Ultrastructural Changes of Hepatic Stellate Cells in the Space of Disse in Alcoholic Fatty Liver
Article information
Abstract
Background :
Hepatic stellate cell (HSC) has been suggested to play a role in fibrogenesis in alcoholic liver disease. We evaluate the correlation with fibrogenesis and ultrastructure of hepatic stellate cells in alcoholic fatty liver.
Methods :
We studied 6 patients with alcoholic fatty liver and 5 non-alcoholic fatty liver. The numbers of fat droplets in hepatic stellate cell was determined by electron microscopy. We also studied the grading of deposition of collagen fibers in the space of Disse. We were to evaluate the structure of hepatic stellate cells in the space of Disse by light and electron microscopy.
Results :
Wider distribution of fat droplets in hepatic stellate cells in alcoholic fatty liver than in normal liver. The hypertrophied endoplasmic reticulum in hepatic stellate cells is a prominent findings in alcoholic fatty liver. We observed basement membrane-like materials in patients with alcoholic fatty liver with hepatic fibrosis.
Conclusion :
The results demonstrate that, in patients with alcoholic fatty liver by alcoholic liver injury, the hepatic stellate cells may play an important role in the fibrogenesis of perisinusoidal spaces in the liver.
INTRODUCTION
Recently it was reported that changes in eating patterns and drinking habits resulted in alcoholic liver disease1). Alcoholic liver disease is divided into alcoholic fatty liver, alcoholic hepatitis and alcoholic liver cirrhosis. The most common form is alcoholic fatty liver in the long-term alcohol drinker. One of the prominent morphologic findings of alcoholic liver damage is fibrogenesis consisting of collagen fibers in normal and edematous liver parenchyma and in the space of Disse2). It is well known which cells are involved in collagenization of hepatic lobules, but collagen fibers are originated from different cells according to the site of hepatic lobules and stages of lesion4). The liver comprises at least five different classes of cells. They are hepatocytes, fat- storing (Ito), endothelial, Kupffer and pit cells6). The main cell in the space of Disse is fat-storing (Ito) cells which are involved in hepatic fibrogenesis11, 12). The purpose of this study was to assess the fibrogenesis in the space of Disse and the role of hepatic stellate cell in patients with normal liver and alcoholic fatty liver under light and electron microscope, and to reveal the ultrastructural changes of hepatic stellate cells in the space of Disse.
MATERIALS AND METHODS
Study population
We studied blind liver biopsy specimens from 13 normal subjects, 6 patients with alcoholic fatty liver, 3 patients with diabetic fatty liver and 2 patients with non-specific fatty liver. Twenty men and four women were enrolled in this study. The ages of the study subjects ranged from 21 to 48 years (mean age: 33.4 years).
Electron microscopy
For electron microscopy, the biopsied livers were rapidly cut into small pieces which were transferred to the following fixatives (pH 7.4) at 4°C: a) 2% glutaraldehyde Sorensen’s phosphate buffer for 3 hours b) 1% OsO4 phosphate buffer for 2 hours, followed by washing with same buffer and further incubated for 24–48 hours in a refrigerator (4°C). Sections were dehydrated with absolute ethanol and embedded in Epon 812. Observations were made on 0.5-μm thick Epon sections from controls that were stained with toluidine blue. After ultra-thin (600 Å) sectioning by ultramicrotome, the sections were lightly counter-stained with uranyl acetate and lead citrate and were analyzed by JEOL-100.
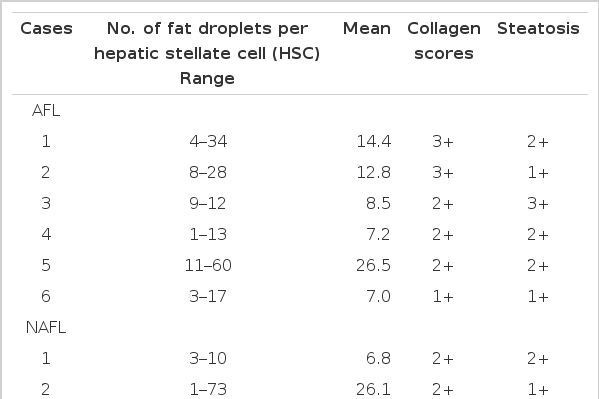
Counting of fat droplets of the hepatic stellate cell
Hepatic stellate cells in the space of Disse were observed and photographed using more than 2 blocks in each case. The numbers of fat droplets in 15–20 hepatic stellate cells, which were found in electron microscopy or photograph, were counted in each case.
Grading of collagenization in the space of Disse
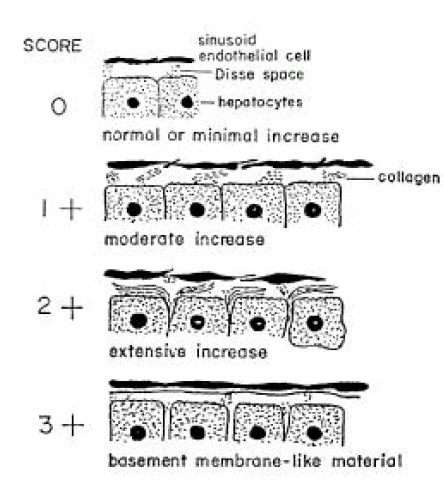
We observed and photographed 10–20 space of Disse in all specimens using more than 2 blocks. Grading of deposition of collagen fibers in the space of Disse was done by using the modified scoring system of Orrego3). The extent and intensity of collagen fibers were graded on a scale of 0–3+ on two separate occasions and average score was calculated. A 3+ grade implies that basement membrane-like materials were found in subendothelial areas. A 2+ grade implies that space of Disse was distended by collagen fiber bundles which were also found in interhepatocellular space. A 1+ grade implies that 2–3 collagen fiber bundles were found in the space of Disse, while 0 implies that collagen fibers were absent throughout the specimen (Figure 1).
Statistical methods
Results were expressed as mean±SEM values. Student t-test or Mann-Whitney U test was used to determine the degree of difference between groups. A p value of less than 0.05 was considered significant.
RESULTS
Biochemical analysis
Major laboratory data was summarized in table 1. The serum albumin levels in two cases of alcoholic fatty liver and non-alcoholic fatty liver groups were below 3.0 g/dl. AST and ALT levels in all of alcoholic fatty liver and 3 of 5 cases of non-alcoholic fatty liver were mildly elevated compared with the control group. The serum bilirubin levels and thymol turbidity tests were not significantly higher than the control group (Table 1).
Distribution of the fat droplets in hepatic stellate cells and grading of collagenization
In comparing the numbers of fat droplets in control group (7.2±2.5), there was a significantly wider range of numbers in patients with alcoholic fatty liver (13.6±7.8) (p<0.05) and non-alcoholic fatty liver (12.0±7.1) (Figure 2).
The grading of collagenization was significantly higher in patients with alcoholic (1+ to 3+, mean 2.2+) and non-alcoholic fatty liver (1+ to 2+, mean 1.4+) than that in control group (0 to 1+) (Table 2). But there was no correlation between fibrosis and steatosis.
Light microscopic observations
Severe fat droplets infiltration throughout the entire hepatic lobules could be seen in patients with alcoholic fatty liver (Figure 3).

Higher magnification of the fatty liver from a patient with alcoholic fatty liver. Large fat vacuoles were distending hepatocytes and several fat vacuoles have coalesced (H & E, ×400).
Mallory body was not seen in any of the cases. Most of the nuclei of hepatocytes were deviated by huge fat droplets (Figure 4). Glycogen nuclei were frequently observed in the cytoplasm of patients with non-alcoholic fatty liver complicated by diabetes mellitus.
Electron microscopic observations
In normal liver, hepatic stellate cells were mainly located in the space of Disse and the perisinusoidal recess (Figure 5, 10, 11,). The nuclei of hepatic stellate cells were heterochromatic and indented by fat droplets. One or two centrioles were observed in near well-developed Golgi apparatus (Figure 5) in patients with alcoholic fatty liver. The dense bodies and microfilament bundles with focal densities, a number of glycogen granules and pinocytotic vesicles were observed in the cytoplasm of hepatic stellate cells. Microfilaments (collagen fiber grading, 2+) in the space of Disse were gathered in bundles parallel to the plasma membrane (Figure 7). Basement membrane-like materials (collagen fiber grading, 3+) beneath the endothelial cells could be seen in patients with alcoholic fatty liver (Figure 8, 9). Also, we observed that bundles of coarse collagen fibers seemed to be arising from fat-storing cell surface in normal liver (Figure 10). Collagen fibers’ deposit in the space of Disse and changes of rough endoplasmic reticulum in hepatic stellate cells were observed in patients with alcoholic fatty liver. The hepatic stellate cells showed short, narrowed cisternae of endoplasmic reticulum and coarse collagen fibers in normal liver (Figure 5, 6). In patients with alcoholic fatty liver, hepatic stellate cells contained a number of endoplasmic reticulum whose cisternae were largely dilated (Figure 11) and filled with amorphous, fibrinoid materials (Figure 12). In patients with alcoholic fatty liver, the mitochondria were small in size and decreased in number. Also, sometimes, microtubules and lysosomes could be seen. Fat vacuoles, interestingly, showed variable sizes and were fused to each other in the liver of patients with alcoholic fatty liver (Figure 8, 13).
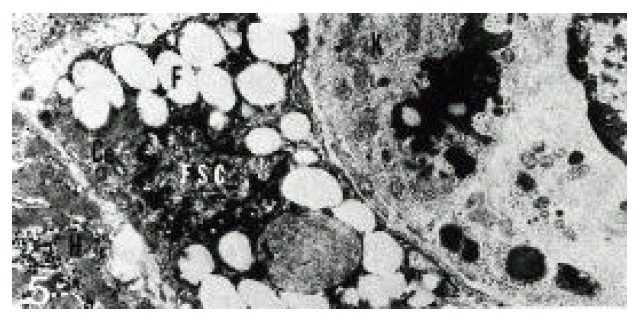
Electron micrograph of a hepatic stellate cell (HSC) in alcoholic fatty liver (case 5). HSC contained fat droplets (F) of variable sizes. HSC was separated from hepatocyte (H), endothelial cells and Kupffer cell (K) by space of Disse (DS) containing collagen fibrils and numerous microvilli. Ce; centriole (×10,000).
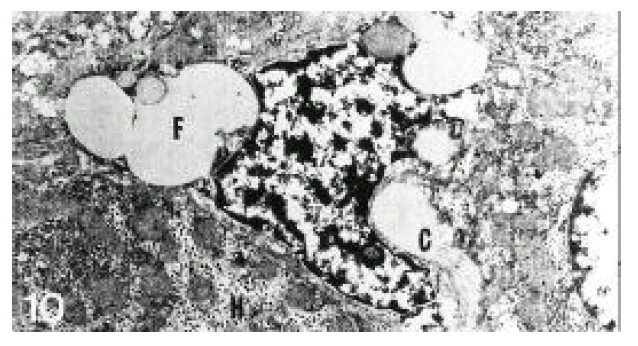
Electron micrograph of a hepatic stellate cell from normal liver. Coarse collagen fiber bundles (C) seemed to be arising from the cell surface. H; hepatocyte, F; fat vacuole (×10,000).
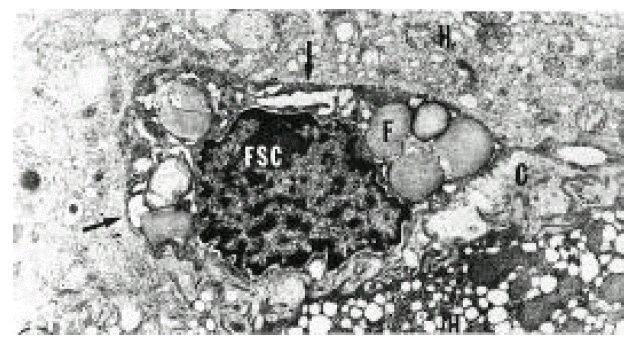
Electron micrograph of the liver in a patient with alcoholic fatty liver. A hepatic stellate cell (HSC) was conspicuous with its abundant and well-developed rough endoplasmic reticulum (arrows). The space of Disse contained many collagen bundles (C) (grade 2+). The smooth endoplasmic reticulum of hepatocytes was hypertrophied. H; hepatocyte, F; fat vacuole (×10,000).

Higher magnification of nuclear margin of hepatic stellate cell (HSC). At the periphery, microfilaments (arrow) were gathered in bundles parallel to the plasma membrane. Dense bodies (D) and pinocytotic vesicles (P) in close proximity to the cell surface were indicated. Bundles of coarse collagen fibers were present in the space of Disse (grade 2+) (×33,332).
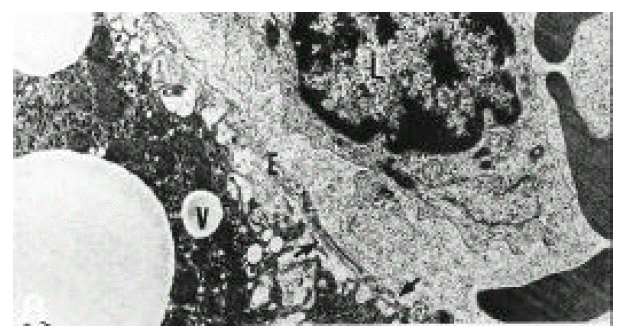
Electron micrograph of the liver of a patient with alcoholic fatty liver (case 4). Basement membrane-like material (arrows) existed (grade 3+) between the sinusoidal lining cell (E) and hepatocytes. Collagen fibers were also present in the intercellular space (grade 2+). A lymphocyte (L) had cytoplasmic projections in close proximity to the sinusoidal lining cell (E). Hepatocytes contained several fat vacuoles (V) (×10,000).

Electron micrograph of the liver of a patient with alcoholic fatty liver (case 4) showing double layered basement membrane-like material (arrows) existing (grade 3+) beneath endothelial lining cell (E). The space of Disse (DS) containing fibers and microvilli was decreased. H; hepatocyte (×20,000).
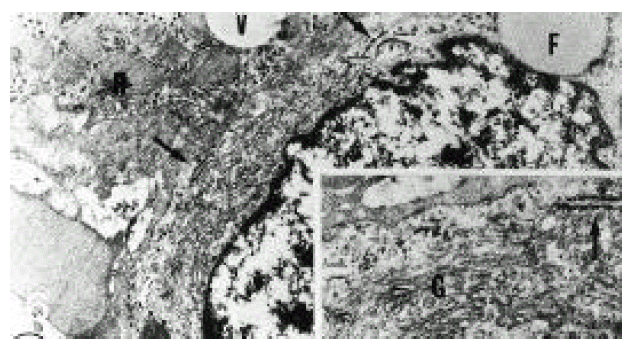
Electron micrograph of hepatic stellate cell (HSC) from normal liver. HSC had well-developed Golgi apparatus and a few rough endoplasmic reticulum (arrows). H; hepatocyte, V; fat vacuole, F; fat droplet (×13,332).
Inset: higher magnification of a part of HSC showing well-developed Golgi apparatus (G), normally short, narrowed cisternae of endoplasmic reticulum (arrow) and glycogen granules in the cytoplasm. The Golgi apparatus consisted of several long and short, flattened cisternae and numerous vacuole (×26,664).

Electron micrograph of a hepatic stellate cell (HSC) from normal liver. The HSC exhibited well-developed Golgi apparatus (G) and dilated endoplasmic reticulum (ER) which contained materials of the fluffy or fibrillar nature. Nc; nucleus (×20,000).
DISCUSSION
Alcohol causes a broad spectrum of liver disease, from fatty liver to cirrhosis. Alcoholic fatty liver is the most common and the least severe among those. One of the prominent histologic characteristics in alcoholic liver disease is interstitial fibrosis by collagen around swelled hepatocytes, which are frequently seen in centrilobular areas and result in bridging fibrosis from central areas to portal areas12). Mallory bodies are another important feature in alcoholic hepatitis, but these are frequently found in alcoholic cirrhrosis10). We could not observe the Mallory bodies in alcoholic fatty liver. The process of fibrosis continued without hepatitis or appearance of Mallory body in analcoholic baboon.
The electron microscopic observation of interstitial fibrosis of the liver is one of the prominent features of collagen deposition in the space of Disse that has profuse hepatic stellate cells and which is more common in alcoholic fatty liver than non-alcoholic one11). Many reports suggest that hepatic stellate cells of the liver (Ito cells or lipocytes) are responsible for this. However, the exact mechanism of collagen synthesis is still uncertain. Hepatic stellate cells of the liver are the principal collagen-producing cells in many liver diseases. Hepatic stellate cell activation and association with fibrosis, necroinflammatory activity, steatosis20). In alcoholic fatty liver, vitamin intoxication and long-term administration of alcohol, the number of hepatic stellate cells is decreased14). We studied to evaluate the correlation between alcohol and fibrosis by ultrastructural changes. We observed that the mean number of fat droplets per cell was significantly increased in patients with alcoholic fatty liver (1–60: mean 13.6) and non-alcoholic fatty liver (1–73: mean 12) than normal (1–33: mean 7.2). In previous studies, down-regulation of hepatic stellate cell may be associated with the appearance of transitional cells which have decreased number of fat droplets. In recent studies, the relationship between alcohol-induced hepatic stellate cells activation and severity of steatosis was shown14). We observed the same results that steatosis was closely related to the development of hepatic fibrosis. This is likely to reflect that both are attributable in part to the metabolic consequences of ethanol metabolism. We did not count hepatic stellate cells in each case. Also, we observed the microfilament bundles and dense bodies in hepatic stellate cells, suggesting transitional cells. Dense bodies may be the site of attaching the filament15). The existence of these organelles suggests that hepatic stellate cells are contractile cells. Contraction of these cells results in the contraction of sinusoids that are involved in the control of microcirculation in hepatic lobules16). The microcystic vesicles can be frequently observed in hepatic stellate cells, but their main function is not clear. Dilated cisternae of short and narrow endoplasmic reticulum in many hepatic stellate cells contain a flocculent material indicative of synthetic activity. In patients with alcoholic fatty liver with hepatic fibrosis, we observed basement membrane-like structure in the space of Disse beneath the endothelial cells. There is a direct correlation between the development of rough endoplasmic reticulum in hepatic stellate cells and the amount of collagen fibers5). We observed that the hypertrophy of rough endoplasmic reticulum in hepatic stellate cell is associated with the morphologic correlative of their activated fibrogenesis in the space of Disse. The microfilament and dense bodies are prominent features of myofibroblast concernied in fibrogenesis in alcoholic liver disease18). The myofibroblast exists in the area of the pericentral and sinusoid and is proliferated after alcohol administration. These cells produce type I, III and IV collagens19). The hepatic stellate cells, transitional cells and myofibroblasts are concerned in the fibrogenesis after liver injury20). It has been suggested that these cells have a common origin18, 21, 22). But the transformation of hepatic stellate cells to myofibroblasts should be further investigated. We observed a basement membrane-like structure in the space of Disse (so called “capillarization”) beneath the endothelial cells23). This capillarization of hepatic fibrogenesis interferes with the blood flow between sinusoid and hepatocytes, resulting in fibrogenesis and hepatic failure24). The preexisting sinusoid is changed functionally by newly developed collagen fibers4). The shunt formation between hepatic vein and portal vein, by overproduction of newly-developed vessels, is a compensatory mechanism for portal hypertension25). Hepatic stellate cell activation is a broad phenotypic response, characterized by distinct functional changes in proliferation, contractility, fibrogenesis26). Further insights into the regulation of hepatic stellate cells are believed to reduce morbidity and mortality in patients with chronic liver injury. The small number of patients was a limitation in this study for result analysis. Future studies, including many enrolled cases, will be needed for certifying our results.