Factors influencing occurrence of metachronous gastric cancer after endoscopic resection: a systematic review and meta-analysis
Article information
Abstract
Background/Aims
Metachronous gastric cancer (MGC) can occur after endoscopic resection for gastric cancer. Further studies on factors other than Helicobacter pylori infection are needed. This systematic review and meta-analysis aimed to evaluate risk factors for metachronous recurrence of endoscopically resected gastric cancer.
Methods
We searched medical literature published by February 2023 and identified patients with MGC after endoscopic resection for gastric cancer. The occurrence of MGC and the presence of intestinal metaplasia (IM), severe atrophic gastritis (AG), and H. pylori infection were quantitatively analyzed.
Results
We identified 2,755 patients from nine cohort studies who underwent endoscopic resection for gastric cancer by 2018. Those with severe AG or presence of IM had a significantly higher incidence of MGC than those without (RR 2.00, 95% CI 1.35–2.98, I2 = 52% for severe atrophy on antrum; RR 7.08, 95% CI 3.63–13.80, I2 = 0% for antral IM). Absolute risk difference of MGC occurrence was 7.1% in those with severe AG and 9.2% in those with IM. The difference in incidence rate per 1,000 person-years was 17.5 person-years for those with severe AG and 24.7 person-years for those with IM. However, H. pylori eradication did not significantly affect the occurrence of MGC (RR 1.18, 95% CI 0.88–1.59, I2 = 10%).
Conclusions
Gastric cancer patients with severe AG or presence of IM had a 2.0-fold or 7.0-fold higher risk of MGC occurrence after endoscopic resection than those without, respectively. They need more stringent follow-up to monitor MGC occurrences (CRD42023410940).
INTRODUCTION
Endoscopic resection (ER) is now a standard treatment modality for gastric cancer (GC) when the GC is small and limited to the mucosa. This treatment has no significant difference in short-term outcomes of overall of disease-free survival compared with laparoscopic gastrectomy in early GC. ER also has fewer late complications and requires a shorter hospital stay than laparoscopic gastrectomy [1,2]. However, local treatments such as ER have a high risk for development of metachronous gastric cancer (MGC) in the residual stomach [3–5].
There are several risk factors for the occurrence of MGC after ER. Although Helicobacter pylori (H. pylori) infection is the most important factor, it can be managed [6]. Atrophic gastritis (AG) and intestinal metaplasia (IM) are precancerous GC lesions in the general population [7,8]. Since advanced AG and IM have a high risk of gastric adenocarcinoma, endoscopic surveillance is recommended [9,10].
Although the influence of AG or IM is considerable in the development of GC, there is no quantifiable evidence to help predict the risk of MGC occurrence in patients who have undergone ER for GC. Thus, this study conducted a systematic review and meta-analysis on the risk of MGC occurrence when AG or IM developed after ER for GC. This systematic review and meta-analysis has been registered in PROSPERO (CRD42023410940).
METHODS
Search strategy and study selection
We searched MEDLINE, Embase, and Cochrane centrally controlled trial registries without language restrictions for studies published according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines from inception through February 2023 to identify potential studies [11]. Suitable studies were investigated for effects of the degree of mucosal atrophy and the presence of IM on MGC occurrence after ER for GC (Supplementary Table 1).
Inclusion criteria for this study were: (i) studies on adults 19 years of age or older with GC who underwent ER, (ii) studies with evaluation of severe AG or IM presence, (iii) studies that clearly cited evaluation method (endoscopy, histology, or serology), (iv) studies on MGC defined as recurrence of GC elsewhere in the stomach at one year after complete ER of GC, (v) studies with follow-up period of at least two years after ER, and (vi) studies with dichotomous assessment of the occurrence of MGC. We extracted all endpoints from the last follow-up using the most recent publication from each trial. Studies in which gastric ‘adenoma’ were resected or metachronous gastric ‘adenoma’ occurred were excluded. Studies using ambiguous methods for assessing severe AG or IM were also excluded.
Two investigators (YC and JMP) and a professional librarian from the Catholic University of Korea performed literature searches independently of each other. Search terms used are detailed in Supplementary File. All potentially relevant references were obtained and evaluated to assess qualifications against the predefined criteria by investigators independently. Titles and abstracts were reviewed to extract relevant studies. Full texts were screened to identify eligible studies that met inclusion criteria. We resolved disagreements among investigators through discussion and, if necessary, consultation with a third reviewer (BWK) who was one of the authors of this study. There were no language restrictions. Foreign language papers were translated when necessary.
Outcome assessment
The primary outcome was the effect of the presence of IM or severe AG on the occurrence of MGC, which was compared with that of patients without IM or with mild to moderate AG. IM was histologically defined by the Sydney classification. Mucosal atrophy was defined variously by three parameters: endoscopy, serology, and histology. Endoscopic evaluation was examined according to Kimura-Takemoto classification (closed type I/II/III and open type I/II/III) or updated Sydney System (none, mild, moderate, or marked) [12,13]. Included studies evaluated open type II and type III as severe AG. Serologically, mucosal atrophy was evaluated by pepsinogen (PG) I and PG II. PG is a proenzyme produced in gastric mucosa. When atrophic change occurs, PG I level produced in chief cells decreases, as does the PG I/II ratio. In general, severe AG is determined when the PG I/II ratio is less than 3.0 [14–16]. Histologically, mucosal atrophy was classified according to the updated Sydney System [13].
Data extraction
All data were extracted independently by two investigators (YC and JMP) with dichotomous results (with or without MGC). The following data were also extracted for each trial: geographical location, country of origin, number of centers, number of patients, sex, age, alcohol intake, smoking history, history of H. pylori infection and eradication, follow-up period, number of patients with MGC, presence of IM, severity of AG at the time of ER, and anatomical, gross, and pathological features of primary cancer. Data were extracted using evaluation parameters of AG and IM.
Quality assessment and data synthesis
Quality and risk of bias were independently assessed by two investigators (YC and JMP). Because included studies were non-randomized studies, the ‘Newcastle–Ottawa Scale for Cohort Study Quality’ was used. If there were differences of opinion between the two investigators, the discrepancy was resolved through discussion. We performed the systematic review and meta-analysis following the Preferred Reporting Items for a Systematic Review and Meta-analysis guideline [17,18].
Data for subsequent development of GC according to the degree of AG and H. pylori infection status were combined using a random-effect model and Mantel-Haenszel estimation method. A random-effect model was also used for MGC occurrence according to subgroup analyses of AG and the presence or absence of IM.
Taylor series and Byar method were used for the incidence rate per 1,000 person-years of MGC by severe AG and presence of 95% confidence interval (CI). I2 was used to assess heterogeneity between studies. To define a significant degree of heterogeneity, we evaluated heterogeneity between studies using both the I2 statistic with a cutoff of ≥ 50% and the χ2 test with p < 0.10. Review Manager V.5.4.1 (RevMan for Windows 2020; Nordic Cochrane Center, Copenhagen, Denmark) and Excel were used for the analysis.
RESULTS
Study inclusion and characteristics
Through the planned search strategy, 96 documents were identified. After reviewing titles and abstracts, 40 studies that appeared to be potentially inclusive were searched and evaluated (Fig. 1). In these studies, 2,755 patients were included from 9 articles reporting relevant data from 8 individual studies [19–27]. After patients were diagnosed with GC, they underwent ER. They were then followed for more than two years at the attending hospital. These patients were evaluated for the presence of IM or degree of AG at the time of ER.
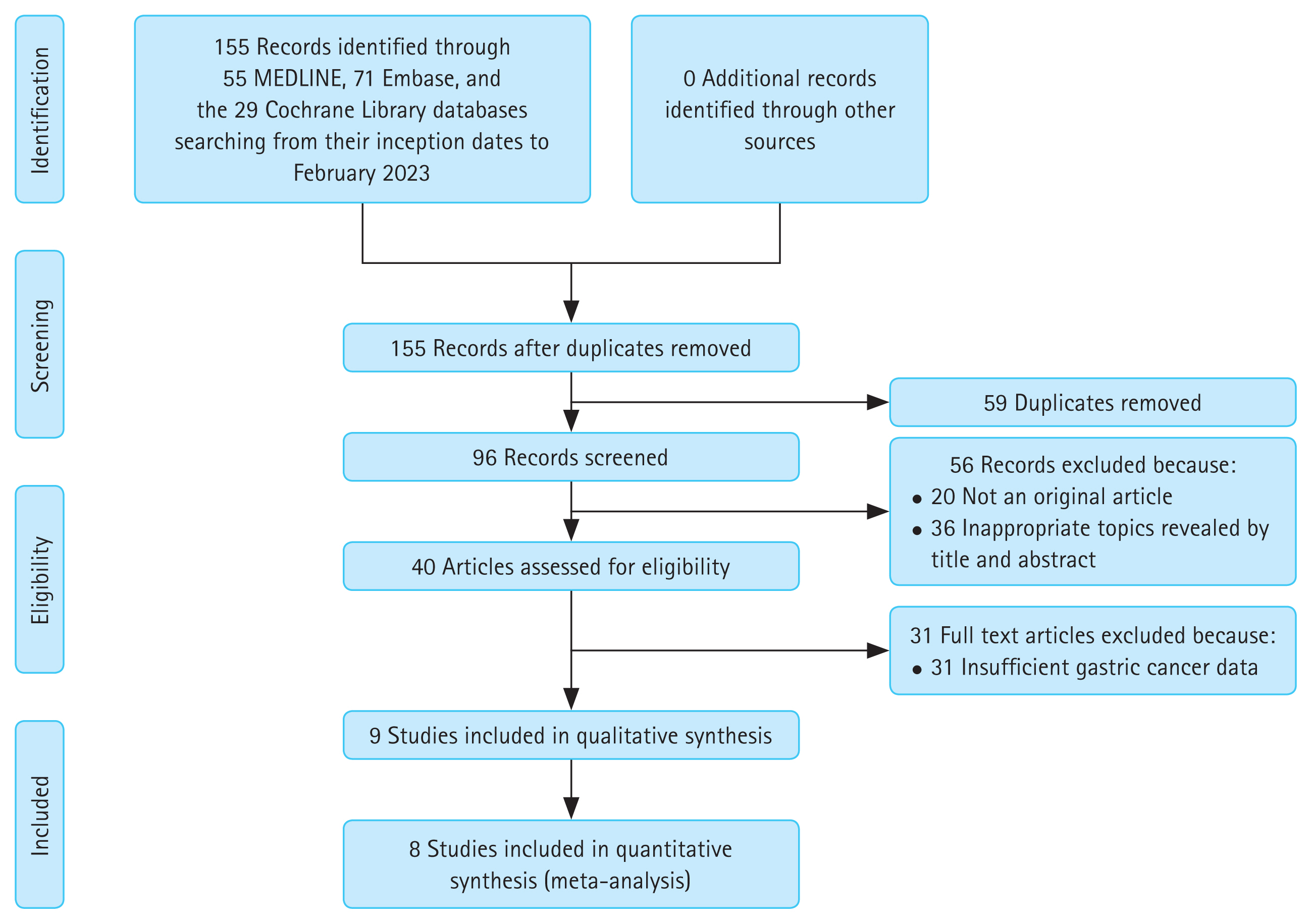
Flow diagram of search and selection of studies on the occurrence of metachronous gastric cancer after endoscopic resection.
Characteristics of studies included based on appropriate criteria are presented in Table 1. Proportions of men in studies ranged from 64 to 83%. At the time of ER, about 67% were negative for H. pylori or had successful eradication treatment of the infection within one year. When analyzing patients who had undergone H. pylori test, 176 patients in the study of Kim et al. [25] and seven in the study of Oura et al. [27] did were not tested for H. pylori infection. The shortest and longest median follow-up periods were 30 and 62 months, respectively.

Baseline characteristics of studies that followed up the occurrence of metachronous gastric cancer after endoscopic resection
This study analyzed severe atrophy based on the definition of each paper’s authors. Among studies that defined atrophy endoscopically, five studies were classified based on the Kimura–Takemoto classification. In four studies, open types II and III were defined as severe. In all other studies, all open types were severe [21]. Two studies classified severe atrophy with a low serum PG I/II ratio. One study defined pathologic severe atrophy by updated Sydney classification. The risk of bias assessments for all included studies was evaluated by the Newcastle–Ottawa Scale for Cohort Study Quality (Supplementary Table 2).
Effect of the degree of mucosal atrophy on occurrence of metachronous gastric cancer
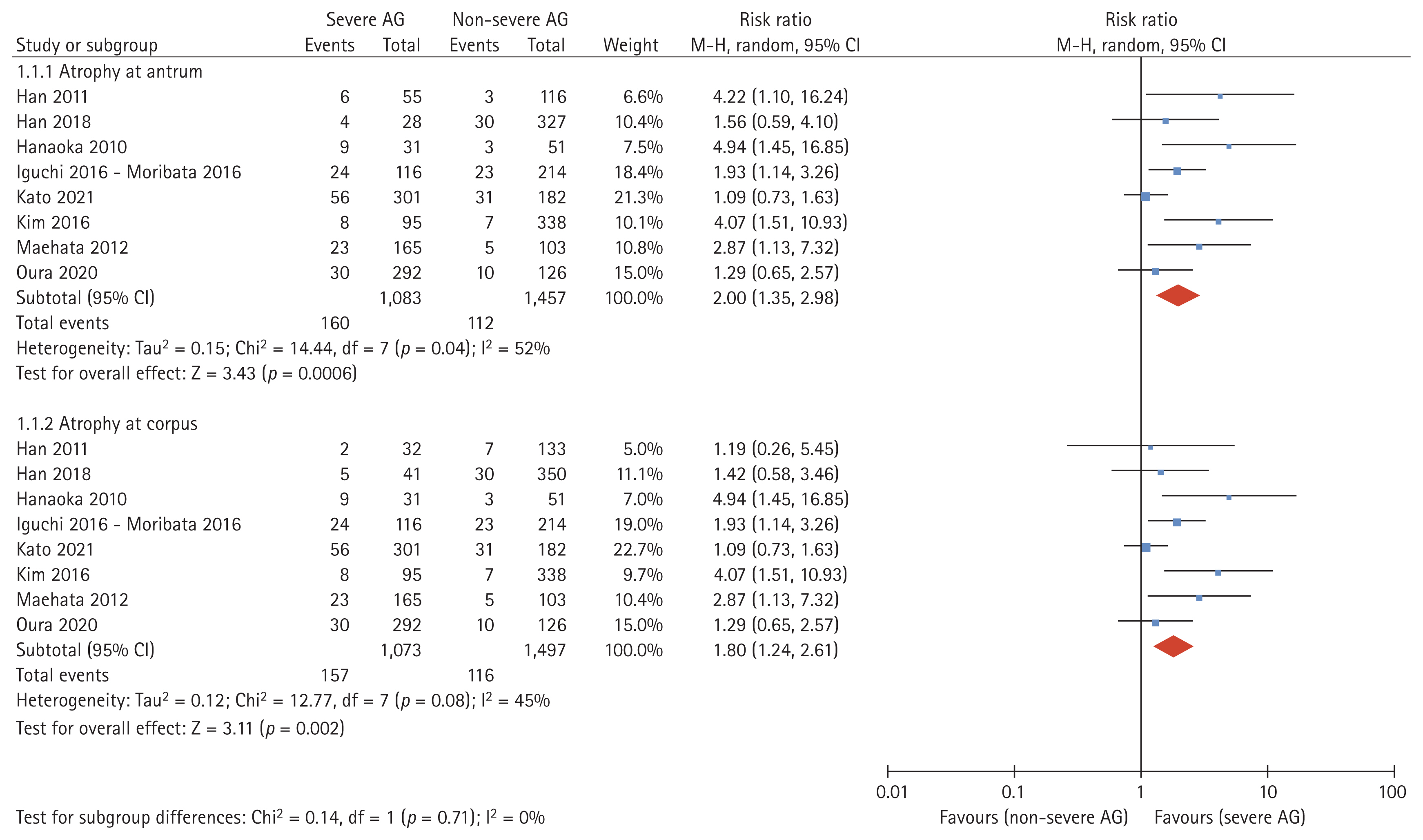
Data obtained from eight studies were pooled [19–27]. Of 2,755 cases, 1,295 cases were classified as severe AG and 1,250 cases were classified as non-severe AG. The incidence of MGC was 14.8% in GC patients with severe AG and 7.7% in GC patients with non-severe AG based on atrophy assessed at the antrum (absolute risk difference: 7.1%; Table 2). Atrophy evaluated at the corpus showed similar results with an absolute risk difference of 6.9%. The incidence rate of MGC was 38.6 (95% CI 32.8–45.0) per 1,000 person-years for those with severe AG and 20.7 (95% CI 17.1–24.9) per 1,000 person-years for those with non-severe AG, with the difference in incidence rate being 17.5 per 1,000 person-years. Patients with severe atrophy of the background mucosa at the ER had a higher risk of MGC occurrence than patients without severe AG (RR 2.00, 95% CI 1.35–2.98, I2 = 52% in the antrum; RR 1.80, 95% CI 1.24–2.61, I2 = 45% in the corpus; Fig. 2).
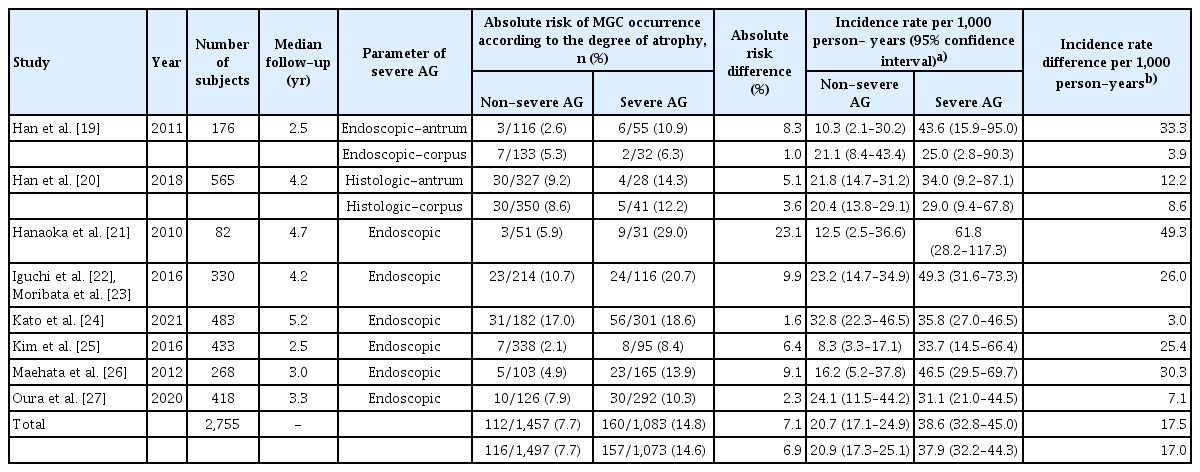
Absolute risk and incidence rate per person-years of occurrence of MGC according to the severity of AG
Subgroup analysis was performed to estimate MGC occurrence according to the parameter for judging the degree of mucosal atrophy. Endoscopically evaluated severe AG had significantly higher MGC occurrence than non-severe AG (RR 2.11, 95% CI 1.35–3.29, I2 = 58%; Fig. 3). Serological (PG I, II) and histological diagnostic methods were also analyzed. Testing for subgroup differences revealed no statistically significant subgroup effect (p = 0.73).
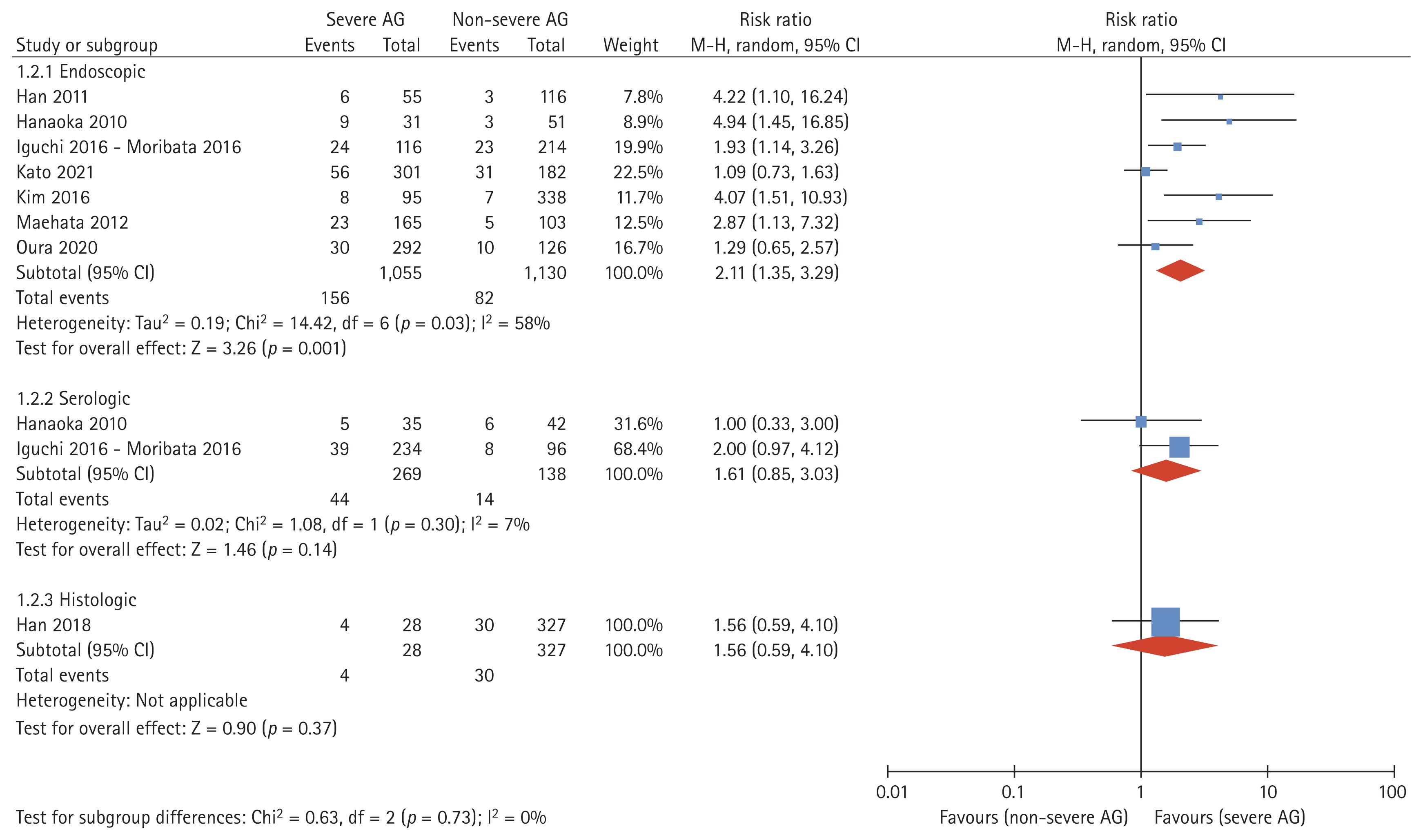
Forest plot analyzing the occurrence of metachronous gastric cancer after endoscopic resection according to endoscopic, serologic, or histologic evaluation of mucosal atrophy. Endoscopic evaluation was analyzed based on antral atrophy. AG, atrophic gastritis.
Countries were also sub-analyzed to examine the possibility of geographically different outcomes (Fig. 4). Five Japanese studies [21–24,26,27] and three Korean studies [19,20,25] were included in this study. In Japanese studies, the number of severe AG patients (n = 905, 57.2%) was higher than the number of non-severe AGs. In Korean studies, the number of severe AGs (n = 178, 18.6%) was less classified. The RR for MGC in severe AG was 1.75 (95% CI 1.11–2.73, I2 = 55%) in Japanese studies and 2.81 (95% CI 1.44–5.47, I2 = 14%) in Korean studies. Test for subgroup difference showed no subgroup effect (p = 0.25).
Effects of intestinal metaplasia on occurrence of metachronous gastric cancer
We investigated whether the presence of IM affected the occurrence of MGC in patients who underwent ER for GC. In four studies, 1,504 patients were included. The median follow-up period after ER was 2.5 to 4.2 years. IM was diagnosed histologically in three studies [19,20,25]. It was diagnosed grossly during endoscopy in one study [22,23]. Two studies divided the biopsy site into antrum and corpus [19,20]. In studies evaluating IM in the corpus, the absolute risk difference of MGC was higher at 9.2% and the incidence rate difference per 1,000 person-years was 24.7 (Table 3). Results evaluated at the antrum were similar.
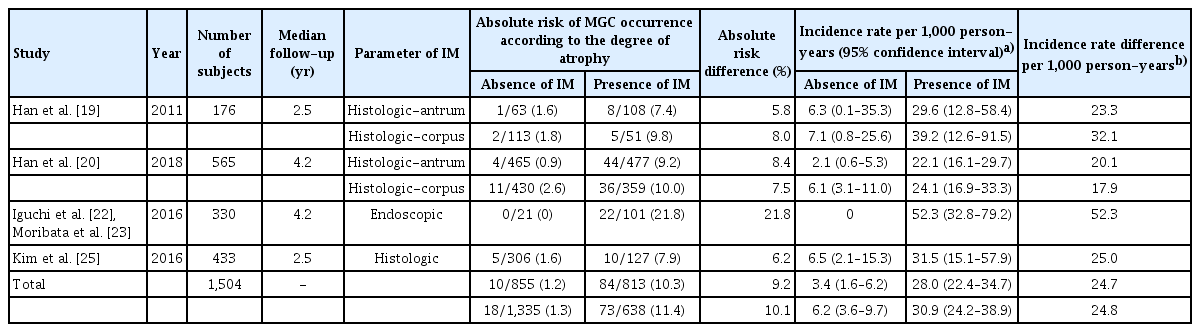
Absolute risk and incidence rate per person-years of occurrence of MGC according to the presence or absence of IM
For GC patients with IM, the RR of MGC occurrence was significantly higher than that in patients without IM (RR 7.08, 95% CI 3.63–13.80, I2 = 0% at the antrum; RR 4.41, 95% CI 2.63–7.41, I2 = 0% at the corpus; Fig. 5).
Relationship between Helicobacter pylori eradication and metachronous gastric cancer
A meta-analysis was performed to evaluate the relationship between H. pylori eradication and MGC occurrence (Fig. 6). Since many people were not clearly evaluated for past eradication of H. pylori, we compared by the presence or absence of H. pylori infection at the time of ER. A total of 660 patients failed in H. pylori eradication while 1,489 patients succeeded. However, there was no significant difference in the risk of MGC between persistent and eradicated groups (RR 1.18, 95% CI 0.88–1.59, I2 = 10%).
Analyses of risk factors affecting the occurrence of metachronous gastric cancer
We performed an analysis to determine whether the risk of MGC occurrence increased according to demographic factors and characteristics of primary cancer. Risks of MGC occurrence according to demographic factors such as sex, age, drinking history, and smoking history are presented in Supplementary Table 3 and Supplementary Figure 1. The risk of developing MGC was significantly higher in males than in females (total number of patients = 2,579, RR 1.54, 95% CI 1.07–2.22, I2 = 22%). Patients with a history of smoking also had a greater incidence of MGC (total number of patients = 412, RR 2.10, 95% CI 1.25–3.53, I2 = 0%). The risk of developing MGC was higher in alcoholics, although the difference was not statistically significant.
A forest plot for the occurrence of MGC according to characteristics of primary cancer is presented in Supplementary Table 4. Primary cancer location, gross type, Lauren’s classification, the degree of differentiation, and the depth of invasion were investigated. Supplementary Figure 2 shows a meta-analysis for each variable. No risk factor showed a statistically significant effect on the occurrence of MGC.
DISCUSSION
This systematic review and meta-analysis showed that severe AG and presence of IM were significant risk factors for MGC occurrence after ER for GC. In particular, patients with severe AG or IM were quantitatively shown to have a 2-fold and a 7-fold higher incidence of MGC, respectively, compared with patients without severe AG or IM. This was consistently observed in sub-analyses according to different methods of evaluating mucosal atrophy and geographical area. There was no significant relationship between H. pylori eradication status and the risk of MGC occurrence in the present study.
AG is usually evaluated endoscopically, serologically, and histologically. Endoscopic biopsy is the gold standard method for diagnosing AG [28]. The Kimura–Takemoto classification is the most commonly used method in clinical practice, is reliable and agrees with the Operative Link for Gastritis Assessment (OLGA) staging system [12,29,30]. Several studies have assessed gastric mucosal atrophy by measuring PG I and PG II. PG is a reliable marker for diagnosing gastric mucosal atrophy [31,32]. Serum PG I and I/II ratio test for OLGA gastritis also showed a strong correlation with the stage [4,33]. A study comparing endoscopic, histologic, and serologic methods together to evaluate AG also showed statistically significant associations among the three [34].
In the present study, analysis was performed to confirm subgroup effects of various methods of evaluating AG. However, most patients were evaluated endoscopically. Other subgroups included a small number of trials and participants. Due to the uneven covariate distribution, it would have been difficult for the analysis to detect differences in subgroups. However, it was identified that severe AG favored MGC generation rather than non-severe AG in all subgroups. This was consistent with changes according to geographic location. We further evaluated results of Japan and Korea separately, considering the possibility of inter-observer variation and educational differences between countries in the endoscopic evaluation of mucosal atrophy. There was no statistically significant subgroup effect in this analysis, showing consistent results between AG and MGC.
MGC occurs after ER for GC, even in patients who have experienced eradication of H. pylori infection. Therefore, identification of potential risk factors for MGC is essential. IM is a precancerous lesion of GC [35,36]. However, to the best of our knowledge, studies that quantify the effect of IM on the occurrence of MGC after ER for GC have not been reported yet. The presence of IM can be more meaningful in the group after ER than in the general population. Our present study showed this through meta-analysis of previous reports.
In a randomized controlled trial that followed GC patients underwent ER [37], the difference in MGC recurrence with or without H. pylori eradication therapy occurred after a longer period, with a median of 5.9 years and a maximum of 13 years. Results showed a more significant difference in the recurrence of MGC after ten years of eradication than 3 to 4 years of eradication treatment. Studies included in our meta-analysis had limitations in evaluating effects of eradicating H. pylori because the follow-up period was not long, usually less than five years. However, recent other studies have shown that the GC preventive effect of H. pylori eradication is more pronounced in people without precancerous lesions after H. pylori eradication [38,39]. It can be assumed that H. pylori eradication might be less effective than the general population.
This study has some limitations. First, included studies might have selection bias as retrospective cohort studies. Second, these studies were conducted only in Japan and Korea. The reason for this is that both counties have a high incidence of GC with screening evaluation for GC performed through a nationwide strategy. Third, the number of patients in whom the presence or absence of IM was confirmed was small. However, since most tissues were evaluated histologically, results were unlikely to be exaggerated or understated. Fourth, past infection or eradication history of H. pylori was unknown. Thus, the analysis was limited. In addition, there were limitations in explaining the impact of AG and IM on MGC and the relationship between H. pylori eradication. Fifth, the definition of severe atrophy was not the same between studies. Thus, there was a limitation in subgroup analysis. However, in all analysis results, the occurrence of less MGC was the same in cases with non-severe AG.
Nevertheless, the strength of this study was its focus on factors other than H. pylori infection as risk factors for MGC. As mentioned earlier, compared with H. pylori, AG and IM are more challenging to study and less attractive. However, since there are H. pylori-negative GC patients, consideration of other causes is necessary.
In conclusion, we found that severe AG and the presence of IM significantly increased the risk of metachronous recurrence in GC patients who underwent ER. In particular, patients with GC and IM occurred about seven times more than those with MGC. These results suggest that patients with severe AG or IM should undergo stricter follow-up endoscopy. Future studies in prospective cohorts with adjustment for baseline characteristics such as age and presence of H. pylori infection are needed.
KEY MESSAGE
1. Early gastric cancer with severe atrophic gastritis increased the risk of metachronous gastric cancer by two times after endoscopic resection.
2. Early gastric cancer with intestinal metaplasia had a 7-fold higher risk of metachronous gastric cancer than those without intestinal metaplasia.
3. The incidence rate of metachronous gastric cancer in endoscopically resected early gastric cancer patients was 17.5 per 1,000 person-years in those with severe atrophic gastritis and 24.7 per 1,000 person-years in those with intestinal metaplasia.
Acknowledgments
The authors thank Professors Hyeon Woo Yim and Hyunsuk Jeong, Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, and Ms. Na Jin Kim, Medical Library, The Catholic University of Korea, for her technical assistance in this study.
Notes
CRedit authorship contributions
Younghee Choe: conceptualization, methodology, investigation, formal analysis, writing - original draft, writing - review & editing, visualization; Jae Myung Park: conceptualization, methodology, investigation, formal analysis, writing - review & editing, supervision; Joon Sung Kim: methodology, investigation, writing - review & editing, project administration; Yu Kyung Cho: methodology, investigation, writing - review & editing, project administration; Byung-Wook Kim: investigation, formal analysis, writing - review & editing, project administration; Myung-Gyu Choi: investigation, formal analysis, writing - review & editing, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
None