were required to fulfill the 1987 RA classification criteria of the ACR or the 2010 ACR/EULAR RA classification criteria in their medical records, as well as to be free of autoimmune diseases and not be involved in a clinical trial. A total of 1,538 TCs in 1,063 patients with seropositive RA who were prescribed bDMARDs (i.v. ABT, s.c. ADA, s.c. ETN, i.v. IFX, and i.v. TCZ) or tsDMARDs (BAR and TOF) were analyzed. Patients treated with biosimilar agents were excluded from the study. To have a follow-up period of at least 1 year, patients who started the drug before December 2020 were included and observed until December 2021. The need for informed consent was waived due to the retrospective nature of the study. This retrospective study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital of the Catholic University of Korea (KC21RISI0131).
Data on the dates of starting and discontinuation for each drug were included, and if discontinued, the main causes for discontinuation were collected by medical chart review. Discontinuation of treatment was defined as interruption of treatment for ≥ 3 months. Temporary discontinuations (restarting the same drug within < 3 months of the last administration) were not recorded as discontinuation. We collected baseline characteristics at drug initiation, including age, sex, RA disease duration, Charlson comorbidity index (CCI), positivity for rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (ACPA), ESR level, CRP level, concomitant use of csDMARDs or corticosteroids, and history of b/tsDMARD use. To compare the 1- and 3-year retention rates of the drugs, the patients were classified into two categories according to the year of treatment initiation: the patients who started each drug between 2008 and 2020 were designated as the “1-year follow-up group,” and those who started each drug between 2008 and 2018 were designated as the “3-year follow-up group.” In both groups, the TCs were divided by previous experience with b/tsDMARDs, naïve and experienced, because a previous study demonstrated differences in drug retention rates of bDMARDs and TOF between naive and experienced groups [14]. The reasons for drug discontinuation were classified as lack of efficacy, toxic adverse events (infusion/injection reaction, skin reaction, infection, malignancy, hepatotoxicity, hematologic adverse events, renal complications, etc.), non-toxic events (patient’s wish to discontinue, transfer to another hospital, cost/insurance, planning for pregnancy, etc.), and remission.
Statistical analysis
Continuous variables were initially assessed using the Kolmogorov–Smirnov test to define the normality of distribution. Student’s t-test and Mann–Whitney U test were used to compare continuous variables and are presented as mean ± standard deviation or median (interquartile range). Categorical variables are presented as frequencies and percentages. The χ2-test (Mantel–Haenszel χ2-test for more than 2 × 2 categorical data) or Fisher’s exact test was used to compare categorical variables. Cox proportional hazard regression analyses were conducted to determine the variables associated with b/tsDMARD discontinuation, providing hazard ratios (HRs) and 95% confidence intervals (CIs). The multivariable model included all significant variables with a p value < 0.2 in the univariate model. All analyses were performed using R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria). A p value < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
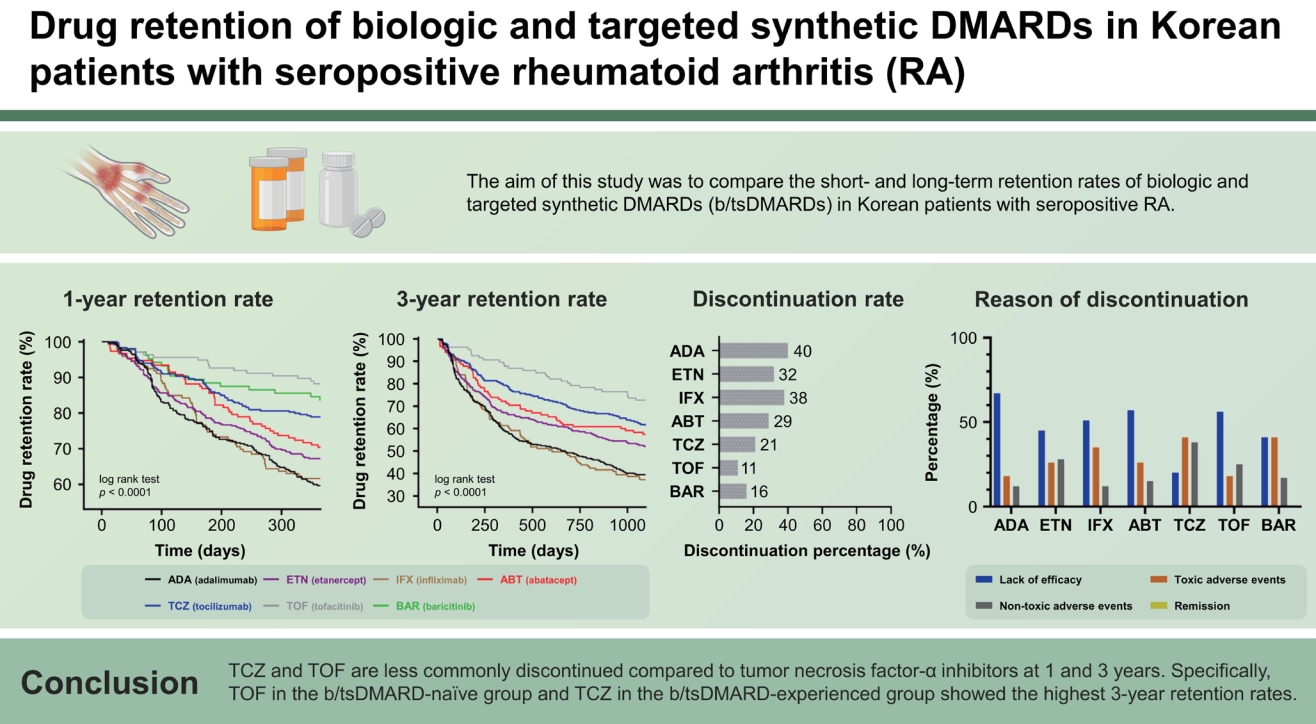
The baseline demographic and clinical characteristics of patients in the “1-year follow-up group” are shown in Supplementary Table 1. A total of 1,538 RA TCs (ADA: 332; ETN: 369; IFX: 146; ABT: 152; TCZ: 299; TOF: 136; BAR: 104) were included. Overall, the median age of the patients was 56.0 years, 86.2% were female, the median RA disease duration was 8.0 years, RF positivity was 94.6%, ACPA positivity was 93.1%, median ESR was 44.0 mm/h, and median CRP was 1.3 mg/dL. The proportion of concomitant medications was 87.0% for corticosteroids and 86.0% for methotrexate. Of the total TCs, 63.8% were b/tsDMARD-naïve. b/tsDMARDs were administered as the second b/tsDMARDs in 24.7% of the total TCs and as the third or more b/tsDMARDs in 11.4%. ETN was the most commonly prescribed drug. The distribution of prescribed b/tsDMARDs before and after 2015 was as follows: 2008–2014 (55.1%) and 2015–2020 (44.9%). After 2015, the frequency of non-TNFi or JAKi use was higher than that of TNFi. The baseline demographic and disease characteristics of the “3-year follow-up group” are also detailed in Supplementary Table 2.
The baseline characteristics of the b/tsDMARD treatment-naive and -experienced groups are shown in Table 1. In the 1-year follow-up group, the median disease duration in the experienced group was 11.0 years, which was longer than that in the naïve group. Naïve TCs were more likely to be treated with a combination of csDMARDs, including methotrexate. In both the naïve and experienced groups, TNFi was used more frequently than non-TNFi or JAKi, and TNFi was more commonly used as the first b/tsDMARD.
Retention rates of b/tsDMARDs during the 1- and 3-year follow-ups
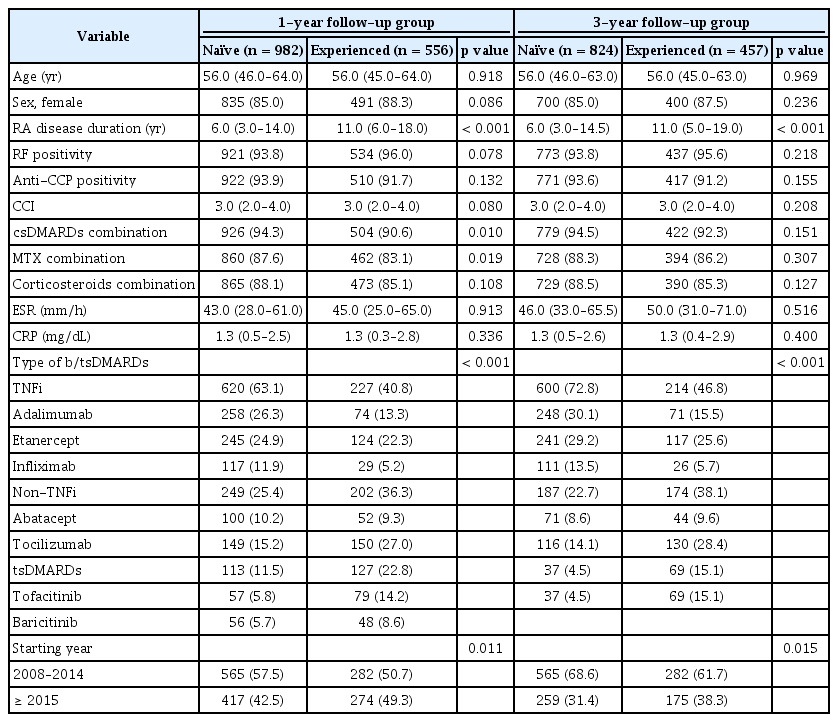
The 1- and 3-year retention rates of b/tsDMARDs from Kaplan–Meier curves in all TCs and by previous experience of b/tsDMARD treatment are shown in Figures 1 and 2. At the 1-year follow-up, the overall treatment retention was 59.6% for ADA, 67.2% for ETN, 61.6% for IFX, 70.4% for ABT, 78.9% for TCZ, 88.2% for TOF, and 83.7% for BAR (log-rank test, p < 0.0001). Pair-wise comparison analysis revealed a statistically significant difference in retention rates between TCZ-ADA, TCZ-ETN, TCZ-IFX, TOF-ADA, TOF-ETN, TOF-IFX, TOF-ABT, BAR-ADA, BAR-ETA, and BAR-IFX. The 1-year retention rates of b/tsDMARD-naïve patients were as follows: ADA (60.1%) ETN (69.8%), IFX (63.2%), ABT (76.0%), TCZ (73.2%), TOF (93.0%), and BAR (83.9%), with significant differences observed between TCZ-ADA, TOF-ADA, TOF-ETN, TOF-IFX, and BAR-ADA in pair-wise comparison analysis. For patients with previous treatment history of b/tsDMARDs, the 1-year retention rates were as follows: ADA (58.1%), ETN (62.1%), IFX (55.2%), ABT (59.6%), TCZ (84.7%), TOF (84.8%), and BAR (83.3%), with significant differences observed between TCZ-ADA, TCZ-ETN, TCZ-IFX, TCZ-ABT, TOF-ADA, TOF-ETN, TOF-IFX, and TOF-ABT in pair-wise comparison analysis.
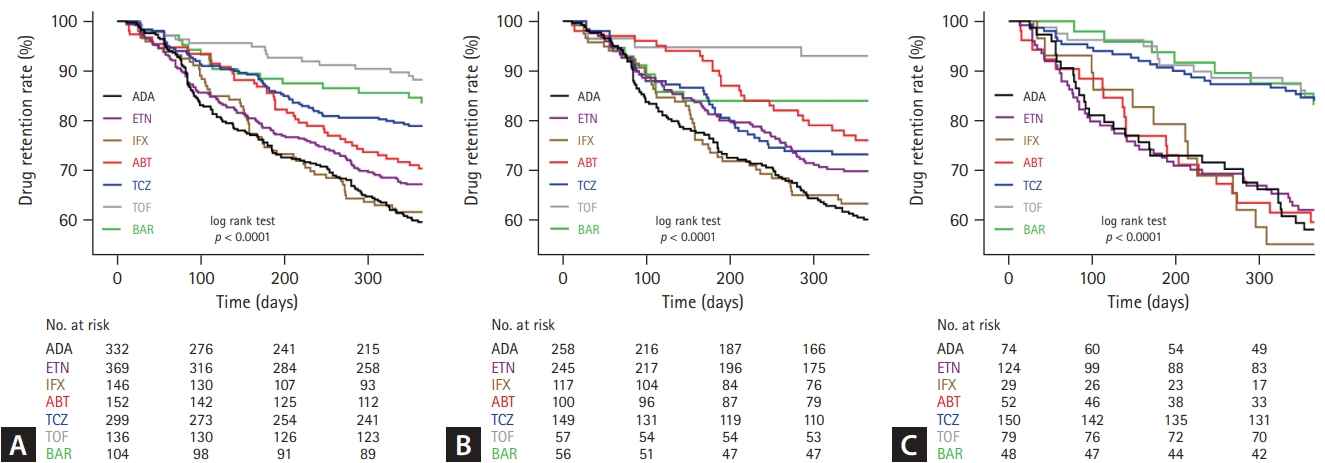
One-year retention rates of each drug in (A) total, (B) b/tsDMARD-naïve, and (C) b/tsDMARD-experienced patients with seropositive RA (Kaplan–Meier survival curves). b/tsDMARD, biological or targeted synthetic disease modifying anti-rheumatic drug; RA, rheumatoid arthritis; ADA, adalimumab; ETN, etanercept; IFX, infliximab; ABT, abatacept; TCZ, tocilizumab; TOF, tofacitinib; BAR, baricitinib.
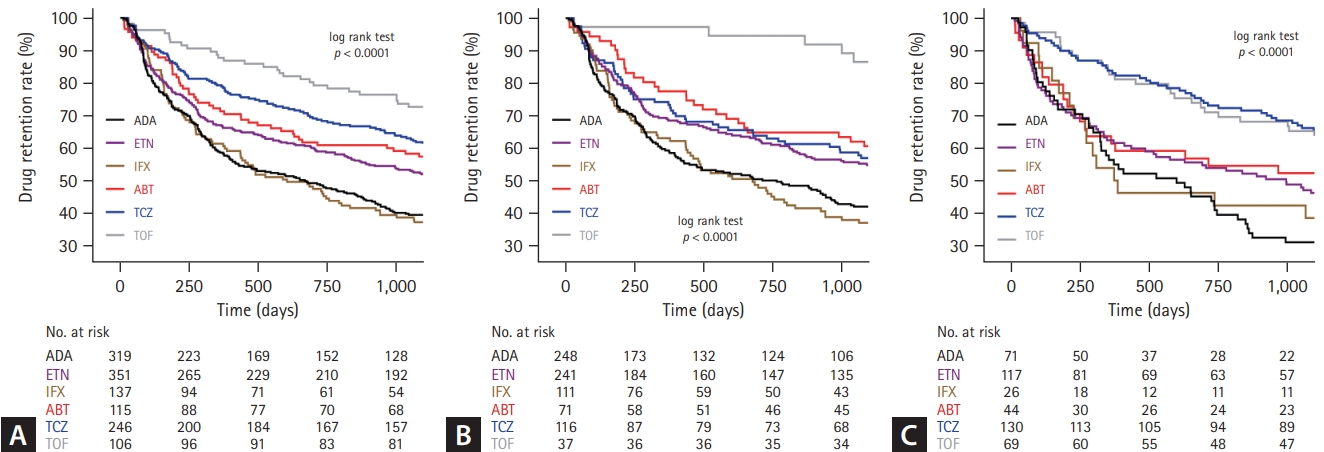
Three-year retention rates of each drug in (A) total, (B) b/tsDMARD-naïve, and (C) b/tsDMARD-experienced patients with seropositive RA (Kaplan–Meier survival curves). b/tsDMARD, biological or targeted synthetic disease modifying anti-rheumatic drug; RA, rheumatoid arthritis; ADA, adalimumab; ETN, etanercept; IFX, infliximab; ABT, abatacept; TCZ, tocilizumab; TOF, tofacitinib.
At the 3-year follow-up, the overall treatment retention rates were 39.5% for ADA, 52.0% for ETN, 37.2% for IFX, 57.4% for ABT, 61.4% for TCZ, and 71.7% for TOF (logrank test, p < 0.0001). Pair-wise comparison analysis revealed a statistically significant difference in retention rates between TCZ-ADA, TCZ-IFX, TOF-ADA, TOF-ETN, TOF-IFX, ADA-ETN, ADA-ABT, and ABT-IFX. The 3-year retention rates of b/tsDMARD-naïve patients were as follows: ADA (41.9%); ETN (54.8%) IFX, (36.9%); ABT (60.6%) TCZ, (56.9%); and TOF (86.5%), with significant differences observed between TOF-ADA, TOF-ETN, TOF-IFX, TOF-TCZ, ETN-IFX, and ABT-IFX in pair-wise comparison analysis. For patients with previous treatment history of b/tsDMARDs, the 3-year retention rates were as follows: ADA (31.0%), ETN (46.2%), IFX (38.5%), ABT (52.3%), TCZ (65.4%), and TOF (63.8%), with significant difference between TCZ-ADA, TCZ-ETN, TCZ-IFX, and TOF-ADA in pair-wise comparison analysis.
Reasons for b/tsDMARD discontinuation during the 1- and 3-year follow-ups
The reasons for 1- and 3-year discontinuation of treatment are summarized in Figures 3 and 4. During the 1-year follow-up period, 29.4% of the TCs were discontinued: ADA (40.4%), ETN (32.8%), IFX (38.4%), ABT (29.6%), TCZ (21.1%), TOF (11.8%), and BAR (16.3%). The most common reason for treatment discontinuation was lack of efficacy (50.9%). In particular, the proportion of lack of efficacy was highest in ADA (67.9%) and lowest in TCZ (20.6%). The proportion of drug discontinuation due to toxic adverse events was highest for TCZ (41.3%) and lowest for ADA (18.7%). In the 3-year follow-up group, 48.8% of the TCs were discontinued: ADA (60.5%), ETN (48.0%), IFX (62.8%), ABT (42.6%), TCZ (38.6%), and TOF (28.3%). In addition, a lack of efficacy was the most common reason for discontinuation. Of the toxic adverse events, the proportion of drug-induced reactions, including infusion/injection reactions and skin reactions, was highest in the IFX group (1-year follow-up group, 25.0%; 3-year follow-up group, 18.6%).
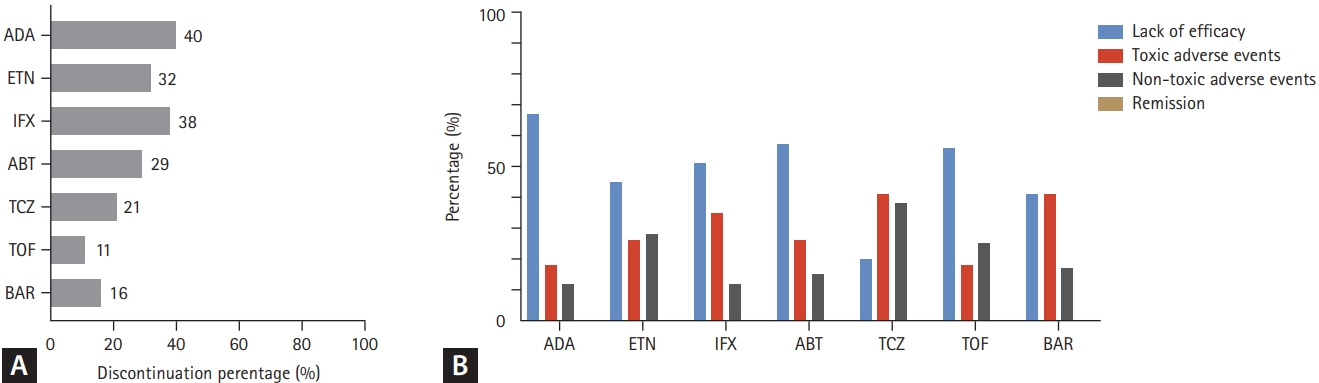
Reasons for treatment discontinuation of each b/tsDMARD in the 1-year follow-up group. (A) The proportion of total drug discontinuation. (B) The proportion of each reason for discontinuation. b/tsDMARD, biological or targeted synthetic disease modifying anti- rheumatic drug; ADA, adalimumab; ETN, etanercept; IFX, infliximab; ABT, abatacept; TCZ, tocilizumab; TOF, tofacitinib; BAR, baricitinib.
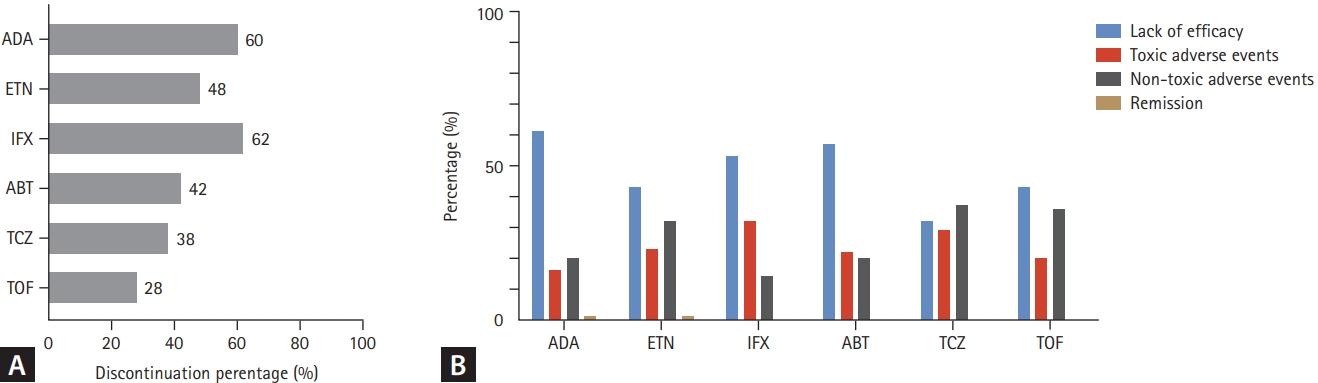
Reasons for treatment discontinuation of each b/tsDMARD in the 3-year follow-up group. (A) The proportion of total drug discontinuation. (B) The proportion of each reason for discontinuation. tsDMARD, biological or targeted synthetic disease modifying anti- rheumatic drug; ADA, adalimumab; ETN, etanercept; IFX, infliximab; ABT, abatacept; TCZ, tocilizumab; TOF, tofacitinib.
Predictors for retention/discontinuation in b/tsDMARDs naïve and experienced patients
Predictors for b/tsDMARD retention/discontinuation were explored by univariable and multivariable Cox regression, with results reported as HRs (1-year follow-up: Table 2, 3-year follow-up: Table 3). For b/tsDMARD-naïve TCs during the 1-year follow-up, an elevated ESR was the risk factor for b/tsDMARD discontinuation (HR, 1.64; 95% CI, 1.29 to 2.09; p < 0.001) at baseline. Furthermore, using ETN (HR, 0.72; 95% CI, 0.53 to 0.97; reference = ADA; p = 0.028), ABT (HR, 0.53; 95% CI, 0.34 to 0.82; reference = ADA; p = 0.005), TCZ (HR, 0.63; 95% CI, 0.44 to 0.91; reference = ADA; p = 0.014), TOF (HR, 0.15; 95% CI, 0.05 to 0.40; reference = ADA; p < 0.001), BAR (HR, 0.36; 95% CI, 0.18 to 0.72; reference = ADA; p = 0.004), and starting agent after 2015 (HR, 0.50; 95% CI, 0.39 to 0.64; p < 0.001) were protective factors for drug discontinuation in univariable analysis. However, in multivariable analysis, ETN (HR, 0.66; 95% CI, 0.49 to 0.89; reference = ADA; p = 0.007), TOF (HR, 0.24; 95% CI, 0.09 to 0.69; reference = ADA; p = 0.008), and starting agent after 2015 (HR, 0.63; 95% CI, 0.50 to 0.88; p = 0.007) were significant factors associated with the persistence of treatment. For b/tsDMARDs-experienced TCs during the 1-year follow-up, the risk factors for b/tsDMARDs discontinuation were older age (HR, 1.02; 95% CI, 1.00 to 1.03; p = 0.011), high CCI (HR, 1.54; 95% CI, 1.12 to 2.11; p = 0.008), and elevated ESR (HR, 1.51; 95% CI, 1.09 to 2.09; p = 0.014) at baseline. We found that TCZ (HR, 0.32; 95% CI, 0.19 to 0.55; reference = ADA; p < 0.001), TOF (HR, 0.30; 95% CI, 0.16 to 0.59; reference = ADA; p < 0.001), BAR (HR, 0.33; 95% CI, 0.15 to 0.72; reference = ADA; p = 0.005), and starting agent after 2015 (HR, 0.47; 95% CI, 0.34 to 0.65; p < 0.001) were protective factors for drug retention in univariable analysis. However, in multivariable analysis, TCZ (HR, 0.34; 95% CI, 0.20 to 0.59; reference = ADA; p < 0.001) and TOF (HR, 0.42; 95% CI, 0.20 to 0.90; reference = ADA; p = 0.025) were significant factors associated with continuing treatment.
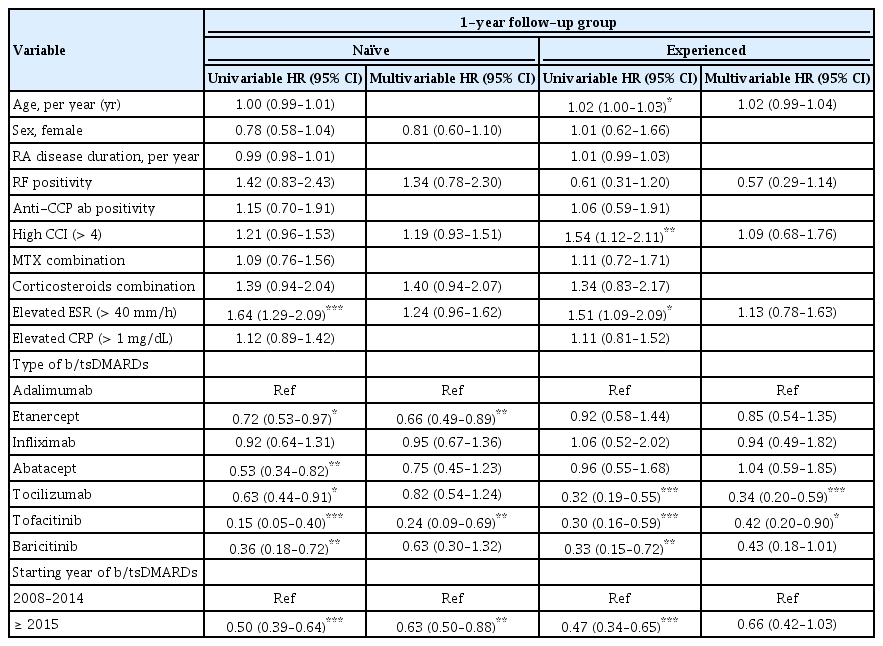
HR of treatment discontinuation in the b/tsDMARDs-naïve and b/tsDMARDs-experienced treatment courses (1-year follow-up group)
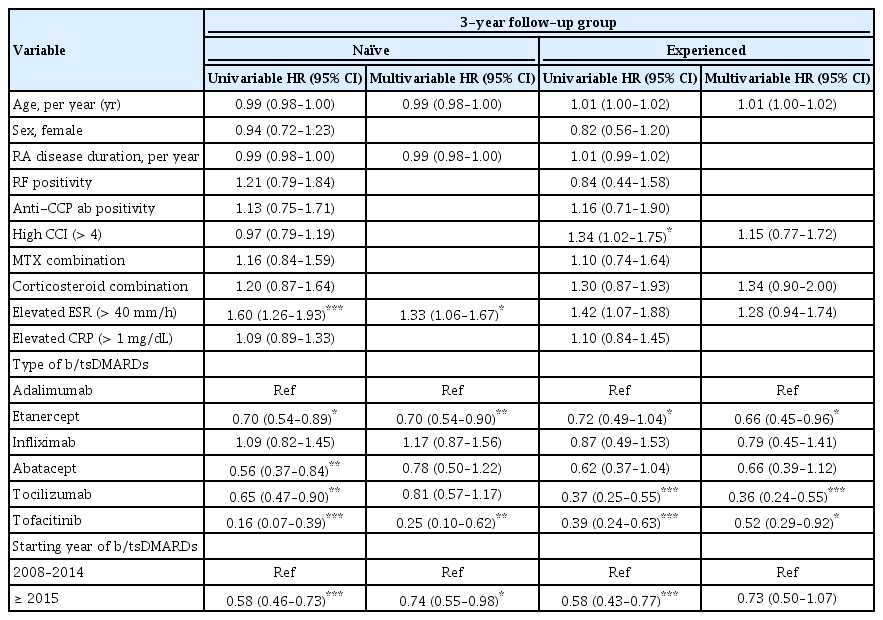
HR of treatment discontinuation in the b/tsDMARDs-naïve and b/tsDMARDs-experienced treatment courses (3-year follow-up group)
In the multivariable Cox regression analysis for the 3-year follow-up group, elevated ESR (HR, 1.33; 95% CI, 1.06 to 1.67; p = 0.014) at baseline, using ETN (HR, 0.70; 95% CI, 0.54 to 0.90; reference = ADA; p = 0.005), TOF (HR, 0.25; 95% CI, 0.10 to 0.62; reference = ADA; p = 0.003), and starting agent after 2015 (HR, 0.74; 95% CI, 0.55 to 0.98; p = 0.36) were independent factors associated with 3-year drug discontinuation in b/tsDMARD-naive-TCs. For experienced TCs, we found that ETN (HR, 0.66; 95% CI, 0.45 to 0.96; reference = ADA; p = 0.29), TCZ (HR, 0.36; 95% CI, 0.24 to 0.55; reference = ADA; p < 0.001), and TOF (HR, 0.52; 95% CI, 0.29 to 0.92; reference = ADA; p = 0.016) were protective factors for drug discontinuation.
DISCUSSION
With regard to the difference in treatment effectiveness between recently approved and existing drugs, the need to select more suitable drugs for patients among various classes of b/tsDMARDs is increasing. However, there is still a need for large-scale, single-center, real-world data on TOF and BAR, especially in Asia. In this study, we aimed to compare the drug retention rates of each b/tsDMARD and to investigate the factors associated with drug discontinuation during the 1- and 3-year follow-ups in patients with seropositive RA using a total of 1,538 TCs from our single-center, real-world data.
We observed the treatment patterns of b/tsDMARDs from the beginning of using the first TNFi to the present day in Korea. Overall, since 2008, the percentage of TNFi initiators (total: 55.0%, ADA: 21.6%, ETN: 23.9%, IFX: 9.5%) has been the highest among all types of b/tsDMARDs. However, since 2015, when there were more drug options, TCZ had the highest proportion (27.4%) of b/tsDMARDs, while the proportions of non-TNFi (43.4%) and JAKi (34.7%) were higher than that of TNFi (21.9%). These changes in treatment patterns based on the time point showed similar tendencies to those of other studies from the observational multicenter registry of Japan and the Korean Health Insurance Review and Assessment Service database [14,15].
Previous studies have reported several results related to the drug retention rate of bDMARDs. Considering the results obtained using data from the Swiss RA (SCQM-RA) registry, the type of bDMARD and the starting year of drugs were associated with drug retention [16]. In addition, higher drug retention rates of TCZ, TOF, and ABT as first- and second-line agents in patients with seropositive RA were confirmed in a recent study from the Korean Health Insurance Review and Assessment Service database [15]. Consistent with these results, our study also showed higher drug retention rates if the starting year of drug prescription was recent and if non-TNFi or JAKi was prescribed. Two factors may have contributed to these results. First, the increased availability of new treatment options in recent years has made rheumatologists change b/tsDMARDs in cases of previous treatment failure. Second, the effectiveness of alternative agents that replace TNFi affects the treatment of patients with RA with TNFi failure [10]. In addition, in our results of patients with previous treatment history of b/tsDMARDs, TCZ and TOF showed higher retention rates than TNFi in both the 1- and 3-year follow-up groups.
Reviewing our results for TNFi alone, the 1-year overall retention rates for ADA, ETN, and IFX were 59.6, 67.2, and 61.6%, while the 3-year overall retention rates were 39.5, 52.0, and 37.2%, respectively. Comparing the difference in the 1- and 3-year overall retention rates for each TNFi, the difference between ADA and IFX was higher than that of ETN. In addition, using ETN in patients with b/tsDMARDs was a statistically significant protective factor for drug discontinuation in the 3-year follow-up group but not in the 1-year follow-up group by Cox proportional hazard regression analysis. The formation of anti-drug antibodies may have contributed to these results. Anti-drug antibodies to ADA or IFX are associated with the efficacy of each drug [17]. According to recent systematic reviews, the incidence of anti-drug antibodies in IFX is the highest, followed by that of ADA, certolizumab pegol, golimumab, and ETN. The risk of developing anti-drug antibodies appears least common with use of ETN, a TNF receptor fusion protein, and most common with the use of IFX and ADA, a chimeric (mouse/human) antibody construct and a humanized monoclonal antibody, respectively [18,19]. In a meta-analysis, the concomitant use of csDMARDs (primarily MTX) reduced the incidence of anti-drug antibodies in patients with RA who were treated with ADA or IFX [20]. Based on these points, the proper selection of a specific TNFi and concomitant prescription of csDMARDs by a clinician improve treatment efficacy.
Lack of efficacy was the most common reason for discontinuation in each of the b/tsDMARDs. Among them, TCZ showed the lowest discontinuation rate due to lack of efficacy in both the 1- and 3-year follow-up groups. In addition, a Japanese cohort study showed lower discontinuation rates of TCZ due to a lack of efficacy compared to TNFi [14]. These results suggest that TCZ generally has better retention than TNFi in real-world settings. Among the toxic adverse events, drug-induced reaction was a reason for discontinuation that showed a significant difference between the drugs. In particular, IFX had the highest rate, which was due to the frequent occurrence of infusion reactions during intravenous infusion of IFX. Patients with RA receiving IFX treatment, who had previously experienced any allergic reactions, had a higher risk of infusion reactions [21]. A patient’s allergic history can be used as a checklist before initiating b/tsDMARDs. Additionally, in this study, IFX was only administered intravenously, but a subcutaneous agent of the same component was recently used in our hospital. The choice of subcutaneous or intravenous administration may also affect the drug retention rate.
A recent study from ORAL surveillance on TOF strongly implicated major adverse cardiovascular events (MACE) and malignancy as risks of JAKi use in elderly patients [22]. No cases of discontinuation of BAR or TOF due to MACE were observed, and discontinuation due to malignancy was observed in only one case in the 3-year follow-up group of TOF. Although our study was based on records prior to the latest warning for JAKi recommending the consideration of patient age and history of thromboembolism or malignancy, MACE or malignancies were rare reasons for drug discontinuation. However, long-term observation was not possible because we limited the follow-up period for each TC to 1 or 3 years. Therefore, it is necessary to investigate the cumulative incidence, not the reason for drug discontinuation, through studies with longer follow-up periods. It is also known that b/tsDMARDs, especially JAKi, increase the incidence of herpes zoster [23,24]. For Korean patients with RA treated with JAKi, a larger number of previously used bDMARDs and higher disease activity could be risk factors for herpes zoster [25]. In the real-world, if herpes zoster infection was observed, the drug was temporarily stopped, but it showed a pattern of restarting it in the near future (within 3 mo). Although the incidence of herpes zoster associated with JAKi may increase, cases leading to permanent discontinuation of JAKi are rare, with one case each of BAR and TOF. Discontinuation of b/tsDMARDs due to remission is very rare, accounting for less than 1% of all discontinuation cases. Indeed, we confirmed that even if remission was reached, the treatment was continued while adjusting the interval or tapering the dose without stopping the drug. This was consistent with the recent EULAR recommendations for the management of RA [26].
Our study has several limitations. First, the conclusions were obtained from single-center observational real-world data, which has intrinsic limitations compared to RCTs. Second, the period of observation was insufficient to obtain a proper conclusion regarding the long-term effectiveness and safety events. Third, several variables that may be important in the analysis were not collected, including the disease activity index represented by DAS28-ESR and radiographic damage to the hands at baseline. However, the present study also has a strength in that it is the first Korean study to evaluate the retention rates of b/tsDMARDs during the total period from the beginning of the first TNFi to the current era with JAKi by considering clinical backgrounds according to prior history of b/tsDMARDs, including BAR, in all spectra of patients with seropositive RA, including those with difficult-to-treat RA [27].
Our study showed that TNFi, non-TNF, and JAKi are safe and widely used in Korean patients with seropositive RA. The 1-year retention rates of TCZ, TOF, and BAR were higher than those of all or some TNFi in b/tsDMARD-naïve TCs. In b/tsDMARD-experienced TCs, TCZ and TOF showed higher 1-year retention rates than any of the evaluated TNFi. Additionally, the 3-year retention rate of TOF was the highest among b/tsDMARD-naïve TCs. In contrast, in b/tsDMARD-experienced TCs, TCZ showed higher 3-year retention rates than all TNFi. We identified that the risk of discontinuation is lower in patients who start b/tsDMARDs treatment after 2015. Additionally, the type of b/tsDMARDs and ESR levels at baseline may be factors associated with treatment discontinuation. These findings may help physicians to plan treatment strategies for the management of patients with seropositive RA in clinical practice.
KEY MESSAGE
1. Non-TNFi and JAKi are widely used as TNFi in Korean patients with seropositive RA.
2. TCZ and JAKi are less commonly discontinued at 1 and 3 years from the first prescription.
3. TOF in the b/tsDMARD-naïve group and TCZ in the b/tsDMARD-experienced group showed the highest 3-year retention rates.
Notes
CRedit authorship contributions
Bong-Woo Lee: investigation, data curation, formal analysis, writing - original draft, visualization; Jennifer Jooha Lee: methodology, validation, writing - review & editing, supervision, project administration; Wan-Uk Kim: conceptualization, resources, writing - review & editing, supervision, project administration, funding acquisition
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by National Research Foundation of Korea grants funded by the Ministry of Science, ICT and Future Planning (Grant 2015R1A3A2032927 to W.U. Kim).