Evaluation of Emphysema in Patients with Asthma Using High-resolution CT
Article information
Abstract
Background
Bronchial asthma is a clinical syndrome characterized by reversibility of airway obstruction. However, many asthmatics have evidence of residual airway obstruction. It has become evident that the repair of the chronic inflammatory process can lead to various irreversible changes. It is generally accepted that the most common cause for the change is cigarette smoking but it is controversial whether asthma progresses to emphysema. High resolution computed tomography (HRCT) is more sensitive and more accurate than chest plain films in determining the type and extent of emphysema. This study was carried out to determine whether asthma can be a cause of emphysema without the effect of cigarette smoking and to evaluate clinical characteristics in asthmatics with emphysema.
Methods
We studied 58 asthmatic patients with reversible airway obstruction and evaluated the presence of emphysema using HRCT and pulmonary function test. According to HRCT findings, they were divided into 2 groups: Asthmatics with emphysema and the ones without emphysema.
Results
Of the 58 patients, 7 were revealed to have emphysema. (1) 6 asthmatics with emphysema were smokers, but one patient was a nonsmoker. (2) Highly significant differences between asthmatics with and without emphysema were found in cigarette smoking (p<0.01) and smoking consumption (p<0.01. (3) There were no significant differences in the duration of asthma, age or sex between patients with and without emphysema. (4) There were no significant differences in FEV1(%), FEV1/FVC (%), diffusing capacity for carbon monoxide (DLco) (%) and DLco/alveolar volume between patients with and without emphysema (5) Differences between asthma patients without emphysema and those with emphysema were found to be significant in bronchial wall thickeness (p<0.05) and in total IgE levels (p=0.07).
Conclusion
These results indicate that smoking is a main factor in causing emphysema in asthmatics.
INTRODUCTION
Bronchial asthma is a clinical syndrome characterized by reversibility of airway obstruction which is caused by increased airway hyper-responsiveness to various stimuli but improved by therapy1). Over last several decades, bronchial asthma has been thought to be a completely reversible airway obstruction, but a certain number of asthmatics have evidence of residual airway obstruction2). Especially, a continuous airway obstruction can be observed in chronic bronchial asthma patients3). Bronchial asthma is known to be caused by chronic airway inflammation with eosinophilic infiltration4). Chronic inflammation causes a remarkable airway remodeling5) and it is closely related to the severity of the disease.
It is generally accepted that cigarette smoking7) or airway inflammation8) causes developing emphysema in asthmatics, However, asthmatics often show the characterristics of emphysema9) and some emphysema patients show asthmatic characteristics such as wheezing or airway hyper-responsiveness so that it is controversial whether asthma progresses to emphysema10, 11). Chest plain X-ray of asthmatics showed normal or nonspecific findings, like pulmonary hyperinflation, which were not helpful in the diagnosis and evaluation of the progression in asthmatics12). However, as the image diagnosis has been developed, the radiographic study of asthmatics is being actively performed, especially by using high-resolution computed tomography (HRCT). The recent study reported that airway inflation and emphysema are observed in HRCT finding and cigarette smoking can cause emphysema in asthmatics8). A computed tomographicpathologic correlation in emphysema was also reported13). So HRCT is known as a useful method in the quantitative and qualitative evaluation14) of emphysema. It is difficult to discriminate between bronchial asthma and emphysema because their clinical features are similar and there is a possibility of a combination between the two diseases15), of which clinical characteristics are not exactly known yet.
The purpose of this study is to evaluate the, clinical characteristics of asthmatics with emphysema and to identify any factors which affect the disease, except smoking. We evaluate the presence of emphysema or not in asthmatics by HRCT and compare the two groups with pulmonary function test and various clinical features.
METHODS
1. Patients
This study included 58 patients with asthma, aged from 17 to 73, who were referred to a tertiary hospital, Soonchunhyang University Hospital.
The number of comprised 22 men and 36 women. Smokers were 20 and nonsmokers were 38. The mean duration of asthma was 6.4±9.1 years with evidence of the patients’ clinical history. Bronchial asthma was defined on the base of characteristic symptoms like dyspnea, wheezing, chest tightness, cough and more than 12% and 200 mL increase in FEV1 after inhalation of short acting bronchodilator, or diurnal variation of peak expiratory flow rate greater than 20% during consecutive 2 weeks. The exclusions were the cases that could not maintain inspiration to undergo HRCT or that had a respiratory infection or circulatory diseases within 6 months before the test. All the patients were maintained free from asthma symptoms by medication.
2. Methods
CT-W2000 (Hitachi Medical, Tokyo, Japan) was used for high-resolution CT scanning and all the patients underwent supine scan at the end of inspiration. Asthmatics and the normal control group were scanned from the apical area of lung to the right diaphragm at intervals of 15 mm with a thickness of 1.0 mm. Based on 120 kVp and 300 mA of high spatial frequency algorithm, images were obtained with 1400 HU and −750 HU of window width and window level, respectively. The field of view was 35 cm.
According to HRCT, intrapulmonary low attenuation area, pulmonary vascular pruning, pulmonary vascular distortion and absence of a well-defined wall were defined as emphysema17). Air trapping was defined as geographic or unclear boundary images of an area scanned when there was no change of lung volume at inspiration and end-expiration at the same place and pulmonary density change at expiration decreased more than 100 HU compared to the surrounding pulmonary area17). Bronchial wall thickening was calculated by the formula that the difference between bronchial external diameter (R) and internal diameter (r) at segmental bronchus and subsegmental bronchus was divided by bronchial external diameter (R). Ratio>0.5 was defined as positive bronchial wall thickening18). To identify the difference of bronchial wall thickening in asthmatics with and without emphysema, they were divided into 3 groups of <0.4, 0.4–0.5, >0.5 according to bronchial wall thickness ratio (R-r/R). One radiologist interpreted the scans without information of the clinical data or pulmonary test results of the patients. Pulmonary function test was performed at the period when their asthma symptoms were controlled. Fukuda-300 (Fukuda Sanyo, Japan) was used and test items were FVC, FEV1, FEV1/FVC, RV, TLC, RV/TLC, DLco, response test to bronchodilator, PC20 (concentration of challenged methacholine until the FEV1 declined by more than 20%). Through a questionnaire, a disease history was compiled for asthma symptoms, smoking experience and previous diseases. In addition, skin test (50 standard inhaled antigens) and serum total IgE value (IU/mL) were included. 1
3. Statistics
SPSSwin, a statistical program for windows, was used for statistical examination and Mann-Whitney U test and chi-square test were used for statistical process and data analysis. Reliability range was 95%. A p value of less than 0.05 was considered significant.
RESULTS
1. Clinical characteristics of asthmatics with and without emphysema
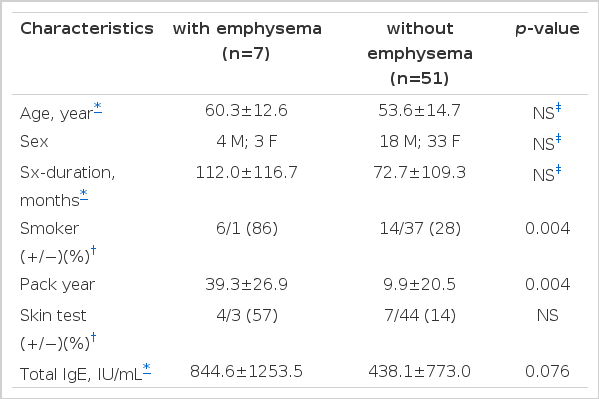
Among the total of 58 asthmatics, 7 patients (12%) were diagnosed as emphysema by HRCT finding of intrapulmonary low attenuation area. 6 (30%) of 20 asthmatics with smoking experience showed emphysema findings. 6 out of 7 asthma patients with emphysema were smokers, but the emphysema finding was also observed in 1 asthmatic who was a nonsmoker (Table 1). With regard to smoking, asthmatics with emphysema were all smokers (6 patients, 86%) except 1, compared to asthmatics without emphysema (14 patients, 28%), which revealed a statistical significant difference (p=0.004). Regarding smoking consumption amount, asthmatics with emphysema (39.29±26.9 packs/year) was higher than that of ashmatics without emphysema (9.9±20.5 packs/year), which revealed a statistical significant difference (p=0.004). Sex ratios of asthmatics without and with emphysema were 18:33 (male/female) and 4:3 (male/female), respectively. Age distribution was 19–76 yrs (mean 53.6 years old) and 34–73 yrs (mean 60.2 years old), respectively. There were no significant differences between age, sex, duration of asthma morbidity in patients with and without emphysema (Table 1). Serum total IgE value was not significantly different in asthmatics without emphysema (438.1±773.0 IU/mL) and with emphysema (844.6±1253.5 IU/mL) (p=0.076). Positive rate to skin tests were not significantly different between two groups (Table 1).
2. Pulmonary function test in asthmatics with and without emphysema
FEV1 (%) was decreased in asthmatics with emphysema (63.1±14.4%), compared to the patients without emphysema (75.2±19.5%), but it was not statistically different. Also FVC (%), FEV1/FVC (%), RV (%), TLC (%) and RV/TLC (%) were not significantly different. DLco/VA (%) was not significantly different between asthmatics without emphysema (103.3±25.4%) and with emphysema (98.3±18.2%)(Table 2).
3. HRCT findings of asthmatics with and without emphysema
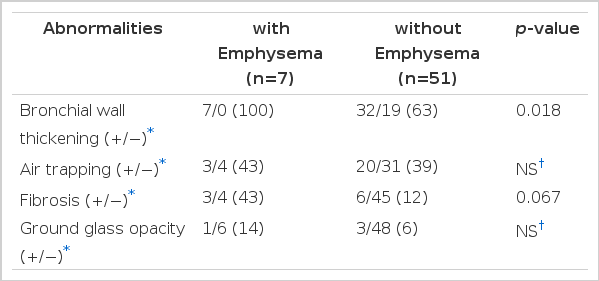
Bronchial wall thickening was observed in all asthmatics with emphysema (7 patients, 100%) compared to those without emphysema (32 patients, 63%), which was statistically significant different (p=0.018)(Table 3).
Fibrosis was not significantly different between asthmatics without emphysema (6 patients, 12%) and with emphysema (3 patients, 43%)(p=0.067).
Air trapping or ground glass opacity was notsignificantly different between the two groups.
DISCUSSION
Bronchial asthma presents chronic airway inflammation which is observed in all patients with asthma4). This inflammation is already developed even in a mild form of asthma and increased according to the severity of the disease. Some damaged tissue, completely repaired in the beginning of the inflammatory response or others, is replaced with connective tissue to form a permanent scar19). Remodeling of airway-like thickening of the smooth muscle with inflammation in the submucous tissue of the 8 airway and mucous gland hypertrophy and reorganization of extracellular matrix are reported5) in patients with asthma. The occurrence of emphysema is closely related to the severity of the asthma and younger asthmatics have healing potential so that they have fewer scars according to the severity of the asthma than older people, which indicates that emphysema is partly related to age20). But, In this study, a significant difference was not observed between the two groups with and without emphysema according to age, duration of morbidity and severity.
It is reported that cigarette smoking is a main risk factor of chronic obstructive lung disease so 26% of heavy smokers developed to chronic obstructive pulmonary disease21). However, its mechanism is not clearly explained yet and smoking is known to cause as imbalance of proteolytic-antiproteolytic activity and lead to pulmonary destruction and airway obstruction22). Auerbach et al.7) reported that emphysema was not observed in 90% of nonsmokers and moderate emphysema was 2.9% of nonsmokers, whereas, in smokers, emphysema was not observed in only 0.3%, moderate emphysema in 32.7% and severe emphysema in 19.2% on autopsy findings, which revealed that smoking consumption and older age were related to the increase of the severity of emphysema. Mochizuki et al.10) observed that among asthmatics, emphysema developed only in the heavy smokers. Lynch et al.23) presented that more significant emphysema was found in smokers with asthma than nonsmokers with asthma, but there were 2 cases of nonsmoker asthmatics which developed emphysema. Our study observed emphysema on HRCT in 6 patients (30%) out of 20 smokers with asthma and closely related with smoking (p=0.004) in case of asthmatics with emphysema. But a finding of emphysema was also observed in one nonsmoker with asthma.
In addition to smoking, there are some factors to cause chronic obstructive pulmonary disease, like old age, male, history of respiratory disease at infancy, occupational inhalation of specific dust, decrease of initial pulmonary function, air pollution and α1-protease deficiency21). Some agents like talc and methylphenidate (Ritalin) are known, but the reason why some smokers have lower pulmonary function is not explained yet. It is accepted that there is a difference of susceptibility related to the unknown genetic factor24).
In this study, a nonsmoking asthmatic with emphysema on HRCT is a 35-year-old woman with 36 months of disease duration. She may have a possibility of passive smoke, but there is no clinically important21) or other factor, except smoking, related to the development of emphysema25). There is a possibility of asthma induced emphysema or α1-antitrypsin deficiency22, 26). However, we did not measure serum α1-antitrypsin so we could not confirm the exact genesis. Cigarette smoking increases IgE and risk of asthma attack in workers who are exposed to specific work allergic antigens, like platinum or moisturizer-related antigens21). It was reported that IgE concentration observed in patients with emphysema is related to the high frequency of smoking27). In this study, serum IgE is not significantly increased in asthmatics with emphysema compared to ones without emphysema, but it increased (p=0.076) so that it is thought to be related to the previous report21) about the role of smoking to increase IgE and the risk of asthmatic attack. Generally, pulmonary function test for the airway obstruction in emphysema does not well reflect the anatomical abnormalities. Although 30% of lung parenchyme changed emphysema, symptoms of airway obstruction may not appear28), which is usually observed with centrilobular emphysema involving the upper lobe29) of the lung. Even in subjects with normal pulmonary function test, we can see the emphysema on HRCT30).
Diffusing capacity (DLco) is useful to diagnose emphysema and it is reported that visual inspection of emphysema in asthmatics is correlated to DLco/VA in reverse8) relation. It is known that mild emphysema can be detected by using HRCT and DLco31). Mochizuchi et al.10) presented that FEV1, FEV1/FVC, DLco/VA were decreased significantly in asthmatics with severe emphysema and FEV1/FVC decreased in a mild emphysema group. But our study did not show significant differences of FEV1/FVC, TLC, DLco between two groups with and without emphysema diagnosed by HRCT. Normal lung function without a significant decrease of DLco may be due to an early period of emphysema31), so it is difficult to evaluate the emphysematous change in asthmatics with pulmonary function test only.
In asthmatics, structural changes of the airway can be estimated by decreases in pulmonary function test and irreversible airflow obstruction, but they mostly show normal findings on chest plain films. It is reported that mild and moderate emphysema could be detected only in 16% based on chest plain X-rays and the severe emphysema could be diagnosed in 41%12). As a result of comparison between chest computed tomography findings and pathologic findings of lung specimens after operations over the last 10 years, chest CT is the most accurate imaging method in diagnosing emphysema32). Recently, HRCT can be an index to evaluate the bronchial wall thickness and airway obstruction with more anatomical and physiological studies on asthmatics by indirect and non-invasive methods33). High-resolution computed tomography is known to be a good method to confirm physiologically significant emphysema23, 36) and a good correlation between the view of HRCT in centrilobular emphysema with remodeling formation and pathologic finding has been reported32). In asthmatics, high-resolution computed tomography shows more abnormal findings related to permanent airway remodelings, like bronchodilation or bronchiectasis, compared to the normal group. Paganin et al.34) observed on HRCT that there were some reversible findings, like mucous impaction, ground-grass opacity, lobular atelectasis, that were not consistent with the degree of disease, whereas there were some irreversible findings like bronchiectasis, bronchial wall thickening, scarred opacity and emphysema that would be serious in severe bronchial asthma and non-allergic bronchial asthma. But the reports about irreversible findings on the bronchial wall thickening reveal different result.35) In our study, air trapping, bronchial wall thickening, fibrosis and ground-glass opacity were observed on HRCT in asthmatics. Lynch et al.23) reported that the incidence of emphysema in asthmatics is 19% on HRCT and our study showed a similar finding with 7 (12.1%) out of 58 patients.
Lynch et al.23) observed bronchial wall thickening in asthmatics by plain chest radiography (71%) and-by HRCT (92%) and presented that bronchial wall was more thickened in asthmatics. Choi et al.18) reported that bronchial wall thickening appears with increased airway hyper-responsiveness at the time of the symptoms and there is no relation between duration of morbidity or age and bronchial wall thickness. They suggested that there is no significant difference in the bronchial wall thickness between asthmatics with and without emphysema. Our study showed a significantly increased finding (p=0.018) of bronchial wall thickening in bronchial asthma patients with emphysema, which is contrary to the result of Choi et al.18) because it is assumed that subjects for our study had more severe asthma than those for the study of Choi et al18).
Paganin et al.34) observed emphysema in nonsmokers with severe bronchial asthma and presented fibrosis in peribronchus and interstitial emphysema findings, which are related to scarred emphysema or airway remodeling. With regard to higher fibrosis appearance in bronchial asthma patients with emphysema, it is assumed that it is related to the fibrosis by tuberculosis in the past37).
Our study has concluded that emphysema in asthmatics is not related to the severity of bronchial asthma or duration of the disease, whereas it is closely related with cigarette smoking, but emphysema can occurr also by asthma itself, except smoking. Therefore, it has been determined that there is no difference of pulmonary diffusing capacity or pulmonary physiological index between the two groups with and without emphysema. But there are some limitations for our study. First, emphysema was not classified according to the severity of the disease so that we could not identify the relation between smoking and the severity of emphysema and the factors to discriminate between patients with mild emphysema and without emphysema. Second, HRCT finding is highly recognized in several studies, but any subjective interpretation can not be completely excluded. Third, serum α1-antitrypsin deficiency was not measured so that the exact development of emphysema by α1-antitrypsin deficiency could not be confirmed. In case of asthmatics with emphysema, FEV1 decreases quickly compared to simple asthmatics and the prognosis is known to be bad due to the lower life expectancy38, 39). As the change of emphysema can be developed by asthma itself except cigarette smoking, more studies should be needed for its genesis.