The effects of remdesivir on mortality and the requirement for mechanical ventilation in patients with COVID-19: a systematic review stratified by disease severity
Article information
Abstract
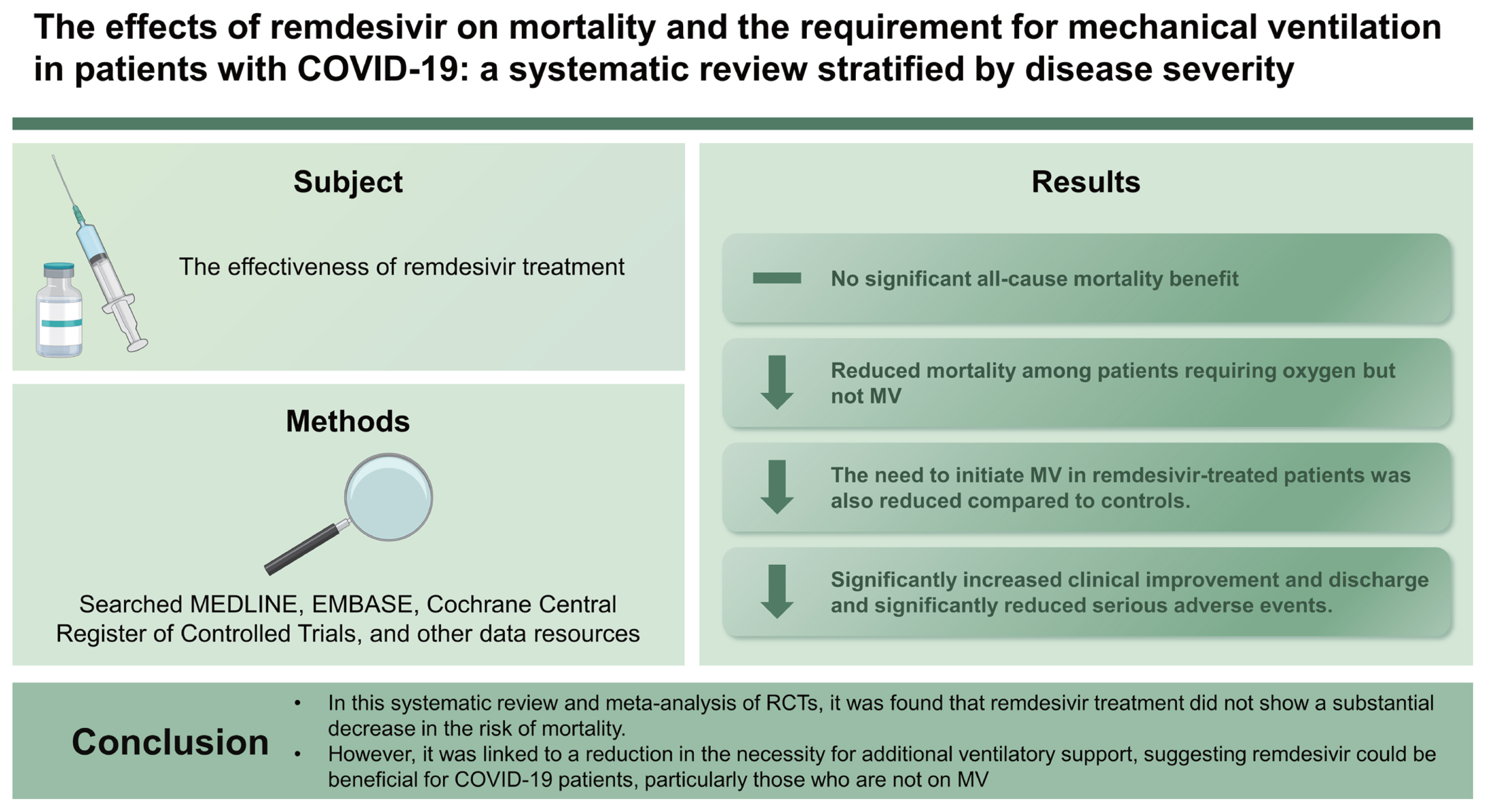
Background/Aims
The effectiveness of remdesivir treatment in reducing mortality and the requirement for mechanical ventilation (MV) remains uncertain, as randomized controlled trials (RCTs) have produced conflicting results.
Methods
We searched MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and other data resources to find RCTs published prior to April 10, 2023. The selection of studies, assessment of risk of bias, and meta-analysis were conducted according to PRISMA guidelines. The primary outcomes were all-cause mortality and the need to initiate MV.
Results
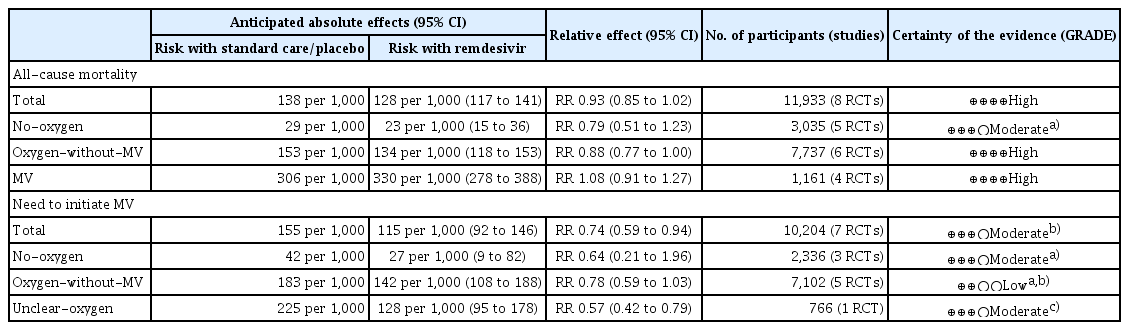
A total of 5,068 articles were screened, from eight RCTs comprising 11,945 patients. The meta-analysis found that, compared to standard care or placebo, remdesivir treatment provided no significant all-cause mortality benefit (pooled risk ratio [RR], 0.93; 95% confidence interval [CI], 0.85–1.02; 8 studies; high certainty evidence), while subgroup analyses revealed a trend towards reduced mortality among patients requiring oxygen but not MV (pooled RR, 0.88; 95% CI, 0.77–1.00; 6 studies; I2 = 4%). The need to initiate MV (pooled RR, 0.74; 95% CI, 0.59–0.94; 7 studies; moderate certainty evidence) in remdesivir-treated patients was also reduced compared to controls. Remdesivir significantly increased clinical improvement and discharge and significantly reduced serious adverse events.
Conclusions
In this systematic review and meta-analysis of RCTs, it was found that remdesivir treatment did not show a substantial decrease in the risk of mortality. However, it was linked to a reduction in the necessity for additional ventilatory support, suggesting remdesivir could be beneficial for COVID-19 patients, particularly those who are not on MV.
INTRODUCTION
The first randomized controlled trial (RCT) evaluating the efficacy of remdesivir treatment in COVID-19 patients was conducted in China, and the results indicated the treatment showed some promise [1]. In a subsequent Phase III RCT, known as the Adaptive COVID-19 Treatment Trial-1 (ACTT-1), remdesivir was found to significantly reduce the median time to recovery, especially among patients who required oxygen support [2]. However, there was no evidence of reduced mortality associated with the treatment. The findings of several subsequent RCTs [3–6] evaluating the effectiveness of remdesivir treatment in reducing mortality and improving clinical outcomes of COVID-19 patients remains controversial. A previous multicenter study conducted in Korea found that administering remdesivir to hospitalized adults with severe COVID-19 requiring low-flow oxygen resulted in clinical benefits, specifically a reduced need for mechanical ventilation (MV) [7]. Otherwise, the World Health Organization (WHO) Solidarity Consortium Trial and meta-analyses [8] showed that remdesivir provided no benefit to hospitalized COVID-19 patients who were already on MV; in non-hospitalized patients, a 3-day course of remdesivir was found to reduce the risk of hospitalization or death [9]. These varying results can be attributed to the heterogeneity in the severity of the disease in patients at the time of remdesivir administration and the timing of drug administration relative to symptom onset. To better understand the impact of remdesivir on COVID-19 patients, for the meta-analyses reported below we stratified patients from the selected RCTs into three categories based on the severity of their illness at randomization for remdesivir treatment: (1) those who did not require oxygen, (2) those who required oxygen but not MV, and (3) those who required MV.
METHODS
The systematic review and meta-analysis were conducted accordance with the recommendations provided in the Cochrane Handbook [10] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11]. The study protocol was approved by Korean COVID-19 guideline development committee [12].
Search strategy
We performed a living systematic review. The initial search was conducted on Jun 14, 2021. The comprehensive search resources were PubMed, Ovid-EMBASE, and CENTRAL, as well as the Korean KMBASE medical database. A hand search through reference lists of relevant primary and review articles was also performed for completeness. After the initial search, we updated the results every month beginning in August 2021 and continuing through to April 10, 2023, using Ovid-MEDLINE. The complete electronic search strategy for each database is presented in Supplementary Table 1.
Eligibility criteria and study selection
Articles that matched the following requirements were considered: (1) the patients were adults with COVID-19; (2) the interventions included remdesivir treatment; (3) the comparator was a placebo or the standard-of-care (SOC) treatment; (4) outcome reporting included primary or secondary outcomes (the primary outcomes included all-cause mortality and the need to initiate MV; secondary outcomes included clinical improvement, serious adverse events, and discharge), and (5) the study was designed as an RCT. Two review authors (SR and SYY) both independently evaluated each publication for inclusion based on title and abstract, and then reviewed relevant full-text articles. Disagreements during the review process were addressed by consensus with the involvement of a third review author (MC).
Risk of bias assessment and data extraction
The authors worked in pairs to independently assessed the quality of the selected studies using the Cochrane risk-of-bias tool [10]. Disagreements were addressed by consensus with the participation of a third review author (MC).
Two review authors (SR and MC) extracted information from each included trial. These evaluations were carried out independently and yielded separate assessments. Any disagreements were resolved by discussion and third opinion (SYY). The data extraction form included items addressing the study characteristics, classification of disease severity, and outcomes.
To ensure that all included studies were assessed using the same criteria, additional data were collected from supplementary materials when available. If supplementary materials were not available, the intention-to-treat principle was used to assess the studies, even if the principle was not explicitly defined in the original articles. To obtain additional information, we also contacted the corresponding authors of the included trials regarding insufficient information.
Rating the certainty of evidence
Certainty of evidence was graded using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach [13]. The factors that were considered to reduce the certainty were risk of bias, inconsistency, indirectness, imprecision, and publication bias. The results were presented as high, moderate, low, or very low quality by outcomes.
Data synthesis and statistical analyses
Synthesis of the extracted data was performed both quantitatively and qualitatively. For the meta-analysis of selected trials, continuous outcomes were presented as mean differences (MD) or standardized MD. Dichotomous outcomes were presented as risk ratios (RRs), and time-to-event data were synthesized as hazard ratios (HRs). We applied a random effects model to assess heterogeneity among the trials. Heterogeneity was resolved in the subgroup and sensitivity analyses.
We classified patients from the selected RCTs into subgroups based on the severity of their illness at randomization for the administration of remdesivir: (1) No-oxygen subgroup, individuals who did not require supplemental oxygen for their medical condition or treatment; (2) Oxygen-without-MV subgroup, individuals who required supplemental oxygen to support their breathing but did not require the use of MV and may have received oxygen through nasal cannula, face mask, or high flow oxygen delivery methods; (3) MV subgroup, individuals who required the use of MV to assist or control their breathing; (4) Oxygen-unclear-MV subgroup, individuals who received supplemental oxygen, but the available information does not specify whether they also received MV ; and (5) Unclear-oxygen subgroup, individuals for whom the available information does not provide clarity on whether they received supplemental oxygen or not. More detailed descriptions of the severity of COVID-19 illness in the study patients are available in Supplementary Table 2. Statistical analyses were performed using Review Manager software version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark).
RESULTS
Description of included studies
A total of 5,055 articles were retrieved from the databases. After excluding duplicates, 4,208 articles were identified. As new evidence regarding COVID-19 treatment emerges continually, a total of 5,068 articles were screened through monthly search updates. Based on the selection criteria, 240 articles were selected for full-text review. A final total of 8 RCTs comprising 11,945 patients were included in this systematic review. Details of the study selection and review flowchart are presented in Figure 1. Among patients enrolled in the eight selected studies, the clinical severity of COVID-19 was reclassified according to pre-defined criteria, as follows: five RCTs for cases requiring no oxygen whether hospitalized or not (no-oxygen subgroup) [2,3,5,8,9], six RCTs for cases requiring oxygen but not MV (oxygen-with-out-MV subgroup) [1,2,4–6,8], three RCTs for cases receiving MV (MV subgroup) [2,5,6], two RCTs for cases receiving oxygen, but it was not clear whether they received MV or not (oxygen-unclear-MV subgroup) [2,6], and one RCT for cases with unclear oxygen supply (unclear-oxygen subgroup) [2]. The characteristics of the included studies are presented in Table 1. The results of the risk-of-bias summary are presented in Supplementary Figure 1. Most studies had a low risk of bias. The GRADE evidence profiles and summary of the findings are presented in Table 2.
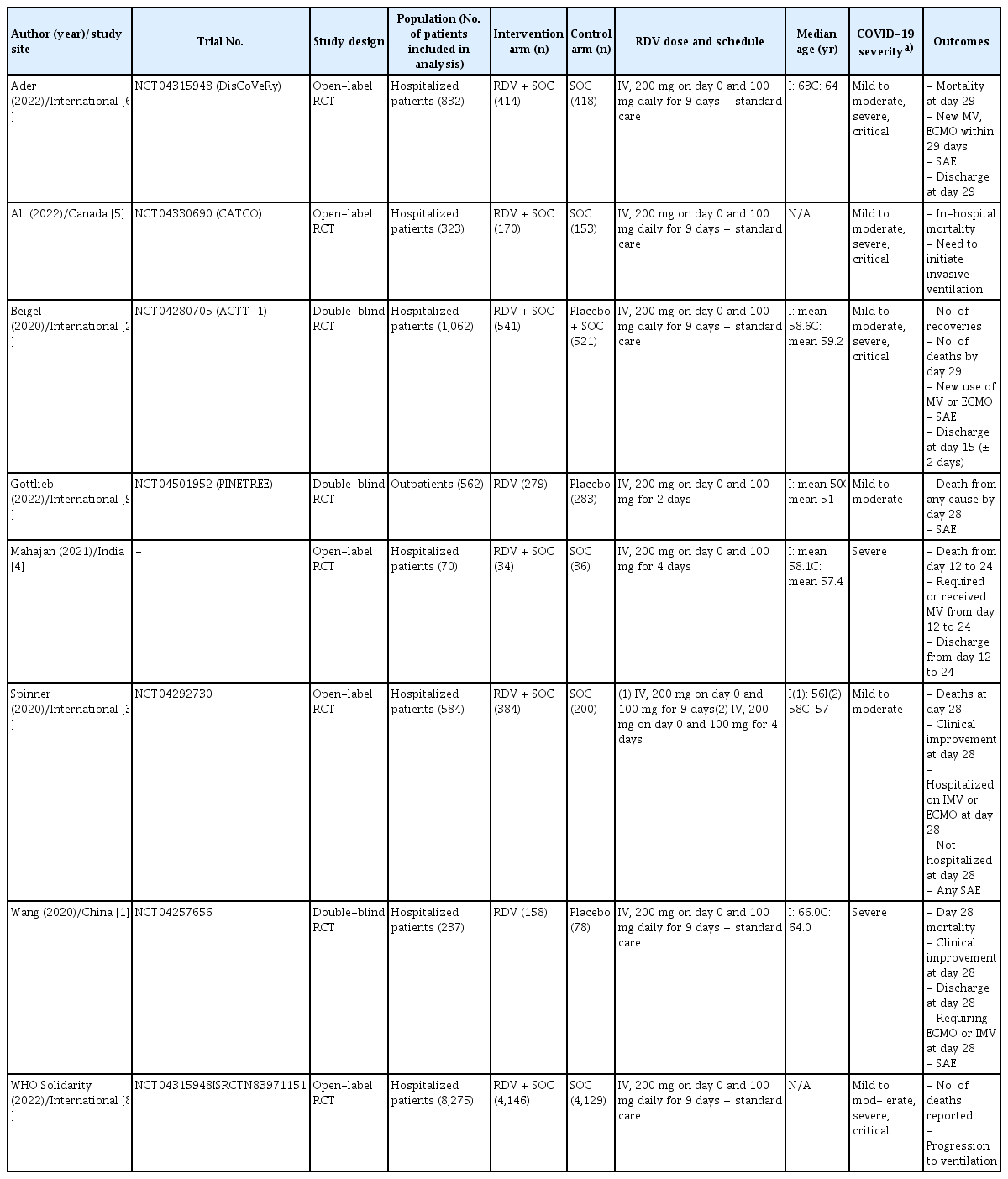
Baseline study characteristics of published randomized controlled trials of remdesivir in COVID-19 patients included in the meta-analysis
All-cause mortality
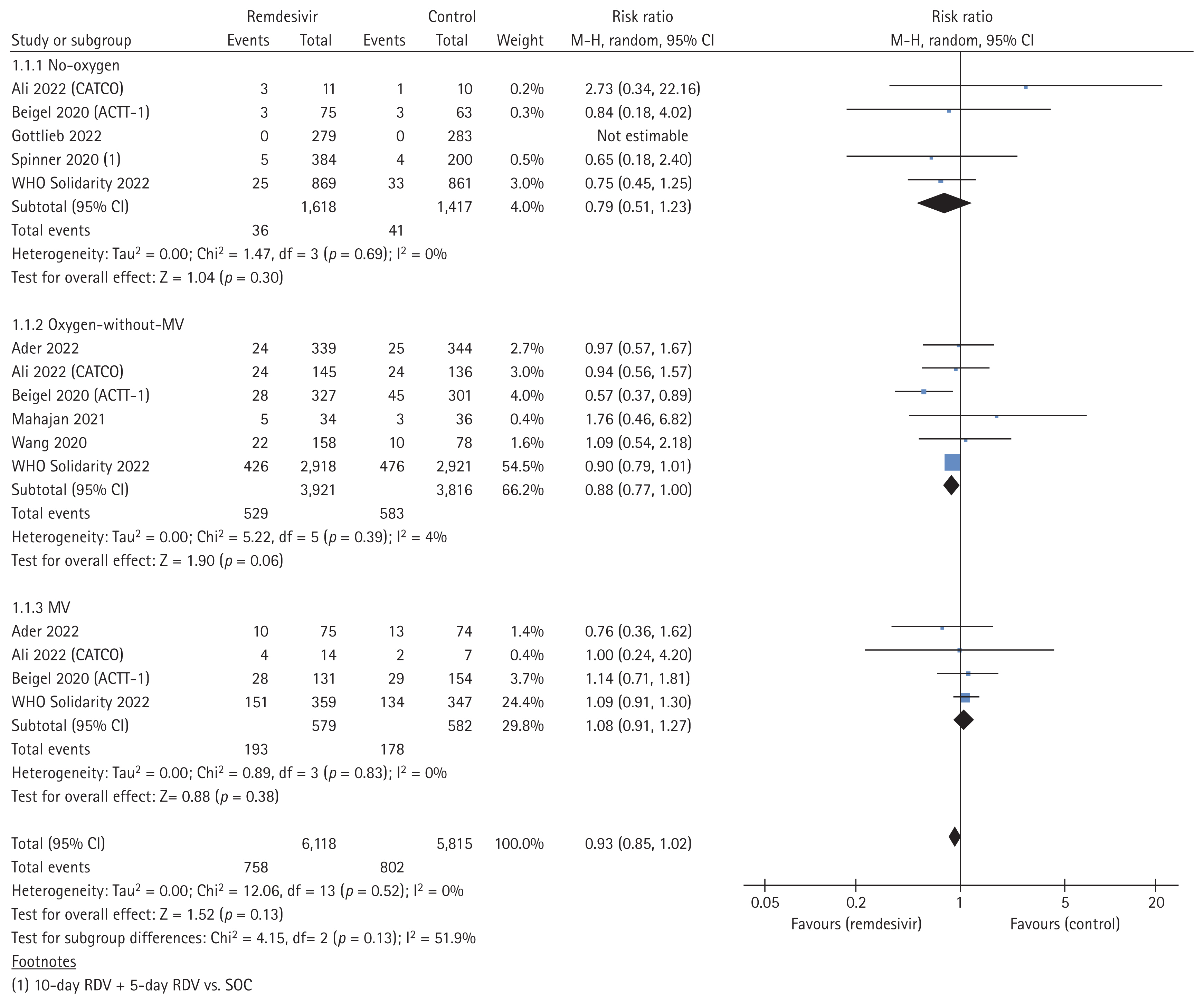
Eight studies, comprising 6,118 cases in the remdesivir arm and 5,815 controls in the placebo or SOC arm, investigated the effect of remdesivir treatment on all-cause mortality. We included mortality data from the studies, primarily reported at 28 days but also included in-hospital mortality data. Compared with the control arm, remdesivir treatment did not significantly reduce mortality (pooled RR, 0.93; 95% confidence interval [CI], 0.85–1.02; I2 = 0%; high certainty evidence; Fig. 2). In the subgroup analyses based on clinical severity of COVID-19, remdesivir treatment failed to reduce all-cause mortality in patients who required no oxygen or receiving MV (no-oxygen subgroup; pooled RR, 0.79; 95% CI, 0.51–1.23; 5 studies; I2 = 0%; MV subgroup; pooled RR, 1.08; 95% CI, 0.91–1.27; 4 studies; I2 = 0%). However, the subgroup requiring oxygen but not MV exhibited a tendency towards decreased all-cause mortality (pooled RR, 0.88; 95% CI, 0.77–1.00; 6 studies; I2 = 4%).
Need to initiate MV
The need to initiate MV was reported as an outcome parameter in seven studies comprising 5,253 remdesivir-treated cases and 4,951 controls. The majority of studies included in the analysis reported the outcome values at day 28, also including studies with unspecified time points. The percentage of patients for which MV was initiated was significantly lower in the remdesivir-treated group than in the control group (pooled RR, 0.74; 95% CI, 0.59–0.94; I2 = 49%; moderate certainty evidence; Fig. 3). Subgroup analyses according to the clinical severity of COVID-19 revealed that remdesivir treatment failed to reduce the percentage of patients requiring initiation of MV among both patients who did not require oxygen at the time of randomization (no-oxygen subgroup; pooled RR, 0.64; 95% CI, 0.21–1.96; 3 studies; I2 = 36%), and patients who received oxygen therapy but not MV (oxygen-without-MV subgroup; pooled RR, 0.78; 95% CI, 0.59–1.03; 5 studies; I2 = 40%). In addition, remdesivir treatment was shown to significantly reduce the percentage of patients for whom MV was initiated in subgroup it was not clear whether they received oxygen or not (RR, 0.57; 95% CI, 0.42–0.79; I2 = not applicable).
Secondary outcomes
Secondary outcomes included clinical improvement, serious adverse events, and discharge. Clinical improvement was defined as improvement on each study-defined ordinal scale at day 28 from randomization. Clinical improvement was evaluated as an outcome parameter in three studies that together comprised 1,075 remdesivir-treated cases and 796 controls. Remdesivir treatment resulted in clinical improvement (pooled RR, 1.08; 95% CI, 1.03–1.13; I2 = 0%; Supplementary Fig. 2): subgroup analyses by clinical severity revealed that this significant result occurred consistently except among patients receiving MV (no-oxygen subgroup; pooled RR, 1.07; 95% CI, 1.02–1.13; 2 studies; I2 = 0%; oxygen-without-MV subgroup; pooled RR, 1.12; 95% CI, 1.03–1.21; 2 studies; I2 = 0%; MV subgroup; RR, 0.96; 95% CI, 0.76–1.22; I2 = not applicable). Five studies, which included 1,756 remdesivir-treated cases and 1,495 controls, reported serious adverse events. The pooled analysis revealed that remdesivir treatment significantly reduced the percentage of patients who experienced serious adverse events as compare with the control arm (pooled RR, 0.73; 95% CI, 0.55–0.98; I2 = 65%; Supplementary Fig. 3). Subgroup analyses according to clinical severity revealed that this statistically significant result was observed only in patients who did not require oxygen (pooled RR, 0.47; 95% CI, 0.29–0.74; 3 studies; I2 = 0%) but not in patients who received oxygen without MV (oxygen-without-MV subgroup; RR, 0.70; 95% CI, 0.43–1.17; I2 = not applicable; any-oxygen subgroup; pooled RR, 0.92; 95% CI, 0.69–1.23; 2 studies; I2 = 77%).
Discharge of the patients as an outcome parameter was analyzed in five studies comprising 1,515 remdesivir-treated cases and 1,249 controls. Remdesivir treatment tended to increase the percentage of patients who were discharged compared with the control group (pooled RR, 1.11; 95% CI, 1.01–1.21; I2 = 46%; Supplementary Fig. 4). Subgroup analyses revealed significant differences between the remdesivir-treated and control arms occurred only among patients who did not require oxygen (no-oxygen subgroup; pooled RR, 1.08; 95% CI, 1.01–1.16; 2 studies; I2 = 0%; oxygen-without-MV subgroup; pooled RR, 1.09; 95% CI, 1.00–1.18; 4 studies; I2 = 7%; MV subgroup; pooled RR, 1.59; 95% CI, 0.51–4.97; 2 studies; I2 = 85%).
Sensitivity analyses
The influence of sponsor support was investigated in the sensitivity analyses. Two of the studies were conducted with funding from pharmaceutical companies [3,9]. After excluding these two studies, there were some changes in the secondary outcomes: remdesivir treatment failed to reduce serious adverse event and increase discharge rates (serious adverse events: pooled RR, 0.86; 95% CI, 0.69–1.09; I2 = 51%; discharge: pooled RR, 1.13; 95% CI, 0.99–1.29; I2 = 52%). In addition, since these two studies included patients who did not require oxygen, there were some changes in the results of subgroup analysis according to the severity of illness. Remdesivir treatment for patients in the no-oxygen subgroup was found to have no benefit with respect to the percentage of patients who experienced clinical improvement or serious adverse events, or were discharged (clinical improvement; RR, 1.06; 95% CI, 0.97–1.15; I2 = not applicable; serious adverse events; RR, 0.56; 95% CI, 0.20–1.59; I2 = not applicable; discharge; RR, 1.13; 95% CI, 0.92–1.40; I2 = not applicable) (Supplementary Fig. 5).
DISCUSSION
Our systematic review and meta-analysis of RCTs found that remdesivir treatment did not significantly reduce the risk of mortality but was effective in reducing the need for additional ventilatory support. Although the treatment failed to reduce all-cause mortality in all populations selected for the RCTs, subgroup analyses revealed a trend towards reduced mortality in patients requiring oxygen but not MV (pooled RR, 0.88; 95% CI, 0.77–1.00; 6 studies; I2 = 4%). The use of remdesivir was also associated with a greater likelihood of clinical improvement and survival to discharge within 28 days. No significant safety signal was evident. These findings suggest that remdesivir could be beneficial for COVID-19 patients, especially those who are not on MV. This meta-analysis is notable for its strength in subgrouping patients based on their oxygen therapy and MV requirements upon admission, allowing for the identification of a beneficial group for remdesivir. Moreover, the inclusion of significant secondary measures, besides mortality, emphasizes the study’s importance.
Several systematic reviews and meta-analyses have assessed the efficacy of remdesivir for COVID-19 treatment; only four out of these studies were current and provided comprehensive evaluation of the efficacy of remdesivir for COVID-19 treatment [14–17]. Lee et al. [14] reviewed eight RCTs published prior to May 2022 and reported that remdesivir reduced mortality in patients requiring supplemental oxygen but not MV (OR, 0.89; 95% CI, 0.79–0.99). The authors conducted a Bayesian analysis to elucidate the probability of remdesivir reducing mortality, which was 93.8%. Similar to our meta-analysis, Lee et al. [14] conducted a matched meta-analysis using studies we incorporated, demonstrating comparable estimated treatment effect. However, Lee et al. [14] concentrated solely on mortality as the outcome measure, not evaluating other significant outcomes such as the need for MV or safety concerns. In Beckerman et al.’s [15] meta-analysis, they focus on a group of patients who required supplementary oxygen but were not initially on MV. It demonstrated a significant decrease in the risk of mortality for individuals treated with remdesivir who received either no or low-flow oxygen. However, this benefit was not observed in those who received “high-flow oxygen”. The definition of the high-flow oxygen therapy group was not clearly specified, and the sizes of the subgroups were relatively small. Grundeis et al. [16] found that remdesivir did not show a significant reduction in mortality. This meta-analysis encompassed studies involving patients described as “hospitalized with moderate to severe COVID-19” in individual trials. As noted by the authors, the definition of severity was heterogeneous throughout the studies. Furthermore, categorizing patients who required “hospitalization but not MV” into a single group of “severe” disease is considered too broad for clinical decision-making and most clinical guidelines categorize severity group based on the degree of respiratory support. Nonetheless, the fact that there was a significantly reduced risk of initiating MV in the remdesivir group, suggesting a potential advantage in using remdesivir, is noteworthy.
In the latest study by Amstutz et al. [17], they collected additional data from clinical trials and conduced meta-analyses using individual patient data (IPD). Their results showed that remdesivir treatment was associated with a reduced requirement for initiating invasive MV and a decrease in overall mortality. These findings are consistent with our own study, indicating a reduction in the necessity for new MV among hypoxic patients treated with remdesivir and a lower risk of mortality in this group, although the difference did not achieve statistical significance. The discrepancy in the effect of remdesivir on mortality between the two meta-analyses can be attributed to differences in methodology. The IPD meta-analysis achieved a more precise categorization of disease severity by obtaining unpublished information directly from the study investigators. This led to larger sample sizes in each group, consequently increasing statistical power to prove mortality benefit. This interpretation is supported by the consistent finding that remdesivir reduced the risk of requiring MV in both studies.
The need to initiate MV is an important intermediate outcome; the requirement for MV represents progression of respiratory failure and has been associated with poor outcomes in severe community-acquired pneumonia [18]. Furthermore, MV itself is associated with various complications, including ventilator-associated pneumonia, lung injury, vascular thromboembolism, muscle wasting, and discharge to long-term care facilities [19]. Reduction of MV leads to significant benefit for patients, which may not be expressed as reduction in mortality. Therefore, our results provide strong evidence for the use of remdesivir in the patients with COVID-19 who require oxygen.
It is crucial to acknowledge several limitations of this review. Firstly, the exclusion of non-published and non-English studies introduces the potential for publication bias, which may affect the validity and generalizability of the findings. However, to ensure the certainty of evidence we made the decision to include only published articles. Moreover, we conducted manual checks to ensure that no important trials were missed during the selection process. Secondly, heterogeneity arises from variations in the specifics of study settings, such as differences in protocols and methodologies, as well as variations in population characteristics, notably severity scales. Moreover, variations in interventions administered to the standard-of-care group can contribute to heterogeneity within the review. Therefore, we categorized the details of severity as best as possible and performed subgroup and sensitivity analyses to account for any variations. Lastly, the included studies were conducted at different stages of the pandemic, thus introducing the possibility of discrepancies in baseline patient characteristics that may have impacted the study outcomes. The pandemic stages could not be classified exactly due to overlapping periods. However, the types of virus and research periods appear to be similar. Therefore, any differences in baseline patient characteristics are unlikely to have a critical impact on the results.
In conclusion, the administration of remdesivir did not significantly reduce mortality risk. There was a tendency towards reduced mortality in patients who required oxygen but were not on MV. Remdesivir also demonstrated effectiveness in reducing the need for additional ventilatory support, suggesting potential benefits for COVID-19 patients, particularly those who are not mechanically ventilated. These findings underscore the significance of early diagnosis and antiviral treatment for COVID-19 patients, especially those with risk factors for severe illness. As our society transitions into an endemic state for this disease, it remains crucial to emphasize the importance of seeking prompt medical care for high-risk populations.
KEY MESSAGE
1. A systematic review and meta-analysis of 8 RCTs involving 11,945 patients assessed the impact of remdesivir on COVID-19 outcomes.
2. Despite not showing a significant reduction in overall mortality, the analysis revealed that remdesivir-treated patients showed a decreased need to initiate MV compared to controls.
3. This suggests that remdesivir could be particularly useful for COVID-19 patients who are not on MV.
Notes
CRedit authorship contributions
Seungeun Ryoo: investigation, data curation, formal analysis, validation, software, writing - original draft, writing - review & editing, visualization; Miyoung Choi: conceptualization, methodology, investigation, data curation, formal analysis, validation, software, writing - original draft, writing - review & editing, visualization, funding acquisition; Su-Yeon Yu: investigation, data curation, formal analysis, writing - original draft; Young Kyung Yoon: conceptualization, investigation, data curation; Kyungmin Huh: conceptualization, investigation, data curation, validation, writing - original draft, writing - review & editing; Eun-Jeong Joo: conceptualization, investigation, data curation, validation, writing - original draft, writing - review & editing
Conflicts of interest
The author discloses no conflicts.
Funding
This research was supported by National Evidence-based Healthcare Collaborating Agency (NECA-P-21-004, NECA-A-22-008, NECA- A-23-010).