Corticosteroid outcome may be dependent of duration of use in severe COVID-19
Article information
Abstract
Background/Aims
For patients hospitalized with coronavirus disease 2019 (COVID-19) who require supplemental oxygen, the evidence of the optimal duration of corticosteroid is limited. This study aims to identify whether long-term use of corticosteroids is associated with decreased mortality.
Methods
Between February 10, 2020 and October 31, 2021, we analyzed consecutive hospitalized patients with COVID-19 with severe hypoxemia. The patients were divided into short-term (≤ 14 days) and long-term (> 14 days) corticosteroid users. The primary outcome was 60-day mortality. We performed propensity score (PS) analysis to mitigate the effect of confounders and conducted Kaplan-Meier curve analysis.
Results
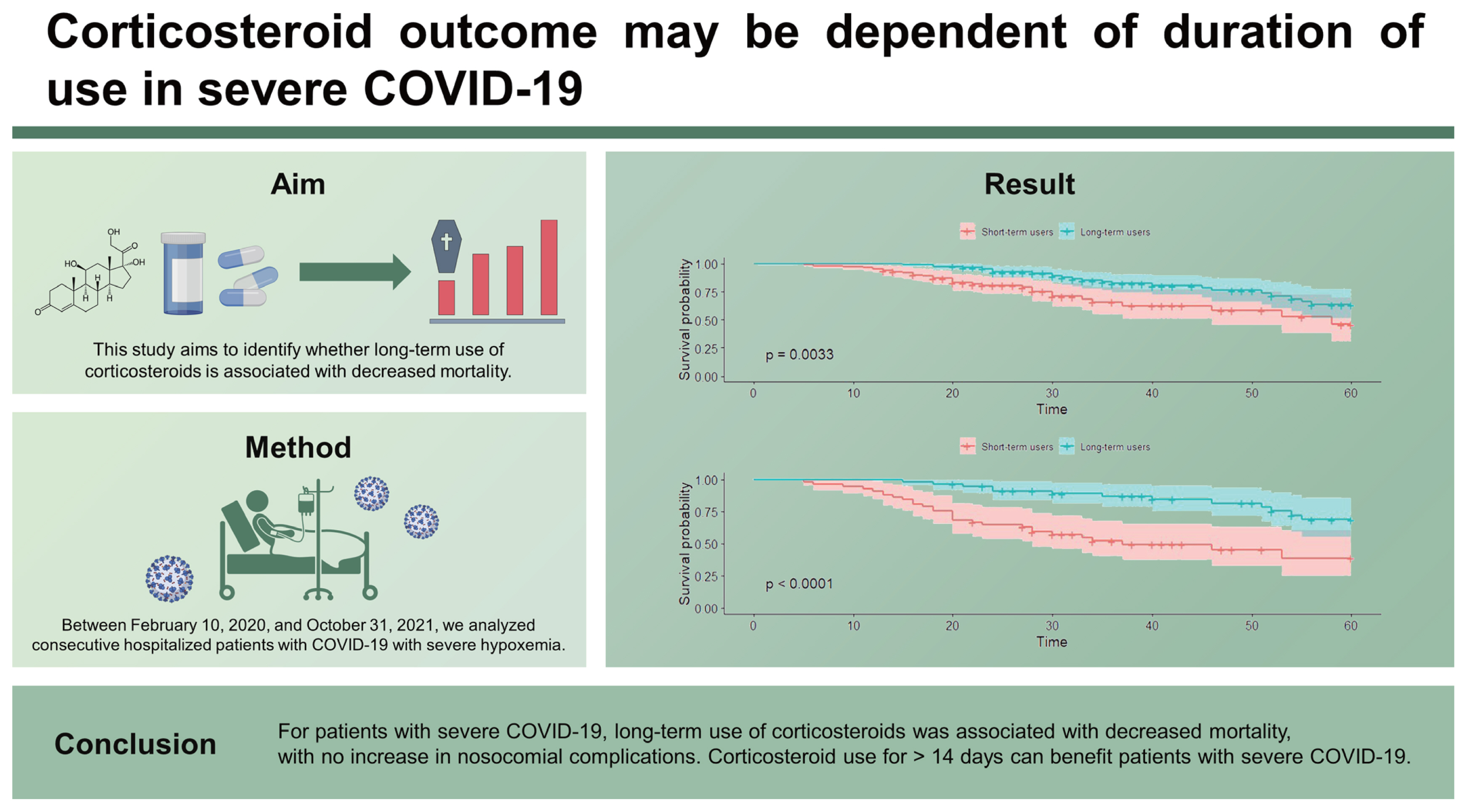
There were 141 (52%) short-term users and 130 (48%) long-term corticosteroid users. The median age was 68 years and the median PaO2/FiO2 at admission was 158. Of the patients, 40.6% required high-flow nasal cannula, 48.3% required mechanical ventilation, and 11.1% required extracorporeal membrane oxygenation. The overall 60-day mortality rate was 23.2%, and that of patients with hospital-acquired pneumonia (HAP) was 22.9%. The Kaplan-Meier curve for 60-day survival in the PS-matched cohort showed that corticosteroid for > 14 days was associated with decreased mortality (p = 0.0033). There were no significant differences in bacteremia and HAP between the groups. An adjusted odds ratio for the risk of 60-day mortality in short-term users was 5.53 (95% confidence interval, 1.90–18.26; p = 0.003).
Conclusions
For patients with severe COVID-19, long-term use of corticosteroids was associated with decreased mortality, with no increase in nosocomial complications. Corticosteroid use for > 14 days can benefit patients with severe COVID-19.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which enters the body primarily via the respiratory tract [1]. Hence, pulmonary manifestations are common, and progressive COVID-19 pneumonia can lead to hypoxemic respiratory failure and the need for mechanical ventilatory support [2]. Markedly elevated inflammatory markers, such as interleukins and C-reactive protein, indicate that the host inflammatory response may play a crucial role in organ injury in severe COVID-19 [2,3]. Therefore, diverse anti-inflammatory and immune modulators, such as corticosteroids [4], interleukin-6 inhibitors [5], and Janus kinase inhibitors [6], have been proposed.
Since the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial [4], the World Health Organization (WHO) recommends 6 mg of dexamethasone for up to 10 days for hospitalized patients with COVID-19 who require supplemental oxygen [7]. Corticosteroids, which have been widely used in the treatment of acute respiratory distress syndrome (ARDS), are relatively inexpensive and have acceptable side effects [8]. Furthermore, they are potentially helpful for mitigating thrombosis and hypercoagulability, other pathogeneses of severe COVID-19 [9]. Neutrophil extracellular traps are associated with hypercoagulability in COVID-19, and are known to be downregulated by dexamethasone [10].
Questions remain about the appropriate dose and duration of corticosteroid treatment. Corticosteroids have a dose-dependent property [11], and higher doses have been traditionally used in ARDS treatment [12]. Thus, the COVID STEROID 2 trial assessed the effects of 12 mg vs. 6 mg of dexamethasone on mortality [13]. Although the trial did not show statistically significant differences in mortality according to corticosteroid dose, secondary Bayesian analysis revealed that 12 mg of dexamethasone had a greater potential for benefit than harm compared to 6 mg [14]. Regarding duration, many clinical studies have used corticosteroids for up to 10 days [4,13,15–19], and WHO recommended that the total duration not exceed this timeframe [7]. However, there is little data on the duration of corticosteroid use and its correlation to mortality in patients with COVID-19. The survival benefit of long-term corticosteroid use is not apparent. This study aims to identify whether long-term use of corticosteroids is associated with decreased mortality.
METHODS
Study design and patients
This multi-center retrospective cohort study was conducted at seven tertiary university-affiliated hospitals in Korea. These hospitals have nationally designated treatment facilities and treat patients with severe COVID-19 requiring intensive care. Among the patients confirmed to have SARS-CoV-2, those who needed diverse life support devices were allocated to the COVID-19-intensive care unit (ICU). Consecutive hospitalized patients (≥ 18 years) between February 10, 2020 and February 28, 2021, with severe disease according to the WHO’s clinical severity scale were eligible for inclusion [20]. Due to the relatively persistent hospitalization of patients with COVID-19 at two hospitals (Chung-Ang University Hospital and Chungnam National University Hospital), we extended the recruitment period until October 31, 2021, at these hospitals. Patients were excluded if they did not receive oxygen therapy or were treated with only a nasal cannula or a reserve mask. We also excluded patients who had a do-not-intubate order, were not treated with corticosteroids, were transferred to another hospital for mechanical ventilation (MV) treatment, or who had missing clinical data.
This study was approved by the Institutional Review Board (IRB) of the Chung-Ang University Hospital (approval number 2103-009-19360), Chosun University Hospital (approval number 2021-04-002), Ulsan University Hospital (approval number 2021-03-015) and other local IRBs of all other participating centers. Informed consent was waived owing to the retrospective nature of this study.
Data collection and definitions
Demographic data, such as age and sex, were obtained through electronic medical records. The following data were recorded at admission: body mass index, time from symptom onset to admission, time from admission to intubation, comorbidities, vital signs, partial pressure of oxygen/fraction of inspired oxygen (PaO2/FiO2), and chest X-ray (stratified as normal, unilateral, bilateral, and multifocal). Treatment (remdesivir, vasopressor, renal replacement therapy, neuromuscular blocker, and prone positioning), corticosteroid-relevant data (initial type, initial dose, maximal dose, and total duration), and oxygen treatment (high-flow nasal cannula [HFNC], MV, or extracorporeal membrane oxygenation [ECMO]), duration of MV, length of hospital stay, and in-hospital mortality were recorded.
The WHO ordinal severity scale classified the severity of a COVID-19 infection using a range from 0 (not infected) to 8 (death) [20]. According to the scale, severe disease was defined as hospitalized patients that received at least one of the following treatments: non-invasive ventilation or HFNC (5), intubation with MV (6), or MV and ECMO (7).
Since some patients who were transferred from other hospitals had previously received corticosteroids, we considered the index date as the date of COVID-19 symptom onset (diagnosis date in the case of asymptomatic patients). The decisions to use corticosteroids as well as the dosage, duration, and tapering methods, were made by the attending physicians at each center. Several regimens for the use of corticosteroid were observed: some patients received 6 mg of dexamethasone for 14 days, and some received a gradual dose-tapering regimen after prolonged high-dose corticosteroids. We divided the patients into short-term users (≤ 14 days) and long-term users (> 14 days). The dose of each corticosteroid was expressed as the converted dose to the methylprednisolone-equivalent dose, and the approximate equivalent doses of corticosteroids were as follows [21]: 20 mg of hydrocortisone, 5 mg of prednisolone, 4 mg of methylprednisolone, and 0.75 mg of dexamethasone.
The primary outcome was 60-day mortality, and we identified 30-day mortality, in-hospital mortality, and nosocomial complications, such as bacteremia, and hospital-acquired pneumonia (HAP). Nosocomial bacteremia was defined as a positive blood culture via central line or peripheral line without clear evidence of contamination for more than 72 hours after ICU admission [22]. According to previous guidelines [23], HAP was defined as pneumonia that was not incubating at the time of hospital admission which occurred 48 hours or more after admission.
Statistical analysis
Categorical variables are expressed as the number (percentage), and continuous variables are expressed as the median (interquartile range, IQR). We used Pearson’s chi-square test or Fisher’s exact test to compare categorical variables, and Student’s t-test or Mann-Whitney U test to compare continuous variables. A McNemar’s chi-square test was used for categorical variables, and a paired t-test was used for continuous variables for the paired data after propensity score (PS) matching. Compared to short-term users, long-term users tended to have a lower PaO2/FiO2, higher severity scale, and higher maximal corticosteroid dose. Therefore, a PS matching analysis was performed to mitigate these confounders, where the nearest neighbor matching algorithm with a 1:1 ratio was the most used strategy. Candidate variables for inclusion in the PS analysis were variables with p < 0.1 in the univariate analysis and were considered clinically important parameters. PS was calculated using logistic regression with age, sex, body mass index, comorbidities, PaO2/FiO2, chest X-ray, remdesivir, vasopressor, renal replacement therapy, severity scale, and maximal corticosteroid dose (Supplementary Fig. 1). Subgroup analysis was performed for mechanically ventilated patients. After PS matching, Kaplan-Meier analysis was performed to assess the 60-day survival, and Kaplan-Meier curves by the duration of corticosteroids were compared using the log-rank test. Conditional logistic regression models were constructed based on the variables used in the PS analysis to determine the impact on outcomes according to corticosteroid duration. p values of < 0.05 were considered statistically significant, and we performed all statistical analyses using R statistical software version 4.1.2 (R Project for Statistical Computing).
RESULTS
Patient characteristics
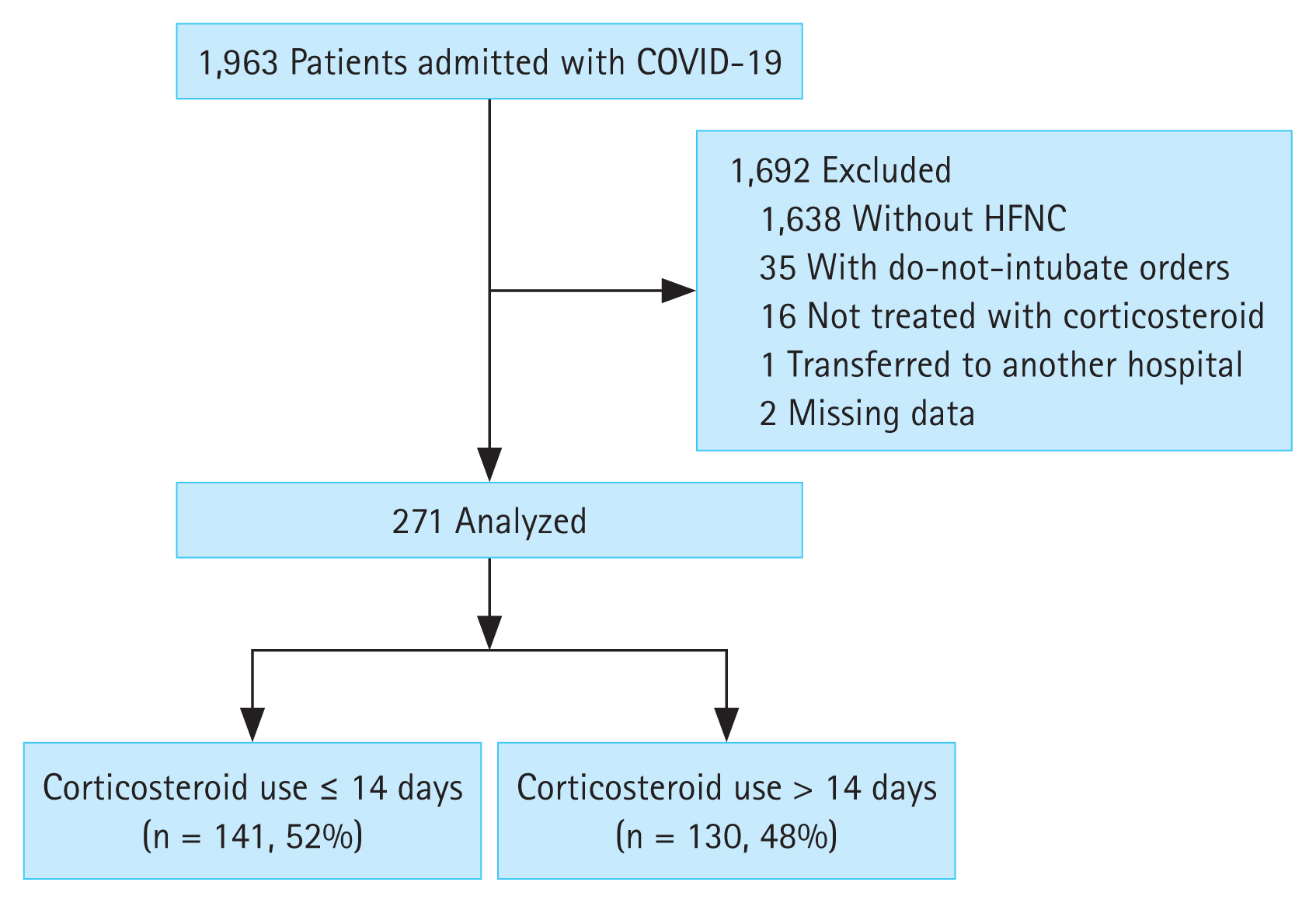
During the study period, 1,963 patients were admitted to the hospitals (Fig. 1). Among them, 1,692 were excluded: 1,638 did not receive HFNC, 35 had do-not-intubate orders, 16 were not treated with corticosteroids, one was transferred to another hospital, and two had missing data. Of the 271 patients who were treated with corticosteroids, short-term users (≤ 14 days) made up 52% of the study population (n = 141) and long-term users (> 14 days) made up 48% (n = 130).
Baseline characteristics of the patients with severe COVID-19 are presented in Table 1. The median age was 68 years (IQR, 58–75), and males made up 59.4% (n = 161) of patients. At admission, PaO2/FiO2 was 138 (IQR, 92–200), and severity scales were as follows: 40.6% required HFNC, 48.3% required MV, and 11.1% required ECMO. Dexamethasone was the most commonly used initial corticosteroid (86.7%, n = 235), and the time from symptom onset to initiation of corticosteroid treatment was 8 days (IQR, 4–11) (Supplementary Table 1). The initial methylprednisolone-equivalent dose was 32 mg (IQR, 32–32), and the maximal dose during hospitalization was 32 mg (IQR, 32–64).
Compared to short-term users, long-term users had significantly lower PaO2/FiO2 (118 [IQR, 80–168] vs. 158 [IQR, 100–220], p = 0.002), and vasopressor, MV, and ECMO were more frequently used in long-term users. The time from symptom onset to initiation of corticosteroid treatment was longer in the short-term users (9 days [IQR, 5–11] vs. 7 days [IQR, 4–9], p = 0.012), and the maximal dose was significantly higher in the long-term users (63 mg [IQR, 32–80] vs. 32 mg [IQR, 32–32], p < 0.001).
Outcomes and complications
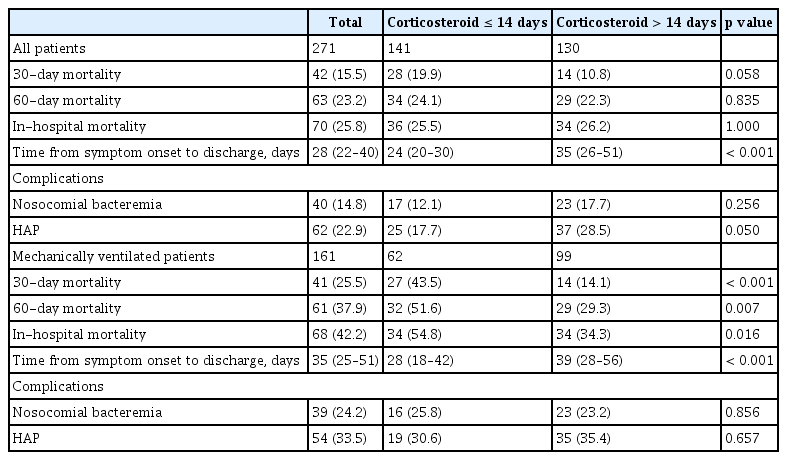
Overall 30-day and 60-day mortality rates were 15.5% and 23.2%, respectively (Table 2). Nosocomial bacteremia was present in 14.8% of patients, and HAP was present in 22.9%. There were no significant differences in the 30-day mortality, 60-day mortality, and nosocomial infections between short-term and long-term users.
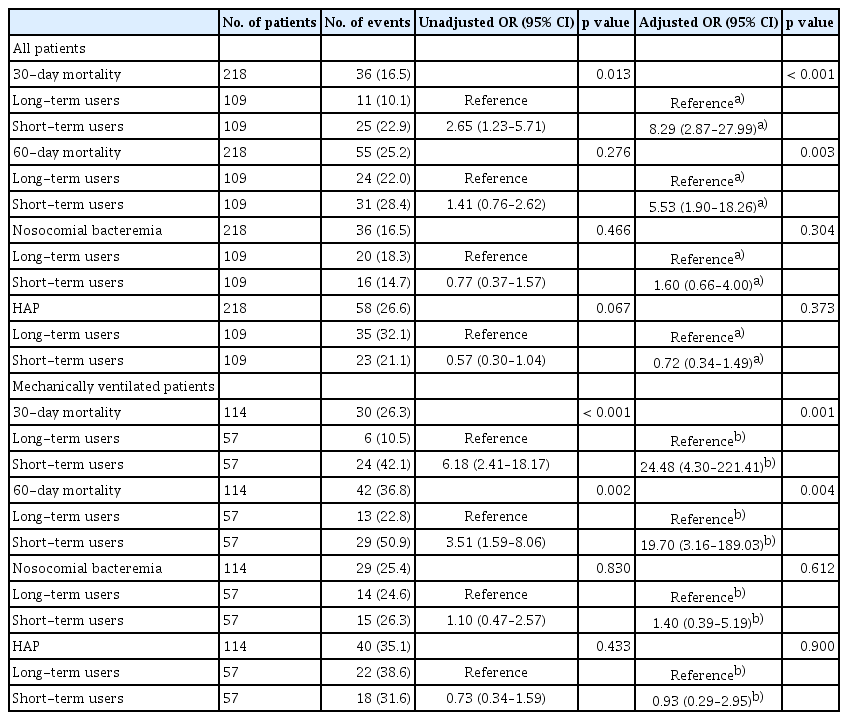
The Kaplan-Meier curve for 60-day survival in the PS-matched cohort showed that corticosteroid treatment for > 14 days was significantly associated with an increased survival rate (p = 0.0033) (Fig. 2). Crude and adjusted odds ratios (ORs) for outcome risks according to the duration of corticosteroid use are demonstrated in Table 3, Supplementary Table 2, and Supplementary Table 3. After adjustment of confounding factors, the probability of death at 60 days was 5.53 times (95% confidence interval [CI], 1.90–18.26; p = 0.003) higher in short-term users than in long-term users. The adjusted ORs for the nosocomial bacteremia and HAP were not significantly different between short- and long-term users.

Kaplan-Meier curve for 60-day survival in the propensity score (PS)-matched cohort. (A) All patients, (B) mechanically ventilated patients.
Subgroup analysis (mechanically ventilated patients)
Baseline characteristics of mechanically ventilated patients are shown in Supplementary Table 4. Short-term users were significantly older than long-term users (71.5 years [IQR, 62–78] vs. 68 years [IQR, 59–74], p = 0.012). However, neuromuscular blockers and ECMO were more frequently required for long-term users than short-term users. The maximal corticosteroid dose was higher in the long-term users (64 mg [IQR, 32–80] vs. 32 mg [IQR, 32–32], p < 0.001) (Supplementary Table 1).
Long-term users had a lower 60-day mortality than short-term users (29.3% vs. 51.6%, p = 0.007). After PS matching, the Kaplan-Meier curve showed that long-term use of corticosteroids was significantly associated with higher survival at 60 days (p < 0.0001) (Fig. 2). Moreover, the adjusted OR for the risk of 60-day mortality was 19.70 times (95% CI, 3.16–189.03; p = 0.004) higher in short-term users (Table 3).
DISCUSSION
In this multi-center, PS-matched cohort study, we evaluated the association between the duration of corticosteroid use and mortality among patients with severe COVID-19 disease. We found that long-term corticosteroid users had severer hypoxemia and required more mechanical ventilatory support. However, there were no significant differences in 30-day and 60-day mortality rates between short-term and long-term users. Rather, after adjusting for several confounders, the 60-day mortality rate was significantly lower in the long-term users. Moreover, the incidences of nosocomial bacteremia and HAP were not significantly higher in long-term users.
Recently, Moreno et al. [24] conducted a large COVID-19 ARDS cohort study in which 61% of 1,835 mechanically ventilated patients used steroids. They suggested that corticosteroid treatment had a biphasic time-dependent effect on mortality. Corticosteroid use improve mortality within 2 weeks, but after that, the mortality rate was higher than in corticosteroid non-users. We suggest that the negative impact on the reported long-term outcomes may be due to their study’s short-term use of corticosteroids. Their study population used corticosteroids for a median duration of 6 days (IQR, 3–10). This duration was much shorter than our study population, which had a median corticosteroid use of 14 days (IQR, 10–22). These results imply that long-term survival increases when a corticosteroid was used for approximately 2 weeks; however, the long-term survival decreases when the corticosteroid was stopped early. The effect of corticosteroids on SARS-CoV-2 infection is quite different from that of conventional viral infections, such as influenza. Corticosteroid use was not associated with reduced mortality in severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome and increased the mortality in influenza ARDS [25]; however, corticosteroids were beneficial for severe COVID-19 patients [26,27]. COVID-19 pneumonia has similar pathophysiology to classic ARDS, such as diffuse alveolar damage [28,29] and endothelial injury and coagulopathy [1,9]. Corticosteroid use longer than 7 days is associated with improved ARDS survival [30,31]. Corticosteroid in ARDS provides anti-inflammation in the acute phase and plays an important role in the optimal restoration of anatomy and function throughout the resolution phase [32]. Therefore, Meduri et al. [32] proposed a gradual dose-tapering regimen after 14 days of high-dose steroids after assessing the restoration of lung function. Given its similarity with ARDS, prolonged use of corticosteroids can be considered for severe COVID-19 pneumonia.
Another possible explanation for the effectiveness of long-term corticosteroid use in the treatment of COVID-19 is the similarity between COVID-19 and organizing pneumonia (OP). There have been several cases of OP after a COVID-19 infection [33–38]. In patients with COVID-19, corticosteroid use had to be maintained for up to 2 months after discharge due to persistent ground-glass opacity (GGO). Patients with diffused GGOs experienced severe dyspnea when corticosteroids were discontinued. Myall et al. [34] identified that 5% of hospitalized COVID-19 patients had an interstitial lung disease 4 weeks after discharge, and the predominant radiological phenotype of the interstitial lung disease was OP (59%). Kory and Kanne [37] suggested similar clinical presentations, radiography, and lung biopsies between COVID-19 and OP. Hypoxemia with an alveolar right-to-left shunt in OP is well tolerable and is similar to silent hypoxemia in COVID-19. Bilateral, lower lung-predominant consolidation or GGOs in the subpleural regions in a computed tomography scan are also collective findings. Although we could not examine the incidence of OP in our cohort, 82% (223/271) of patients showed bilateral or multifocal infiltration on a chest X-ray. Corticosteroids are effective in OP treatment, and the recommended duration is 1–3 months [39]. Moreover, relapses are common after stopping corticosteroid treatment for OP [40]. Given the possibility of severe COVID-19 pneumonia being OP, prolonged corticosteroid treatment in severe COVID-19 should be considered.
Despite the potential positive effect of prolonged corticosteroid therapy, concerns remain. COVID-19 is associated with various complications, such as nosocomial and opportunistic fungal infections [7]. Fungal infections can worsen the prognosis of COVID-19 [41]. However, in a previous meta-analysis for ARDS, corticosteroids did not increase nosocomial infections [42]. Similar to this finding, our study results revealed that long-term use of corticosteroids was not associated with increased nosocomial bacteremia or HAP in mechanically ventilated patients (p = 0.856 and p = 0.657, retrospectively). A logistic regression analysis performed on the PS-matched cohorts showed similar results. Nonetheless, co-infections and other complications, such as myopathy, hyperglycemia, or gastrointestinal bleeding, should be carefully monitored during corticosteroid therapy [43]. As dexamethasone can increase clotting factors and fibrinogen [44], it is necessary to identify how the long-term use of corticosteroids affects thrombotic complications in patients with COVID-19.
This study has some limitations. First, this is a retrospective study, and some variables, such as sequential organ failure assessment scores, were not obtained. Second, although we performed PS matching with clinically important variables, there may be residual confounders. High doses of corticosteroids, which are potentially beneficial, were used significantly more often in the long-term users. Even after PS matching, the maximal dose was significantly higher in the long-term users. Moreover, it was difficult to adjust the duration of the high doses. Third, a significant standardized mean difference (> 0.1) was observed after PS matching in both overall and mechanically ventilated patients. This may be due to the large number of variables used for the PS matching. Although the options can be changed to improve matching performance, there was a problem that the sample size was too small. Therefore, while maintaining a moderate sample size, the matching method with the default option was finally applied. Furthermore, the results of the analysis after PS matching were not that much different from those before PS matching. Fourth, we did not present the data for causes of death. Therefore, it is uncertain whether the higher mortality in short-term users was due to aggravated respiratory failure after corticosteroid discontinuation or withholding life-support due to the patients’ age. Many questions remain regarding the long-term use of corticosteroids, such as how long they should be used and in which subgroups this treatment is more effective. Mishra and Mulani [45] suggested long-term use for prophylaxis in patients at risk for post-COVID-19 fibrosis. However, it is difficult to clinically determine who is at risk for lung fibrosis. Our study revealed that long-term use of corticosteroids in mechanically ventilated patients is associated with greater survival. The long-term use of corticosteroids for patients with COVID-19 requiring MV is warranted. Further prospective randomized controlled trials are needed to elucidate the impact of the long-term use of corticosteroids in patients with severe COVID-19 infection.
In patients with severe COVID-19, corticosteroid treatment for > 14 days was associated with decreased mortality rate compared to use for ≤ 14 days. Nosocomial complications including bacteremia and HAP did not increase in long-term corticosteroid users. Considering the risks and benefits of prolonged corticosteroid therapy, it can be utilized for severe COVID-19 patients requiring HFNC or MV.
KEY MESSAGE
1. For patients with severe COVID-19, long-term use of corticosteroids was associated with decreased mortality, with no increase in nosocomial complications. Corticosteroid outcome may be dependent of duration of use in severe COVID-19.
Notes
CRedit authorship contributions
Jin Hyoung Kim: conceptualization, data curation, writing - original draft, writing - review & editing; Yong Sub Na: conceptualization, data curation, writing - original draft, writing - review & editing; Song-I Lee: data curation; Youn Young Moon: formal analysis; Beom Seuk Hwang: formal analysis; Ae-Rin Baek: data curation; Won-Young Kim: data curation; Bo Young Lee: data curation; Gil Myeong Seong: data curation; Moon Seong Baek: conceptualization, data curation, formal analysis, writing - original draft, writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
None