A Large Malignant Insulinoma: Case Report with Endosonographic, Immunohistochemical and Ultrastructural Features
Article information
Abstract
Malignant insulinoma in the beta cells of the pancreatic islet is rare and usually presented as hypoglycemia. We report a case of large malignant insulinoma in a 53-year-old Korean woman. A presumptive clinical diagnosis was made before surgery, based on the high plasma insulin-to-glucose ratio and a large solitary heterogeneous pancreatic mass by abdominal computed tomography and endosonography. The tumor measured 5.8×4.7×4.5 cm in dimension and showed capsular invasions and metastases in two of four peripancreatic lymph nodes. The tumor cells were strongly immunoreactive to insulin and had a high Ki-67 labeling index (13%) and atypical membranous electron-dense granules, ranging from 120 to 400 nm in diameter, in the cytoplasm on electron microscopy. The patient was treated by distal pancreatectomy with splenectomy and rapidly recovered without neurohypoglycemic symptoms. This case showed not only lymph node metastases, the most reliable parameter for malignancy in pancreatic endocrine tumors, but also other valid diagnostic clues, such as high Ki-67 labeling index, heterogeneous enodosonographic findings, capsular invasions with large tumor and pure atypical secretory granules.
INTRODUCTION
Insulinomas are uncommon endocrine tumors arising from the pancreatic β cells. Most insulinomas are benign, single and small, measuring less than 2 cm in diameter. Only 8% were greater than 5 cm1–5). Malignant insulinomas are rare and few cases have been reported in Korea2,6–9). Differentiation between benign and malignant insulinomas by histologic finding was difficult, therefore malignant insulinomas were diagnosed only by metastasis to lymph nodes or other organs3,5). Hence we report a patient of a large malignant insulinoma with peripancreatic lymph node metastases and characterize it’s endosonographic, immunohistochemical and electron microscopic features.
CASE
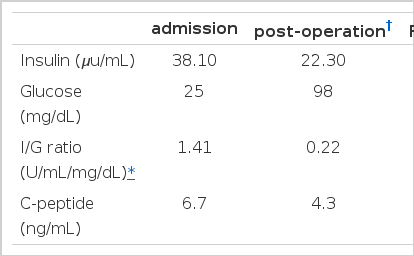
A 53-year-old woman presented with recurrent dizziness and loss of consciousness upon skipping meals for several months. She was known as a ‘shaman’ in her neighborhood because of frequent faintness. On admission, the results of complete blood counts, tumor markers, thyroid function test, parathyroid hormone, calcium, gastrin, prolactin and brain computed tomography (CT) were normal, but the random glucose level was 39 mg/dL. On 72-hour fasting test, she demonstrated cold sweat, disorientation, and dizziness at 5 hours with 25 mg/dL of serum glucose level. The plasma insulin-to-glucose ratio was 1.41 (Table 1) and symptoms were immediately relieved following glucose administration (Figure 1). Preoperative localization was done by abdominal CT and endosonography (EUS). A hypoechoic, heterogeneous echoic mass was discovered at the tail of the pancreas (Figure 2, 3). Distal pancreatectomy with splenectomy was done.
Measurement of serum glucose on 72-hour fasting test. The arrow indicates the time-point of glucose administration

Abdominal CT showed a single, round, well-enhanced mass in the tail of the pancreas, measuring 6 cm in diameter. Neither lymph node enlargement nor metastatic lesion was visible.

Hypoechoic, heterogeneous with multiple anechoic spots and a smooth delineation without other intra-pancreatic occult mass were detected by EUS at a frequency of 7.5 MHz (G: stomach, M: mass, P: pancreas, S: superior mesenteric artery, V: splenic vein).
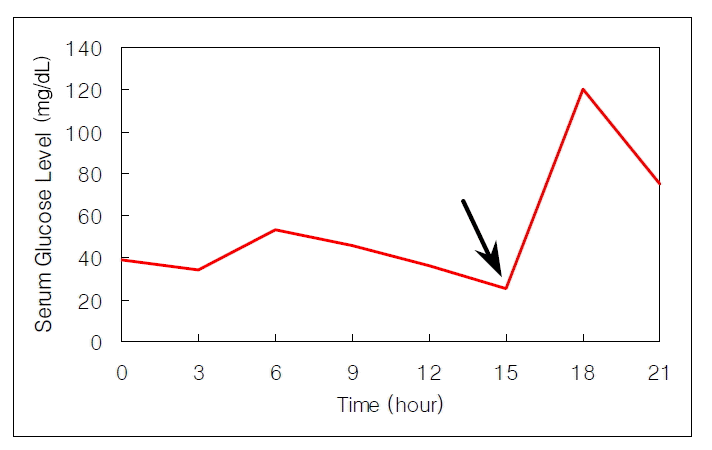
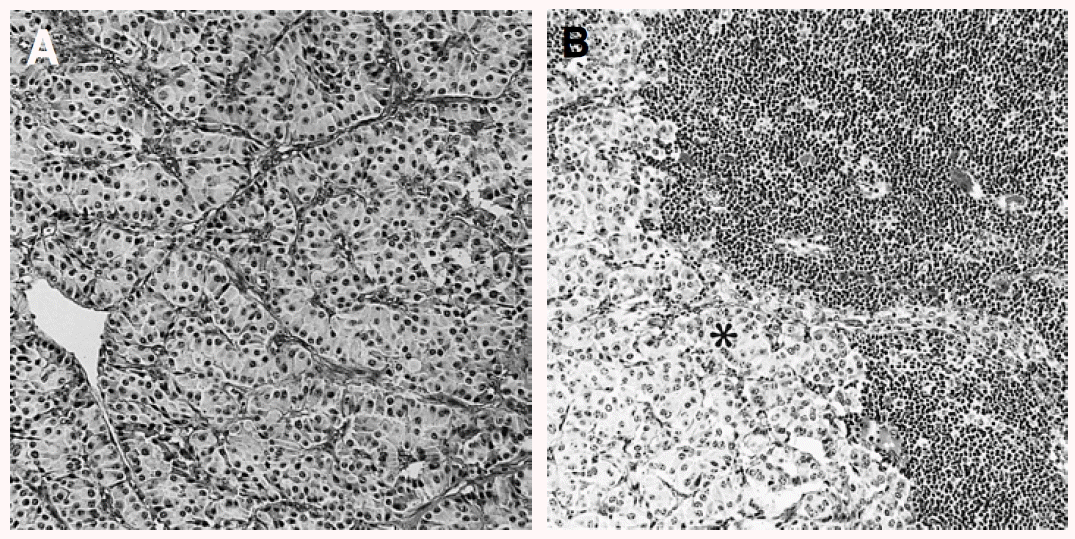
The mass measuring 5.8×4.7×4.5 cm in dimension (Figure 4) was composed of uniform bland cuboidal cells with granular eosinophilic cytoplasm and round nuclei (Figure 5A). Two of four peripancreatic lymph nodes were metastasized (Figure 5B). Immunohistochemically, cytoplasm of tumor cells were strongly immunoreactive to insulin (Figure 6A) but not to somatostatin and glucagon. The Ki-67 labeling index (LI) was approximately 13% (Figure 6B). On ultrastructural study, atypical secretory granules were easily found in the cytoplasm (Figure 7A). Amyloid deposits were showed both by electron microscopy (EM, Figure 7B) and congo red stain under polarizing microscopy (Figure 8). Immediate after surgery, the levels of insulin and glucose were normalized and the postoperative course was uneventful and without any complication (Table 1). During 18-months of follow-up, she remains with no evidence of recurrence.

The tumor mass was oval, well-demarcated solid, measuring 5.8×4.7×4.5 cm in dimension. Two foci of capsular invasion (arrowheads) were observed in the cut surface.
The tumor demonstrated small nests of uniform bland cells septated by thin fibrovascular stroma (A). A peripancreatic lymph node was metastasized by tumor cells (asterisk) (B), (H&E. ×100).
The cytoplasm of the tumor cells was strongly immunoreactive (dark-brown) for the insulin (A). A few tumor nuclei showed immunoreactive (dark-brown) for Ki-67 (B), (×200).
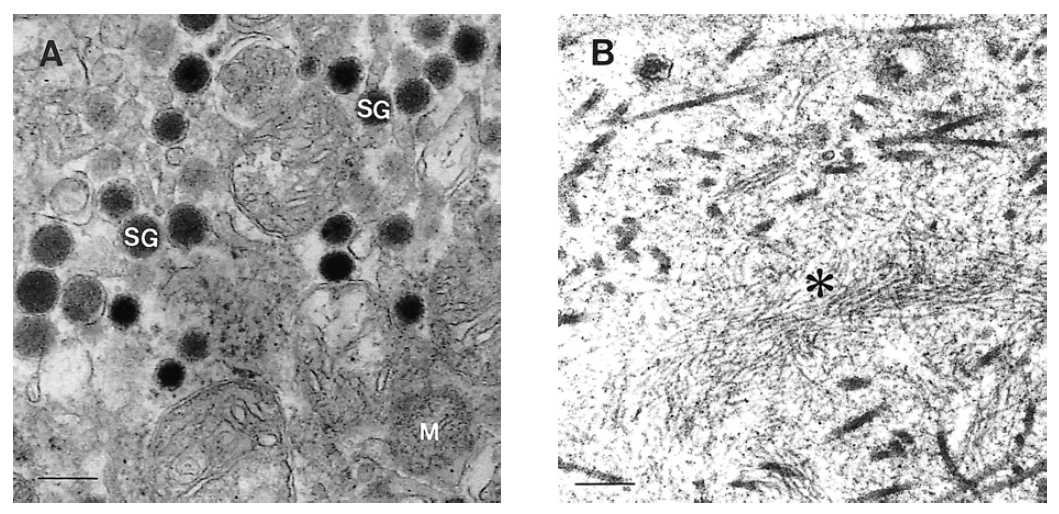
EM demonstrated well-developed, membranous, electron-dense atypical secretory granules (SG) in the cytoplasm measuring 120 to 400 nm (A). There were wisps of non-branching intermediate amyloid filaments (asterisk) in the extracellular spaces (B). (M: mitochondria. Scale bar: 0.5 μm).
DISCUSSION
Insulinomas are the most common pancreatic endocrine tumors (PETs) and only less than 10% are malignant. Malignant insulinoma could be suspected clinically if the size of the tumor is 3 to 6 cm or larger and confirmed bythe evidence of metastasis which is mostly found in the liver, lymph nodes, or both1–4).
A large variety of imaging modalities for the preoperative localization have been used, but the average accuracy remains low5,10,12,19). Recently, EUS is a new convenient diagnostic method for the localization of a pancreatic mass10–14). The sensitivity and specificity of EUS are up to 82–93% and 95%, respectively10–12).
Differentiations between benign and malignant insulinoma by EUS depend upon size, heterogeneity of internal echo, multinodular structure, cystic transformation or necrosis and vascular invasion11). In our case EUS findings that demonstrated large-sized, low-echoic mass with heterogeneous internal echo evoked a possibility of malignancy. The Ki-67 LI is known to be less than 5% in benign tumors, but it was estimated as 13% in this tumor which could be a predictable feature of a malignant tumor15).
More than half of the PETs secrete multiple hormones (insulin, glucagon, somatostatin), nevertheless, the clinical manifestations are nearly always derived from hypersecretion of only one of these hormones16). In our case, the tumor cells were only immunoreactive to insulin, therefore, seemed to be as a pure insulinoma.
Ultrastructurally, our tumor was devoid of typical insulin-secreting β granules and only showed a decreased number of atypical secretory granules comparing with normal islet β cell. Because atypical granule could be regarded as an incomplete form in the maturation process and stored with a small amount of functional insulin, these findings might be correlated with long, insidious, endurable clinical symptoms and malignant potential. Metastasis is the most reliable parameter for malignancy in PETs. However, high LI, heterogeneous EUS findings, capsular invasion with large tumor and pure atypical secretory granules might be other valid diagnostic clues for diagnosis of malignant insulinoma. Amyloid deposits were shown under polarized light after Congo red stain and electron microscopy. Those were thought to be the result of uncontrolled insulin or proinsulin release with decreased storage capacity17).
Insulinoma is usually managed by surgical excision, laparoscopic enucleation14) or medical treatment. Distal pancreatectomy with splenectomy was performed because of the unfavorable EUS findings. For patients with benign insulinoma, the long-term cure rate is about 88.6–97.5% by surgical treatment18,19), and patients with malignant insulinoma have a 49–66% of 5-year survival20,21) with 7.5-year of overall survival3). The patient has remained well for 18 months after surgical excision of the tumor, despite some unfavorable prognostic factors such as lymph node metastases, capsular invasions and high LI.