Changes of Glomerular Basement Membrane Components in Vacor-Induced Diabetic Nephropathy
Article information
Abstract
Objectives
The thickening of the glomerular basement membrane in rats after Vacor ingestion was examined by electron microscopy. This study was performed to elucidate which biochemical components changed in the glomerular basement membrane after Vacor-induced diabetic glomerulopathy.
Methods
Immunohistochemical analyses of type IV collagen, laminin, fibronectin and chondroitin sulfate proteoglycan were performed. A single dose of Vacor (molecular weight 272), 80 mg/kg, was administered to adult male Wistar rats by orogastric canule, and the animals were sacrificed at 0.5, 1, 3, 7, 14, 28 and 56 days after administration.
Results
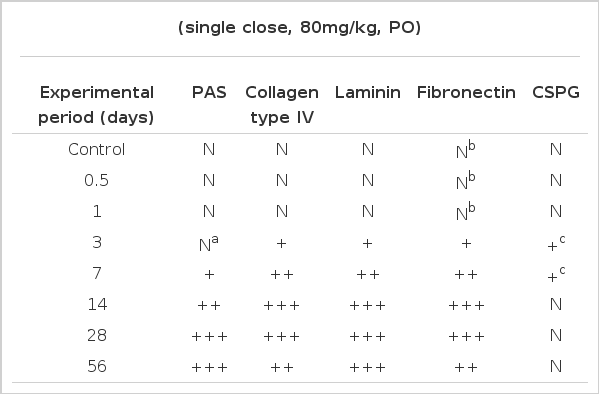
Mild thickening of the glomerular basement membrane was evident 7 days after Vacor administration, and the width of the glomerular basement membrane was more than twice that of normal controls at 28 and 56 days. Significantly increased expressions of type IV collagen, laminin, fibronectin and neutral polysaccharide in the thickened glomerular basement membrane were noted 14 to 56 days after administration, and a mildly increased expression of chondroitin sulfate proteoglycan appeared between 3 to 7 days.
Conclusion
These abnormally increased glomerular basement membrane components might be part of what causes diabetic nephropathy after Vacor administration
INTRODUCTION
Diabetic patients, who consume Vacor (N-3-pyridylmethyl-N’-p-nitrophenylurea, MW272), experience microangiopathy causing retinopathy and nephropathy1). The width of the capillary basement membrane in these patients thickens significantly compared to normal controls after 6 years of Vacor ingestion, on average.
A number of animal models of diabetes have suggested that hyperglycemia can produce microangiopathic changes2). Rodents made hyperglycemic with streptozotocin (STZ) or alloxan developed glomerular changes that were similar to, but not strictly comparable to, those of humans3). Vacor-induced diabetic rats developed acute glomerulopathy within a few months and the glomerulus revealed prominent thickening of the basement membrane4).
The glomerular filtration barrier consists of complex matrix constituents interposed between the glomerular endothelial and epithelial cells, which constitute the lining of the glomerular capillaries. They include collagen (mostly type IV), laminin, polyanionic proteoglycans (mostly heparan sulfate), fibronectin, entactin and several other glycoproteins5). These individual components of glomerular basement membrane (GBM) were immunohistochemically tagged using specific monoclonal antibodies and observed by light and electron microscopy 6–8). Changes of four major components of GBM, (type IV collagen, laminin, fibronectin and proteoglycan), were studied in Vacor-induced diabetic rats in this study.
MATERIALS AND METHODS
Adult male Wistar rats (n=42), weight 200–250g, were used for this study. A single dose of Vacor, 80 mg/kg of body weight, was administered to the experimental animals by an orogastric canule, while distilled water was given to controls. Blood glucose was measured by glucose oxidase before and after the treatment.
The experimental animals were sacrificed 0.5, 1, 3, 7, 14, 28 and 56 days after the administration of Vacor. Animals were perfused through the jugular vein with 300 ml of physiologic saline followed by an equal amount of 10% buffered formalin. Both kidneys were taken, and then routine formalin-fixed, paraffin-embedded tissue blocks were made for the immunohistochemical studies of glomerular basement membrane (GBM) components. Tissue sections, 4 μm in thickness, were obtained, and stained with hematoxylin-eosin (H&E) and periodic acid Schiff (PAS) stain.
For immunohistochemical studies of the GBM components, monoclonal antibodies for collagen type IV (Monosan, Netherlands), laminin (Monosan, Netherlands), fibronectin (Sigma Bioscience, U.S.A.) and proteogylcan chondroitin sulfate (CSPG, Biogenesis, U.K.) were used. Immunohistochemical studies were performed by using the peroxidase-antiperoxidase technique9). Paraffin sections were cut to 4μm in thickness. Deparaffined sections were soaked in absolute methanol containing 0.3% hydrogen peroxide for 30 minutes at room temperature in order to block endogenous peroxidase activity. After washing, the sections were incubated with 0.4% pepsin (Sigma) in 0.01 N HCI for 1 hour at 37°C, then treated with 0.05% protease type VII (Sigma) in phosphate-buffered saline (PBS), pH 7.2, for 15 minutes at 37°C. With these enzyme pretreatments, consistent visualization of collagen type IV in the formalin-fixed tissues was possible. Enzyme digestion was terminated with cold running tap water. After further washing in PBS, the sections were exposed to 1:20 diluted normal swine serum (DAKO-Immunoglobulins Ltd., Denmark) for 30 minutes at room temperature. Then sections were incubated with each primary antibody at a dilution of 1: 500 overnight at 4°C. After washing with PBS, treatment followed with anti-rabbit IgG swine serum (at a dilution of 1:20, DAKO), and PAP solution (at a dilution of 1:80, DAKO), for 30 minutes, respectively, at room temperature. Finally, the sections were soaked in 0.05 M Tris-HCI buffer, pH 7.6, containing 3,3′-diaminobenzidine hydrochloride (40 mg/100 ml) and hydrogen peroxide (0.015%) for 10 minutes, and counterstained with hematoxylin. Vascular walls and renal tubules in the specimens served as positive controls for the primary antibodies. As the negative control, the primary antibodies were replaced by nonimmune rabbit serum by the same procedure.
RESULTS
Glomerular changes examined by H&E and PAS stains
The glomeruli in control animals revealed patent capillary loops demarcated by a thin, delicate glomerular basement membrane. Mesangium was located among the capillary loops. The basement membrane and mesangium were positively stained by PAS stain. In the experimental groups, no histologic change was noted until 1 day after Vacor treatment. Mild mesangial widening was observed 3 days after treatment by PAS stain. Mild thickening of the GBM was evident at 7 days by H&E and PAS stains. Increased thickening of the GBM and mesangial widening were noted at 14 to 56 days by PAS and H&E stains (Fig. 1). The width of the GBM had more than doubled at 28 and 56 days compared with normal controls. Focal nodular proliferation of both glomerular endothelial and mesangial cells in the glomerulus was observed (Fig. 2).

Vacor-induced acute nephropathy in Wistar rats by H&E (A, B) and PAS stains (C, D) (X400) revealed prominent thickening of the GBM at 56 days (B, D) compared with normal controls (A, C).
Changes of GBM components by immunohistochemical stains
The glomeruli in control animals show thin linear staining along the GBM after immunohistochemical staining for collagen type IV and laminin (Fig. 3). Fibronectin and proteoglycan were also identified along the GBM, but they stained more faintly than collagen type IV and laminin.

Expression of collagen type IV in a control rat (A), 7 days (B) and 56 days (C) after Vacor administration (X400). The expression initially developed from the peripheral loop of the GBM and subsequently became diffuse and stronger along the GBM.
Immunohistochemical staining for collagen type IV was the most sensitive way to demonstrate GBM thickening, evident 3 days after a single, large dose (80 mg/kg) of Vacor treatment. The GBM was moderately thickened at 7 days, and was maximally thickened 28 to 56 days after the treatment by collagen type IV immunohistochemistry. The pattern was initially focal and global from the GBM peripheral loop, and became subsequently diffuse (Fig. 3). Moderately thickened immunoreactivity with collagen type IV was noted after as many as 56 days. Laminin in the GBM also thickened after Vacor treatment, and behaved similarly to collagen type IV (Fig. 4).
Fibronectin in the GBM appeared faintly at 12 hours and 1 day after Vacor treatment. Mild, focal and segmental thickening of fibronectin was evident at 3 days. From 7 days after Vacor treatment, the fibronectin presence in the GBM began to resemble that of collagen type IV and laminin (Fig. 5).
Chondroitin sulfate proteoglycan (CSPG) in the GBM did not change so prominently as type IV collagen, laminin and fibronectin. Only a mild focal thickening of CSPG was noted along the GBM at 3 to 7 days after Vacor treatment. No identifiable changes of proteoglycan in the GBM were noted from 14 to 56 days after Vacor treatment (Fig. 6).

Mildly increased expression of CSPG in mesangium at 7 days (A, X400), and normalized 28 days (B, X120) after Vacor administration.
The results are summarized in Table 1.
DISCUSSION
In humans, characteristic morphological changes of the glomeruli in diabetes mellitus include GBM thickening, diffuse glomerulosclerosis and nodular glomerulosclerosis 10). Diffuse thickening of the GBM occurs in virtually all diabetics, irrespective of the presence of proteinuria, and constitutes a part of diabetic microangiopathy. GBM thickening can be detected only by electron microscopy. Careful morphometric studies demonstrate that this thickening begins as early as 2 years after the onset of type I diabetes and, by 5 years, develops to about a 30% increase11). Diffuse and nodular glomerulosclerosis are late events for diabetics, and become pronounced after 10 to 20 years of diabetes10).
In Vacor-treated rats, the glomeruli reveal prominent thickening of the GBM within a short period. The width of the GBM is more than twice that of normal controls, 28 and 56 days after Vacor administration. Increased expressions of collagen IV, laminin, fibronectin and neutral mucopolysaccharide engendered the thickening of the GBM in this study. Proteoglycan immunoreactivity was not significantly different between Vacor-treated and normal control groups. In a study of human diabetic diffuse glomeruloscleosis, the enlarged mesangial matrix also revealed increased staining reactions for collagen IV, V, laminin and fibronectin, whereas the staining pattern for heparan sulfate proteoglycan (HSPG) was markedly reduced12). These data suggest that Vacor-induced glomerulopathy in rats can provide a useful experimental model to study human diabetic glomerulopathy.
Expression of type IV collagen and its mRNA in glomeruli and the interstitium of diabetic rats increased at 3, 7 and 14 days after streptozotocin (STZ) administration13). The expression was associated with up-regulation of transforming growth factor (TGF)-β. Administration of TGF-β increased collagen synthesis in normal rat glomeruli in a dose-dependent manner up to 5 ng/ml14). These data indicate a regulatory role for TGF-β in renal glomerular collagen synthesis in normal and diabetic rats. Inhibition of TGF-β and type IV collagen expression by insulin treatment suggests that they may be useful structural markers for determining the efficacy of therapeutic intervention during early diabetic nephropathy13). Concomitant increased staining patterns for laminin and type IV collagen have been reported in human diabetic glomerular lesions12) and experimental diabetic nephropathy15). Over-expression of TGF-β was also partly associated with the up-regulation of glomerular laminin gene expression in diabetic mice. The occurrence of collagen type III in late diffuse glomerulosclerosis could be interpreted as an irreversible change in glomerular structure16).
Fibronectin also increases in the GBM and mesangial matrix in human diabetic nephropathy17) and STZ-induced diabetic rats18,19). Fibronectin is a multifunctional matrix protein important in wound healing, and is markedly increased in glomerular crescents20). There are two phases of fibronectin metabolism in anti-GBM models of rapidly progressive glomerulonephritis (RPGN) in the rabbit20). Phase I is associated with increased glomerular fibronectin content from plasma, and phase II with increased fibronectin mRNA in glomeruli at days 7 to 14. This finding suggests that fibronectin synthesis may take place in glomeruli and that it may also be a molecule regulating glomerular cell proliferation and fibrosis.
Two types of proteoglycan, HSPG and basement membrane-specific CSPG, have been studied in human diabetes21) and diabetic rats22). The expression of HSPG decreases in the GBM16,23) and other capillaries24) in diabetes, while CSPG increases in STZ-diabetic rats22). A mildly increased expression of CSPG was noted only after 3 to 7 days of Vacor administration in this experiment. Other studies suggest that changes of CSPG displace the capillary endothelial cells from the GBM causing loss of fenestrae, while loss of the HSPG results in alteration of GBM charge-selectivity23). The abnormal levels of type IV collagen, laminin, fibronectin and proteoglycan may, in part, be the cause of diabetic nephropathy induced by Vacor administration.
CONCLUSION
To elucidate the biochemical component changes in the GBM in Vacor-induced diabetic glomerulopathy, immunohistochemical analyses of type IV collagen, laminin, fibronectin and CSPG were performed in adult male Wistar rats.
Mild thickening of the GBM was evident 7 days after Vacor administration, and the width of the GBM was more than twice that of normal controls at 28 and 56 days. Significantly increased expressions of type IV collagen, laminin, fibronectin and neutral polysaccharide in the thickened GBM was noted 14 to 56 days after the administration, and the mildly increased expression of CSPG was present after 3 to 7 days. These abnormal levels of GBM components might, in part, be the cause of diabetic nephropathy induced by Vacor administration.