Pulmonary hypertension in systemic lupus erythematosus: an independent predictor of patient survival
Article information
Abstract
Background/Aims
We investigated whether transthoracic echocardiography-suspected pulmonary hypertension (PH) affects survival in systemic lupus erythematosus (SLE) patients and examined factors associated with PH occurrence and survival.
Methods
This retrospective single-center study included 154 Korean SLE patients fulfilling the American College of Rheumatology criteria (January 1995 to June 2013). Student t test, Mann-Whitney U test, Kaplan-Meier curves, and log-rank tests were used for comparisons.
Results
A total of 35 SLE patients with PH (SLE/PH+) and 119 without PH (SLE/PH-) were analyzed. Higher percentages of interstitial lung disease, Raynaud's phenomenon (RP), World Health Organization functional classification III/IV, and cardiomegaly were found in SLE/PH+ compared to SLE/PH-. Furthermore, the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index was significantly higher in SLE/PH+ (2.46 ± 1.245 vs. 1.00 ± 1.235), whereas survival rates were significantly higher in SLE/PH- in log-rank tests (p = 0.001). In multivariate analysis, the adjusted mortality hazard ratio (HR) for SLE/PH+ patients was 3.10. Subgroup analysis demonstrated a higher percentage of lupus nephritis in the SLE/PH+ patients who died (p = 0.039) and low complement-3 levels (p = 0.007). In univariate analysis, the mortality HR for SLE/PH+ patients with lupus nephritis was 4.62, whereas the presence of RP decreased the mortality risk in multivariate analysis; adjusted HR, 0.10.
Conclusions
PH is an independent factor predicting survival in SLE patients. The presence of lupus nephritis resulted in an increased trend for mortality, whereas coexistence of RP was associated with a better survival prognosis in SLE/PH+ patients.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disorder involving various organ systems, including the pulmonary system. Transthoracic echocardiography (TTE)-suspected pulmonary hypertension (PH) is a fairly frequent pulmonary manifestation of SLE. Since Perez and Kramer [1] first reported four PH cases in SLE in 1981, many reports of PH in SLE have documented its clinical manifestations, associated factors, and predictors of mortality. The prevalence of PH in SLE is estimated to be 1.8% to 14% [1,2,3,4,5,6,7,8,9]. Furthermore, PH in SLE has been reported to be associated with poor prognosis by several studies [3,5,9,10]. In one study, PH was the third leading cause of mortality among SLE patients [11], while another report demonstrated that PH was the most frequent cause of mortality among SLE-related deaths [12]. These results demonstrated the importance of PH in SLE in relation to survival.
To date, several factors have been reported to be associated with SLE-related PH. Several studies reported that Raynaud's phenomenon (RP), antiphospholipid antibody (aPL), anti-U1 ribonucleoprotein (anti-U1RNP), and serositis may contribute to the development of PH or be associated with its pathogenesis in SLE [2,3,5,9,13,14]. However, the associations between these factors and PH in SLE remain unclear, because of the small sample sizes of these studies, with the majority drawing conclusions from a case series.
We investigated whether PH is an independent risk factor for mortality in SLE. Furthermore, we investigated the clinical characteristics and risk factors for PH in SLE patients. Finally, we determined the predictors of mortality in SLE patients with PH.
METHODS
Patients and controls
We retrospectively reviewed the medical records of SLE patients treated at Seoul St. Mary's Hospital, Korea, a tertiary care university hospital and referral center, between January 1995 and June 2013. Of the 2,432 patients who fulfilled the 1982 revised criteria for the classification of SLE [15], 160 patients were identified to have undergone echocardiography at least once. The patients had undergone two-dimensional and Doppler echocardiogram for one of the following reasons: unexplained dyspnea; evaluation of chest discomfort or pain; clinical suspicion of pericardial effusion; a loud audible pulmonic second heart sound on auscultation; prominent pulmonary conus on chest radiographs; or evaluation for syncope. Subjects were excluded from analysis when they had multiple-organ failure, overlapping syndrome, incomplete clinical data, or died within 1 month of echocardiography. To exclude patients with PH induced by interstitial lung disease (ILD), patients with ILD were included only when they had no interval changes for 6 months before and after PH diagnosis in pulmonary function tests and high-resolution computed tomography. From the 160 SLE patients, six were excluded according to the described exclusion criteria, while 154 were analyzed.
This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital.
Definitions and variables
PH was defined as a right ventricular systolic pressure (RVSP) > 40 mmHg [16,17]. RVSP was estimated as the sum of the systolic right-atrial-ventricular pressure gradient (PG) and the right atrial pressure. The PG was calculated from the modified Bernoulli equation using the tricuspid regurgitation maximal velocity (TRVmax): PG = 4 × TRVmax2. The TRVmax was estimated from the Doppler echocardiogram. The right atrial pressure was presumed to be 5 mmHg. Patients with an RVSP > 50 mmHg were defined as severe PH patients [18].
Demographic, clinical, and laboratory profiles
The demographic characteristics (age, sex, and disease duration), echocardiography findings, and radiological, clinical, laboratory, and autoantibody data of the patients were obtained at the time of echocardiography. The systemic lupus erythematosus disease activity index (SLE-DAI) scores were calculated retrospectively by reviewing patient records. In addition, the Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) damage index and World Health Organization (WHO) functional class were obtained.
Statistical analysis
Comparisons of continuous values between two groups were performed using Student t test or Mann-Whitney U test. Results are presented as the means ± standard deviation. Categorical variables, including the proportions between groups, were compared using chi-square test or Fisher exact test. A value of p < 0.05 was considered to indicate statistical significance. Survival rates were determined using the Kaplan-Meier method and compared between two groups using the log-rank test. In survival analyses, the time scale was defined as the time from the first echocardiogram. Factors associated with survival were determined using univariate Cox proportional hazards regression analysis. Variables with p ≤ 0.2 on univariate analysis were included in multivariate analysis. All of the tests were performed using the SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Comparisons of baseline demographic, clinical, laboratory, and autoantibody profiles between the TTE-investigated and control SLE patients.
A total of 154 patients underwent TTE during the study period. Among the remaining 2,272 SLE patients enrolled, 960 age- and sex-matched patients were selected randomly as controls to compare baseline demographic, clinical, and laboratory profiles. The SLE group that underwent TTE displayed higher percentages of lupus nephritis, neuropsychiatric lupus, and serositis and elevated anti-Ro/La levels, whereas the control group showed a higher aPL-positive frequency (Table 1). The time period between diagnosis and the final follow-up or mortality in SLE patients who underwent TTE and control patients was 147.97 ± 82.78 and 144.12 ± 74.57 months, respectively.
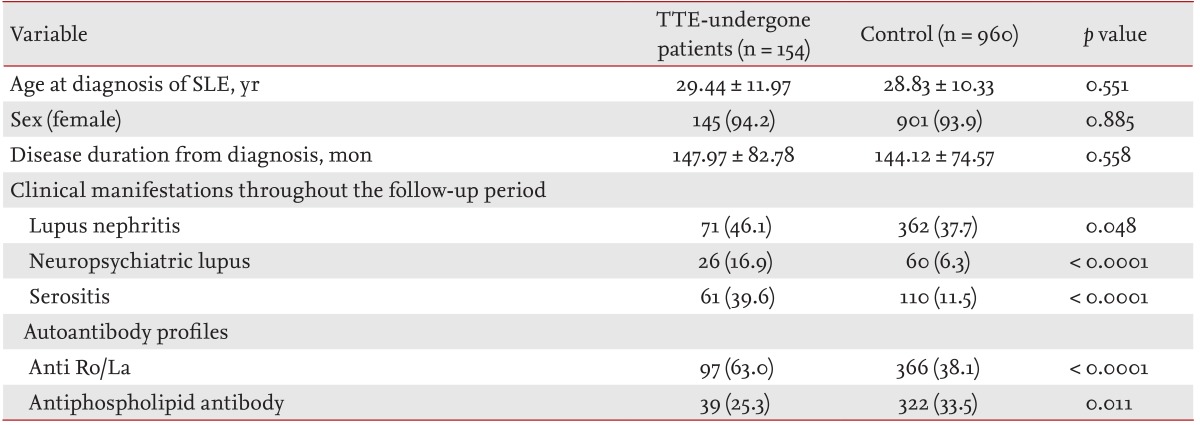
Comparison of baseline demographics, clinical, and laboratory data between the TTE-undergone and control SLE patient
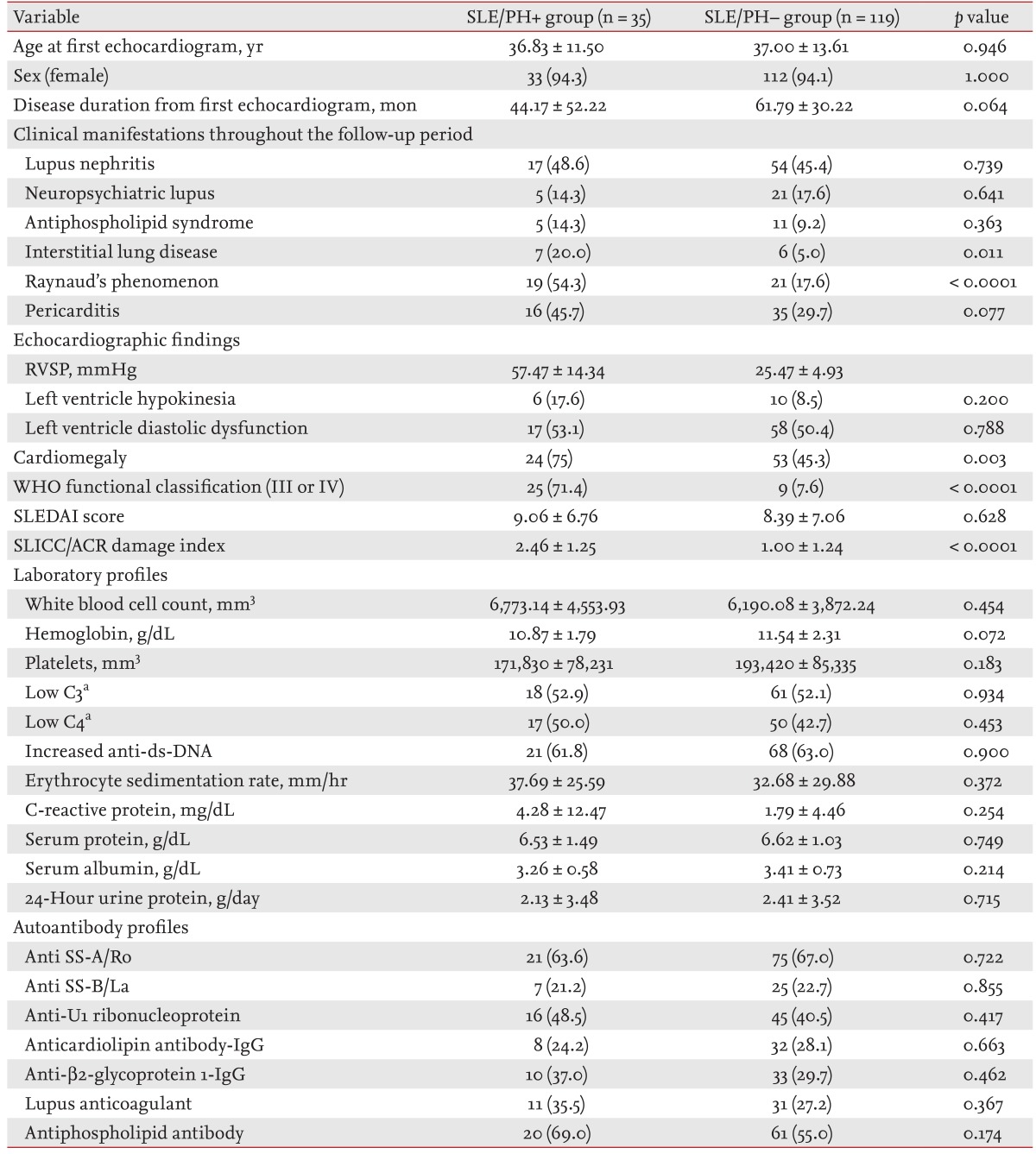
Comparisons of baseline demographic, clinical, laboratory, and autoantibody profiles between SLE patients with and without PH
Among the 2,432 enrolled SLE patients, we identified 35 subjects with PH (SLE/PH+ group) and 119 without PH (SLE/PH- group). Of the 35 SLE/PH+ patients, six were lost to follow-up, and repeat follow-up echocardiograms were available in 25 of the remaining 29 patients. Right heart catheterization (RHC) was performed in four patients of the SLE/PH+ group based on clinical judgment. The baseline demographic characteristics and the laboratory and echocardiogram profiles of the SLE/PH+ and SLE/PH- groups are presented in Table 2. All of the patients in the present study were Korean. The mean age at the first echocardiogram in the SLE/PH+ and SLE/PH- groups was 36.83 ± 11.50 and 37.00 ± 13.61 years, respectively (p = 0.946). The time period between the first echocardiogram and the final follow-up or mortality in the SLE/PH+ and SLE/PH- groups was 44.17 ± 52.22 and 61.79 ± 30.22 months, respectively (p = 0.0064). Echocardiogram-determined RVSP in the SLE/PH+ and SLE/PH- groups was 57.47 ± 14.34 and 25.47 ± 4.93 mmHg, respectively. Regarding clinical manifestations, significantly higher percentages of ILD (20.0% vs. 5.0%, p = 0.011), RP (54.3% vs. 17.6%, p < 0.0001), WHO functional classification III or IV (71.4% vs. 7.6%, p < 0.0001), and cardiomegaly (75.0% vs. 45.3%, p = 0.003) were observed in the SLE/PH+ group. Furthermore, the SLICC/ACR damage index was significantly higher in the SLE/PH+ group (p < 0.0001), whereas the SLEDAI score was not significantly different (p = 0.628). No significant differences were observed in the laboratory findings or autoantibody profiles between the two groups (Table 2).
Causes of mortality and survival rates in the SLE/PH+ and SLE/PH- groups
In the SLE/PH+ and SLE/PH- groups during the follow-up period, 11 patients (31.4%) and 13 (10.9%), respectively, died. In the SLE/PH+ group, the causes of mortality included bleeding in four patients, infection in three patients, heart failure in three patients, and PH itself in one patient. The causes of mortality in the SLE/PH- group comprised infection in five patients, bleeding in five patients, myocarditis in one patient, thrombotic thrombocytopenic purpura in one patient, and acute respiratory distress syndrome caused by chemotherapy for secondary lymphoma in one patient. The survival rates at 1, 3, and 5 years in the SLE/PH+ group were 83.7%, 79.0%, and 60.2%, respectively, whereas in the SLE/PH- group they were 94.0%, 92.2%, and 88.1%, respectively (Fig. 1). Therefore, the survival rate was significantly higher in the SLE/PH- group by log-rank test (p = 0.001).

Survival curves with respect to the presence of pulmonary hypertension (PH) in systemic lupus erythematosus (SLE) patients. The cumulative survival rates in the SLE/PH+ and SLE/PH- groups were compared during the follow-up period. The survival rate was significantly lower in the SLE/PH+ group compared to the SLE/PH- group using the log-rank test (p = 0.001).
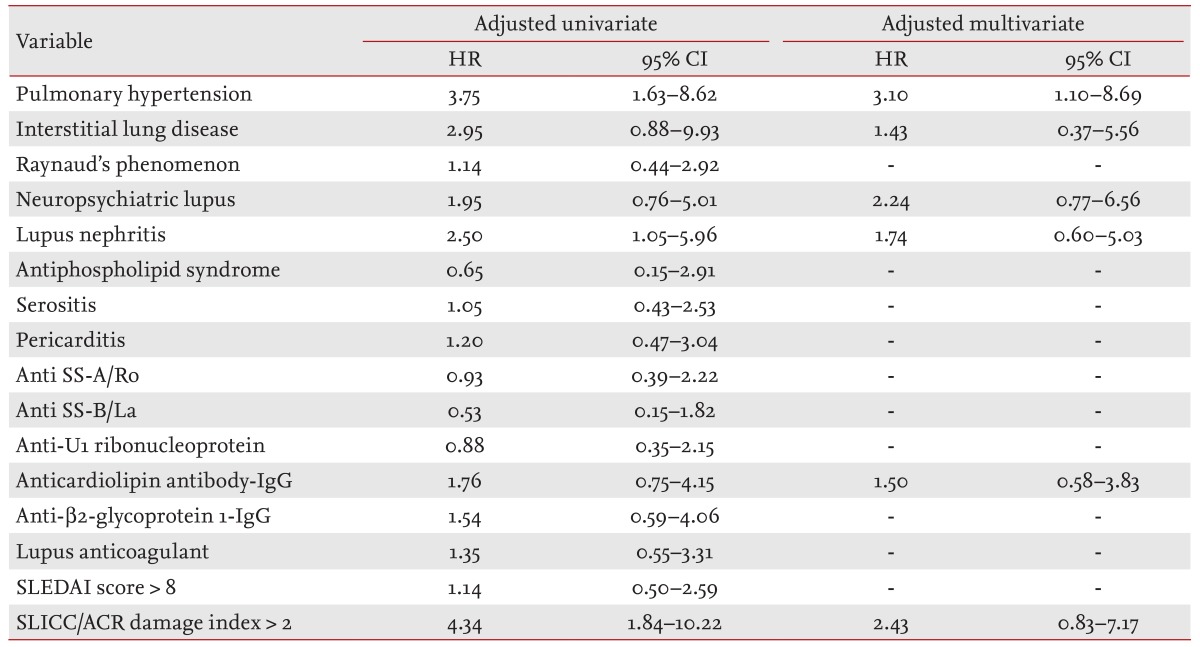
Univariate Cox proportional hazard regression analysis demonstrated that variables significantly associated with mortality included the presence of PH (p = 0.002) and lupus nephritis (p = 0.039), and a SLICC/ACR damage index > 2 (p = 0.001) (Table 3). In multivariate analysis, the presence of PH persisted as an independent risk factor for mortality (adjusted hazard ratio [HR], 3.10; 95% confidence interval [CI], 1.10 to 8.69; p = 0.032).
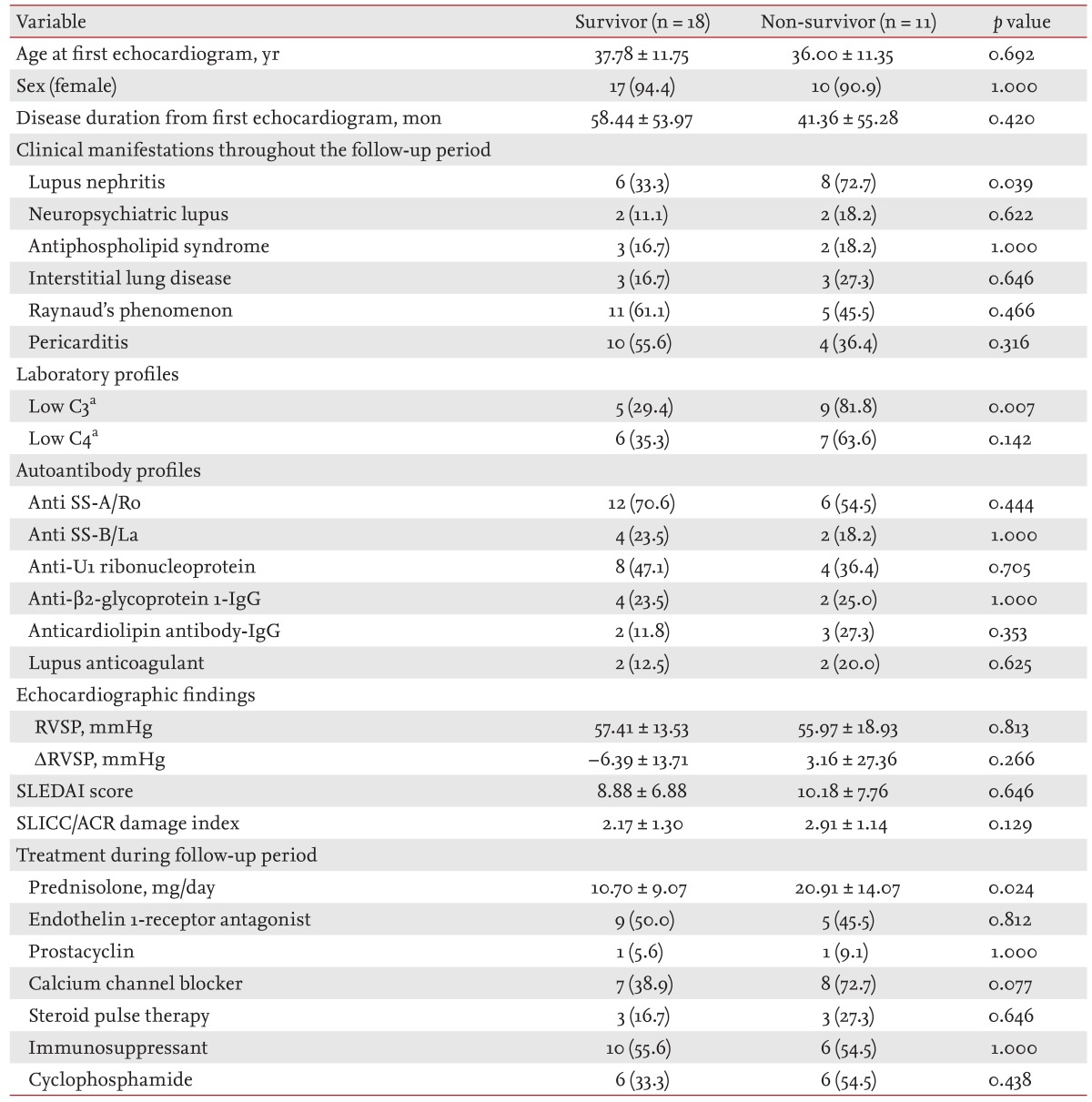
Subgroup analysis of SLE/PH+ patients
Table 4 shows a comparison of the clinical characteristics, SLEDAI score, SLICC/ACR damage index, laboratory results, autoantibody levels, echocardiogram profiles, and pharmacologic therapy between the SLE/PH+ patients who survived and those who died. The baseline clinical characteristics did not differ significantly between these two groups, except for a higher frequency of lupus nephritis in the patient group who died (p = 0.039). The incidence of low complement-3 levels was higher in the SLE patients who had died (p = 0.007), whereas the SLEDAI score and SLICC/ACR damage index were not significantly different. The patients who had died had received a higher daily prednisolone dosage (20.91 ± 14.07 mg/day vs. 10.69 ± 9.07 mg/day, p = 0.024).
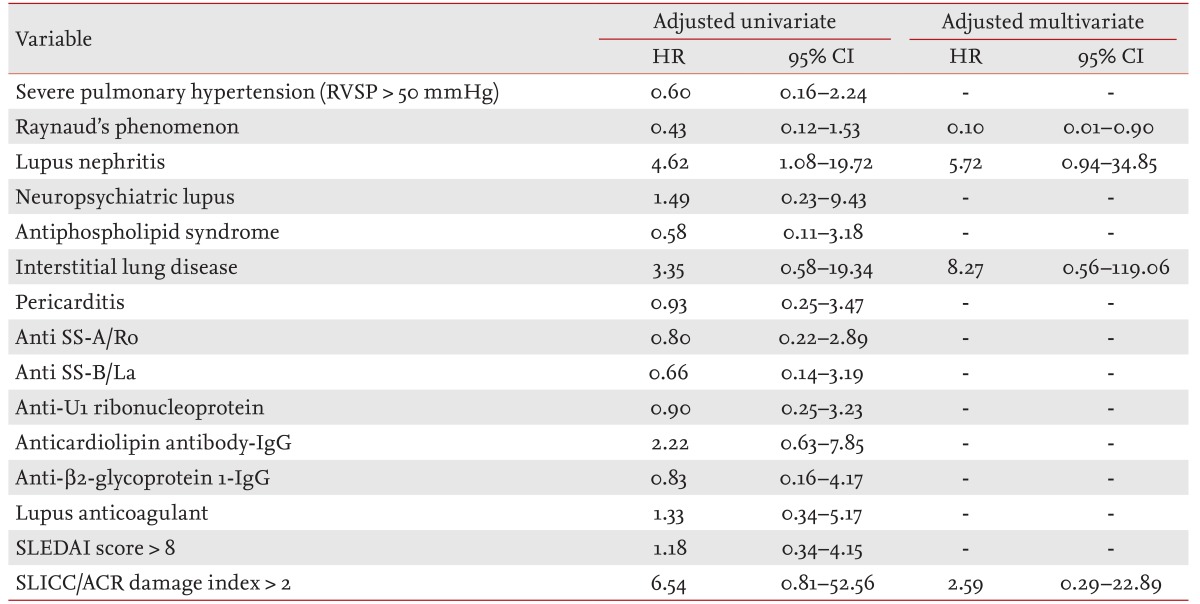
Predictors of mortality in SLE-PH
The mortality associated factors on univariate and multivariate analyses in SLE/PH+ patients are presented in Table 5. Univariate analysis demonstrated that only lupus nephritis was significantly associated with mortality (HR, 4.62; 95% CI, 1.08 to 19.72; p = 0.039). On multivariate analysis of factors with p ≤ 0.20 on univariate analysis, the presence of lupus nephritis showed a trend for an increased relative HR (adjusted HR, 6.14; 95% CI, 0.94 to 34.85; p = 0.059), whereas coexisting RP was the only significant factor for a favorable outcome (adjusted HR, 0.10; 95% CI, 0.01 to 0.90; p = 0.041).
DISCUSSION
PH is a progressive disease that can accompany SLE. Since Perez and Kramer [1] first recognized PH as a complication of SLE, further studies involving Doppler echocardiography have demonstrated that PH is not infrequently associated with SLE. The reported prevalence of PH in SLE patients ranged from 1.8% to 14% [1,2,3,4,5,6,7,8,9], because the cutoff value for PH diagnosis from echocardiography differed among the studies. Nevertheless, the results suggested that SLE/PH+ patients showed a poorer prognosis than SLE/PH- patients [3,5,9,10]. However, it was unclear whether PH was an independent prognostic factor in SLE. Chung et al. [19] demonstrated that SLE with PH had a poorer prognosis than with idiopathic pulmonary artery hypertension (iPAH). Although the prognosis of iPAH was poor, the use of novel therapeutics, including endothelin-1 antagonists, has improved survival rates in iPAH [20]. To improve outcomes in SLE/PH+, it is important to determine SLE-specific risk factors that can be treated in addition to administering iPAH medications.
Several studies have investigated the factors associated with PH development in SLE. Simonson et al. [2] reported a higher frequency of RP in SLE with than without PH. Subsequently, several further factors, including anticardiolipin (aCL) antibody, anti-U1RNP, and serositis, were proposed to be associated with SLE/PH+ [3,5,9]. In contrast, a further study reported that aCL was not associated with PH in SLE [8]. A systematic review of SLE/PH+ in China demonstrated an association between PH development and RP, serous effusion (pericardial effusion and pleural effusion), serositis, aCL, anti-β2-glycoprotein I, and anti-U1RNP [14]. In the present study, only the frequency of RP was significantly higher in SLE/PH+ patients than in SLE/PH- patients. Although higher incidences of the presence of aPL, anti-U1RNP, and serositis were noted in SLE/PH+ patients, these differences between the groups were not significant. The discrepancy between our findings and those of other studies may be due at least in part to selection bias. We selected control patients who were found not to have PH on echocardiography. However, echocardiograms were obtained only in patients who had experienced cardiopulmonary-system-related symptoms or in the presence of radiologically abnormal findings. Therefore, patients who had undergone echocardiography may have had more active disease than those who did not require examination. This was supported by the fact that SLE patients with PH in the present study were more positive for aPL than the 1,010 SLE patients who were enrolled regardless of PH in our previous study (69.0% vs. 36.0%) [12]. The association of RP and aPL with PH requires further investigation, with a focus on its role in SLE/PH+ pathogenesis.
In the present study, PH was demonstrated to be an independent risk factor for mortality in SLE with an adjusted HR of 3.10 (95% CI, 1.10 to 8.69; p = 0.032). Furthermore, the 1, 3, and 5-year survival rates in SLE/PH+ patients of 83.7%, 79.0%, and 60.2%, respectively, were lower than the 94.0%, 92.2%, and 88.1%, respectively, in SLE/PH- patients. We previously reported that the overall 5-year survival rate in SLE patients was 97.8% [12]. In the univariate Cox proportional hazard analysis in the present study, lupus nephritis was the only significant predictor for mortality in SLE/PH+ patients. In contrast, multivariate analysis demonstrated that RP was the only factor significantly associated with an increased survival rate. As with iPAH, the level of the RVSP was not associated with mortality [18]. Echocardiography is a non-invasive method for detecting PH that can also identify secondary causes of PH. However, the RVSP estimated using echocardiography could not predict the prognosis of SLE/PH+. In contrast to a previous study, which suggested that pericardial effusion found on echocardiography was correlated with a poor prognosis in iPAH [18], our results demonstrated that pericardial effusion was not associated with mortality in SLE/PH+ patients. Chow et al. [21] systematically reviewed the available literature to identify prognostic factors in SLE/PH+ patients, and found several such possible factors, including RP, thrombocytopenia, and aCL. However, none of these factors were associated with decreased survival in SLE/PH+ patients in the present study. Although the frequency of the presence of aCL was higher in the SLE/PH+ patients who died, the difference was not significant. Moreover, the presence of RP in SLE/PH+ patients was associated with an increased survival rate. The discrepancies between the present study and previous studies may be because of the aforementioned selection bias and the small sample size in previous studies. SLE patients with RP had a greater possibility of undergoing TTE at an earlier stage than SLE patients without RP because of the clinical impression of PH. Therefore, we suggest that SLE patients with RP had a better prognosis because of the early treatment initiation. In addition, because calcium-channel blockers administered to RP patients are helpful in PH patients with vasoreactivity, these patients may have shown a better prognosis because of the early treatment. A prospective cohort study with a larger number of subjects is needed to clarify this issue.
With respect to the therapeutic approach, none of the medications conferred a survival benefit in SLE/PH+ patients. Interestingly, the daily prednisolone dosage was higher in the patients who had died, which may indicate that patients with a more active disease died despite treatment. However, there were no differences in the SLEDAI score or the SLICC damage index between the survivors and non-survivors. The role of prednisolone in SLE/PH+ needs further investigation. The most common medication used in SLE/PH+ patients was an immunosuppressant followed by an endothelin-1 receptor antagonist. In the present study, the 1, 3, and 5-year survival rates of SLE/PH+ patients were better than those reported by Chung et al. [19], of 50.5%, 44.9%, and 16.8%, respectively. The improved survival rate in SLE/PH+ patients in the present study may have been due to various medications, including immunosuppressants and novel iPAH-targeted medications.
The prevalence of SLE/PH+ in the present report was approximately 1.4%. Li and Tam [5] proposed that the prevalence of SLE/PH+ might differ dependent on ethnicity. Estimated prevalence rates in Asians have been reported to be 4.2% to 6.2%, while many studies involving Caucasian cohorts have reported prevalences of up to 4.2% to 14% [2,3,4,8,16,22,23]. Moreover, by defining PH using the same cutoff value (i.e., RVSP > 30 mmHg), prevalences of 4.2% to 14% in Caucasian patients, 4.2% to 5.9% in an Asian population, and 1.8% in a Turkish study were reported [2,4,5,6,9,20]. These differences in the prevalence of PH among SLE patients from various ethnicities suggest that the genetic background may contribute to PH development in SLE.
This study had certain limitations. First, the control group, which comprised SLE patients without PH, was selected from SLE patients who had undergone echocardiography (i.e., patients who presented with cardiopulmonary symptoms or abnormal radiographic findings). Therefore, it is very likely that patients with a more severe disease than general SLE patients were included as disease controls. However, a comparison of the survival rates between the two groups showed statistically significant differences, while the difference in the survival rate between the SLE/PH+ group in the present study and our historical cohort [12] was even greater, suggesting that the difference between the two groups reported here was valid. A further limitation was the diagnosis of PH being defined by echocardiographic findings. Therefore, mild asymptomatic SLE/PH+ patients were not enrolled in this study. Moreover, the RVSP may have overestimated the pulmonary artery pressure determined during RHC.
In conclusion, we demonstrated that PH is an independent prognostic factor in SLE. Additionally, SLE patients with RP have a higher risk of developing PH. Conversely, RP was associated with a better prognosis for survival in SLE/PH+ patients.
KEY MESSAGE
Pulmonary hypertension is one of independent risk factors of mortality in systemic lupus erythematosus (SLE) patients.
Coexistence of RP showed better survival prognosis in SLE/pulmonary hypertension (PH)+ patient.
Lupus nephritis was associated with increased risk of mortality in SLE/PH+ patients.
Notes
No potential conflict of interest relevant to this article was reported.