Drug-Resistant Pulmonary Tuberculosis in a Tertiary Referral Hospital in Korea
Article information
Abstract
Objectives
To estimate the resistance rate and to correlate the clinical characteristics of resistant tuberculosis with the patients of pulmonary tuberculosis who were referred to the university hospital.
Methods
We prospectively performed sensitivity tests for all patients who were diagnosed as active tuberculosis by sputum smear or sputum culture from January, 1995 to June, 1996. Patients profile, previous treatment history, patterns of drug resistance, initial chest films and other clinical findings were analysed.
Results
Overall, 24(26.0%) of the 92 patients had resistance to at least one drug and 8(8.6%) had resistance to isoniazid (INH) and rifampin (RFP). Among the 66 patients without previous tuberculosis therapy, 11 (16.6%) were drug-resistant and 2 (3.0%) were multi-drug resistant. Among the 26 patients with previous therapy, 13 (50.0%) were drug-resistant and 6 (23.0%) were multi-drug resistant. For all 92, resistance to INH was most common (19.5%), followed by RFP (9.7%) and ethambutol (9.7%). Drug resistance was significantly high in previously treated patients and in cavity-positive patients. Treatment failure was also high in previously treated patients with resistant tuberculosis. In patients with primary resistance, treatment failure was not observed.
Conclusion
Sensitivity tests are strongly recommended in all culture positive patients with previous therapy but, in patients with primary resistance, sensitivity tests are not required. Proper combination chemotherapy should be given under careful surveillance.
INTRODUCTION
Although the prevalence rate of pulmonary tuberculosis is decreasing due to the National Tuberculosis Control Programme, drug resistant pulmonary tuberculosis has been a longstanding public health problem in Korea.1) The major concerns over drug resistance were a fear of spread of drug-resistant organisms and the ineffectiveness in chemotherapy of the patients infected with them. In general, the prevalence of drug resistance shows a close inverse relationship with the efficacy of antituberculosis treatment regimens, even though there were several reports that the patients with initial drug resistance responded fairly well with the conventional triple combined regimens and as good as sensitive cases with the intensive, short course regimens2,3). Also, multidrug resistant tuberculosis is a fatal disease because of the high mortality rate reported as 20% to 70%, depending on underlying diseases, especially AIDS, which is equivalent to the outcome for untreated tuberculosis4–6).
The nation-wide tuberculosis prevalence survey (NTPS) in Korea was performed at 5-year intervals since 1965, and the results show a decreasing tendency but still rather high in both prevalence rate and drug resistance7). From a practical point of view at university hospital level, the 3rd referral center, drug resistant pulmonary tuberculosis is somehow different from that of the nation-wide survey of tuberculosis based in various settings. So, we conducted this investigation, prospectively, to estimate resistance rate and to correlate the clinical characteristics of resistant tuberculosis with the patients of pulmonary tuberculosis who were referred to the university hospital.
MATERIALS AND METHODS
M. Tuberculosis isolates
The study population consisted of 92 patients who were diagnosed as pulmonary tuberculosis by sputum culture with sensitivity test at Chungnam National University Hospital, from January, 1995 to June, 1996.
Drug sensitivity tests
When M. tuberculosis was identified by routine culture, multi-drug sensitivity test was done at the laboratory of the Reasearch Institute of Korean National Tuberculosis Association (KNTA). The procedure of drug sensitivity test was based on the absolute concentration method described by Canetti et al8,9), with a little modification of inoculum preparation and size10). The tests were done in the Lowenstein-Jensen Medium and the drugs were added before inspissation at the concentration shown on the table. We have defined multi-drug resistance (MDR) as resistance to both INH and RFP or more drugs.
Severity and chest X-ray
Severity was classified by NTA method11). We interpreted chest radiographs taken at the time of diagnosis. Presence of cavity was determined by only simple chest radiographs.
Anti-tuberculosis chemotherapy
We administered standard regimen (2HREZ+4HRE or 9HRE) to all patients with or without previous therapy. Follow-up chest X-ray and sputum smear were examined completely. We defined treatment failure as smear positive after 6 months or more therapy.
Statistical analysis
Data are presented as number and percentage. Resistance ratio was compared using the student’s t-test or chi square test, when appropriate. A p value < 0.05 was considered significant.
RESULTS
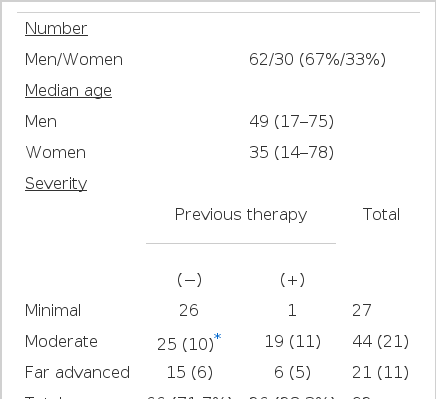
We diagnosed 92 patients as active tuberculosis by either sputum AFB smear or culture. The patients characteristics are shown in Table 1. Among 92 patients, 62 were men and 30 were women, with a mean age of 49 and 35 years, respectively. The 66 patients without previous anti-tuberculosis therapy were made up of 26 minimal, 25 moderately advanced and 15 far advanced disease patients. Of 26 patients with previous therapy, only 1 patient was minimal and the others were moderately advanced19) or far advanced6). Cavitary lesion was found in 16 patients (24.2%) of the group without previous therapy, and in 16 patients (61.5%) of those with previous therapy.
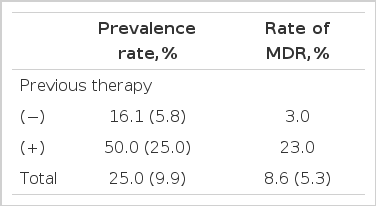
Overall, 24 (26.0%) of the 92 patients had resistance to at least one drug. Among 66 patients without previous therapy, 11 (16.7%) patients had resistance to at least one drug, and among 26 patients with previous therapy, 13 (50.0%) patients had resistance. Rate of multi-drug resistance is 3.0% in patients without previous therapy, and significantly high (23.0%, p<0.05) in patients with previous therapy (Table 2).
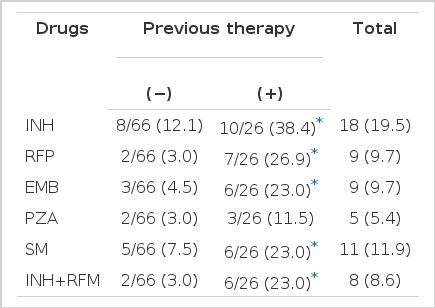
The rate of resistance to five first-line drugs are summarized in Table 3. For all 92, resistance to INH was most common (19.5%) followed by SM (11.9%), RFP (9.7%), EMB (9.7%), PZA (5.4%). Generally, resistance of patients with previous therapy is higher than those of patients without therapy.
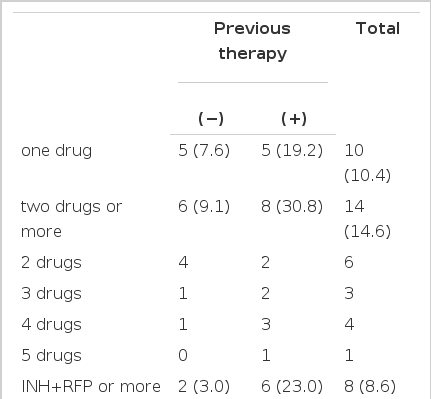
Resistance to 2 or more drugs are shown in Table 4. One patient had resistance to all 5 first-line drugs. Patients with resistance to RFP (n=9) also have resistance to other first-line drugs (INH 8, other drug 1). Resistance to second-line drugs are summarized in Table 5.
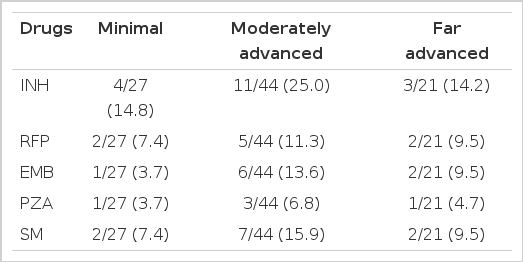
Resistance according to the radiologic severity are shown in Table 6. The difference in resistance rate according to the severity was not significant
Resistance in patients with cavity is higher than in patients without cavity, in case of INH (Table 7).
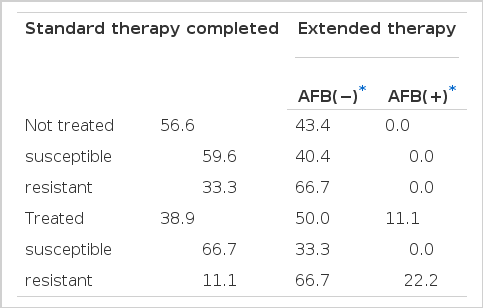
Outcome of chemotherapy was shown in Table 8. More extended therapy is needed in patients with previous therapy and with presence of cavity. Treatment failure was found only in patients with previous therapy (11.1%). All patients associated with treatment failure have one or more drug resistance. However, treatment failure in patients with primary resistance was not found.
DISCUSSION
Drug resistant tuberculosis is one of the most important factors in treatment failure. Treatment of patients with tuberculosis resistant to RFP, INH and other medications is risky, and results in limited efficacy. In western countries, introduction of RFP in 1971 gave short-term treatment to anti-tuberculosis therapy. It is very hopeful but, with the advent of AIDS, not only increasing prevalence of tuberculosis but also emergence of the drug resistant tuberculosis became an important health care issue even in western countries12–15).
The nationwide survey of tuberculosis in Korea showed a decreasing tendency of prevalence. But drug resistance tuberculosis is still high, and has been a major problem.
Reports on drug resistance were so different because of difference in study area, in time of study and in study population etc.
By NTPS in 1995, overall drug resistance was 9.9%, and resistance in patients without previous therapy was 5.8%. In patients with previous therapy, resistance was up to 25.0%7). Overall resistance rate in our study (26%) was higher than in NTPS. This difference may be due to difference in severity of patients. By NTPS, patients made up 40% of moderately advanced disease and 10% of far advanced disease, while patients of this study made up 48% of moderately advanced and 23% of far advanced disease. So, patients visited at our tertiary referral center were more likely to have severe disease.
Among 66 patients without previous therapy, 11 (16.7%) patients had resistance to at least one drug and, among 26 patients with previous therapy, 13 (50.0%) patients had that. Rate of MDR is 3.0% in patients without previous therapy and 23.0% in patients with previous therapy. So, the prevalence of the single-drug and multi-drug resistance in patients with previous treatment is significantly higher than in patients without previous therapy.
For prevention of emerging resistance, maintaining good compliance of the patients and appropriate prescription are needed. If retreatment is required, a susceptibility test should be done. Futhermore, introduction of directly observed therapy may be helpful in lowering resistance rate16,17).
Resistance to INH is the most frequent. The reported prevalence of INH resistance was variable from nation to nation and time to time. Recently, M. Demissie reported it as 8.4% in Ethiopia18), and M.T. Mendoza found that 17% of Phlippine patients19) had it. In our patients, an average of 19.5% had resistance to INH. Primary INH resistance was found to be 12.1% and highest, followed by streptomycin, ethambutol, pyrazinamide, rifampicin.
Resistance to RFP is most harmful and closely related to treatment failure. Cauthen reported the rate of RFP resistance as 0.6% with previously untreated patients and 3.3% with previously treated patients in the US20). In our study, resistance to RFP is 3.0% in patients without previous therapy and 26.9% in patients with previous therapy. Moreover, all patients with RFP resistance also have MDR. So, RFP resistance was a more serious problem in Korea.
Presence of cavity may be associated with a slow regression of the lesion and with more emerging of resistant strain21). In our study, cavity-positive patients had higher resistance to INH. More extended therapy is needed.
Treatment of primary resistant patients or selection of the drug is not firmly established17). In our study, a small but significant percentage of the patients without previous therapy also had drug resistance, but no treatment failure is developed in the observed period. So, somehow, extended therapy is needed in some patients and regimen change is not needed in any patients. But the follow-up period is short and further long-term observation of patients with primary resistance will be performed.
Sensitivity tests are strongly recommended in all culture-positive patients with previous therapy. But recommendation of a sensitivity test for patients without previous therapy is still debatable. Treatment failure may be seen in these patients22). A guide for optimal drug selection may be needed. For this reason, a susceptibility test is also needed in case of no history of previous therapy. In our study, however, treatment failure was not observed in patients with primary resistance, so a susceptibility test may not be required. Further large studies for recommendations of susceptibility tests of primary resistance are also needed.
In conclusion, sensitivity tests are strongly recommended in all culture-positive patients in those with previous therapy but, in cases of primary resistant tuberculosis, sensitivity tests are not required and proper combination chemotherapy should be given under careful surveillance.