Expanding the therapeutic landscape: ezetimibe as non-statin therapy for dyslipidemia
Article information
Abstract
Dyslipidemia is a significant risk factor for atherosclerotic cardiovascular disease (ASCVD), and statins are the primary therapeutic options for reducing low-density lipoprotein cholesterol (LDL-C) levels. However, it can be challenging to achieve optimal LDL-C goals with statin monotherapy. Ezetimibe, a cholesterol absorption inhibitor, offers a potential non-statin therapy to optimize LDL-C management. Key clinical trials, such as IMPROVE-IT and RACING, have demonstrated that the addition of ezetimibe to statin therapy leads to further decreases in LDL-C or significant decreases in major adverse cardiovascular events (MACEs), particularly in patients with high ASCVD risk. Subsequent meta-analyses and clinical trials have further supported the beneficial effect of ezetimibe, suggesting additive decreases in LDL-C and MACEs, as well as pleiotropic effects. This review provides a comprehensive analysis of the clinical implications of ezetimibe for managing dyslipidemia; it also evaluates the available evidence that supports the role of ezetimibe as an adjunct non-statin therapy for long-term use. However, the long-term pleiotropic effects of ezetimibe remain controversial because of limited clinical data. Therefore, additional research is needed to clarify its potential benefits beyond LDL-C reduction. Nonetheless, an understanding of the role of ezetimibe in dyslipidemia management will help clinicians to develop effective treatment strategies.
INTRODUCTION
Despite a sustained effort to treat dyslipidemia, atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of death worldwide [1]. Until 2010, ASCVD mortality decreased in South Korea, followed by a steady increase, reaching 123 per 100,000 persons in 2018 [2]. Dyslipidemia, a major risk factor for metabolic diseases and ASCVD, is characterized by an abnormal lipid profile, including high levels of low-density lipoprotein cholesterol (LDL-C) (≥ 160 mg/dL), low levels of high-density lipoprotein cholesterol (HDL-C) (< 40 mg/dL), or high levels of triglycerides (≥ 200 mg/dL). A high LDL-C level is the main risk factor for ASCVD [3]. The prevalence of dyslipidemia has increased by eightfold from 1.5 million in 2002 to 11.6 million in 2018 [4]. The most recent international guidelines for managing dyslipidemia recommend reducing LDL-C based on the principle of “lower is better” [5–8]. Recent epidemiological studies and clinical trials have consistently demonstrated that reductions of LDL-C level lower the risk of ASCVD in both primary and secondary settings [9,10]. Among lipid-lowering drugs, statins, which inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (an early enzyme in the cholesterol biosynthetic pathway), are the most cost-effective drugs for preventing ASCVD; they are commonly prescribed worldwide, including in Korea [11]. However, the proportion of patients achieving their target LDL-C goal remains low, and only a small percentage of very high-risk patients reach their target LDL-C goal during treatment with statins [12]. Despite sufficient reduction in LDL-C, patients experience an elevated risk of ASCVD, which is attributed to changes in lipid components such as triglycerides, lipoprotein (a), HDL-C, and chylomicrons [13–15]. Therefore, high-intensity statin therapy or combination therapy involving ezetimibe is increasingly prescribed [16]. Previous studies have shown that the effects of statin/ezetimibe combinations are superior to statin monotherapy in terms of achieving target LDL-C goals in patients with ASCVD or ASCVD risk equivalents. Furthermore, an updated consensus addressed evidence concerning novel medications beyond statin or ezetimibe combination therapy. This review evaluated alternative therapeutic options for non-statin treatments with a focus on ezetimibe, based on the most recent and relevant evidence.
GUIDELINES FOR MANAGING DYSLIPIDEMIA
Global clinical guidelines recommend statins as first-line treatment for reducing the LDL-C level, which is a major target of lipid-lowering therapy. Many studies have established an association between reducing LDL-C and preventing ASCVD, thereby emphasizing LDL-C management as the primary strategy. Although there are some similarities among clinical guidelines for dyslipidemia, the recommendations display substantial variation. The 2018 American College of Cardiology and American Heart Association (ACC/AHA) guidelines prioritize statin therapy to reduce LDL-C levels, without emphasizing specific lipids or lipoprotein targets [17]. However, for patients with very high ASCVD risk, an LDL-C threshold of 70 mg/dL is recommended as an indicator of the need for additional non-statin therapy. The 2019 European Society of Cardiology/European Atherosclerosis Society (ESC/EAS) guidelines [18], the American Association of Clinical Endocrinologists and American College of Endocrinology (AACE) guidelines [19], and the Endocrine Society Clinical Practice Guidelines [20] encourage reducing LDL-C and non-HDL-C levels to targeted thresholds based on a patient’s cardiovascular risk profile. However, the most reliable risk estimate system should be based on specific population characteristics and corresponding data. The ESC/EAS guidelines utilize the Systematic Coronary Risk Estimation (SCORE) system, whereas the AHA/ACC guidelines use the Framingham Risk Score (FRS). Nevertheless, most guidelines define the very high-risk group as individuals with documented ASCVD, including acute myocardial infarction, ischemic stroke, and peripheral arterial disease. Numerous lipid experts suggest targeting LDL-C levels below 70 mg/dL and non-HDL-C levels below 100 mg/dL in patients with clinical ASCVD across all levels of baseline LDL-C or in patients with very high risk. The AACE and ESC/EAS guidelines recommend even lower LDL-C levels, below 55 mg/dL, for patients with very high ASCVD risk. The high-risk group is characterized by chronic kidney disease, diabetes mellitus (DM) with target organ damage, type 1 DM with duration > 10 years, familial hypercholesterolemia, SCORE ≥ 5%, and FRS ≥ 20%. The goal for the high-risk group should be an LDL-C level of < 100 mg/dL and a non-HDL-C level of < 130 mg/dL. The ESC/EAS recommended target LDL-C level for high-risk groups is < 70 mg/dL. Thus, the European guidelines establish a more stringent LDL-C target, compared with other guidelines. In Korea, the delineation of target LDL-C levels based on the presence of cardiovascular disease (CVD) risk factors is consistent with international guidelines. The recommended target LDL-C levels vary according to the level of risk: for the very high-risk group with CVD, the target LDL-C is < 70 mg/dL; for the high-risk group with carotid disease or DM, it is < 100 mg/dL; for the moderate-risk group with more than two major risk factors, it is < 130 mg/dL; and for the low-risk group with one or no major risk factors, it is < 160 mg/dL. However, immediate statin therapy is indicated for acute myocardial infarction, regardless of the baseline LDL-C level [21].
ROLES OF NON-STATIN THERAPIES IN CLINICAL GUIDELINES
In the last few years, complementary therapies for HMG-CoA reductase inhibitors have emerged as additional options for LDL-C reduction. These therapies include the use of ezetimibe, an inhibitor of the Niemann-Pick C1-Like 1 (NPC1L1) transporter responsible for cholesterol absorption [21], and the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, which interferes with the degradation of LDL receptors in hepatocytes [22]. By targeting different metabolic pathways, these therapies offer new treatment options for patients with dyslipidemia who have a high ASCVD risk. Recent studies regarding the addition of ezetimibe to statin therapy, including Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) [23], and studies focused on PCSK9 inhibitors [24–26] revealed further decreases in LDL-C levels and CVD events. Current guidelines recommend these non-statin therapies when maximally tolerable statin dosage has been reached or when a switch to another class has not achieved sufficient reduction in LDL-C; thus, the strength of the recommendation is increased. Among newly developed treatments, ezetimibe and/or PCSK9 inhibitors have emerged as the primary non-statin therapies. In 2022, the ACC released the expert decision pathway for non-statin therapy, which recommends novel therapies (e.g., bempedoic acid, inclisiran [small interfering RNA], and evinacumab) but prioritizes ezetimibe and/or PCSK9 inhibitors because of their superior outcome data [6]. Global perspectives based on all clinical guidelines are consistent in recommending statins as first-line treatment; non-statin therapies, such as ezetimibe and/or PCSK9 inhibitors, remain second-line options. More options are available to achieve target LDL-C levels and improve CVD outcomes with the emergence of novel therapies for dyslipidemia.
EZETIMIBE AS FIRST-LINE NON-STATIN THERAPY FOR LDL-C MANAGEMENT: EVIDENCE AND IMPLICATIONS
Considering the large amount of data supporting the clinical effectiveness of statin therapy and the current guidelines that prioritize statin prescriptions, the number of clinical trials assessing ezetimibe monotherapy has been relatively limited. A meta-analysis of eight randomized controlled trials (RCTs) regarding the clinical effectiveness of ezetimibe monotherapy (10 mg/d) for 12 weeks demonstrated a mean LDL-C level reduction of 18.58%. Additionally, there were substantial reductions in total cholesterol (13.49%) and triglycerides (8.6%), compared with the placebo group [27]. According to a recent meta-analysis, the pooled prevalence of statin intolerance is 9.1% [28]. In patients who cannot tolerate statin therapy, non-statins are recommended as alternative treatment options. In confirmed CVD cases with statin intolerance or contraindications, ezetimibe monotherapy is cost-effective relative to no treatment. Ezetimibe monotherapy reduces LDL-C levels by 18.56% relative to no treatment [29]. The GAUSS-3 trial investigated the effectiveness of two non-statin therapies, ezetimibe (10 mg/d) and evolocumab (a PCSK9 inhibitor, 420 mg/mo), in 419 patients with statin intolerance [30]. At week 24, the average percentage changes in LDL-C level were −16.7% with ezetimibe and −52.8% with evolocumab. One study (EWTOPIA 75), conducted among Japanese patients aged ≥ 75 years, demonstrated the effectiveness of non-statin monotherapy using a 10 mg dose of ezetimibe to prevent CVD. The study revealed a hazard ratio of 0.66 (95% confidence interval [CI] 0.50–0.86), indicating a reduced risk of CVD, along with a substantial reduction (25.9%) in LDL-C levels [31]. Nevertheless, evidence regarding ezetimibe monotherapy as first-line treatment for primary prevention and its effectiveness in preventing ASCVD is insufficient, compared with statin therapy. Consequently, further research is needed to better understand the potential benefits and efficacy of ezetimibe in these areas.
ADD-ON EZETIMIBE WITH STATIN THERAPY: EFFECTS ON ASCVD OUTCOMES
LDL-C levels are affected by endogenous cholesterol synthesis, cholesterol absorption, and clearance [32]. Statin-mediated inhibition of hepatic cholesterol synthesis can paradoxically enhance cholesterol absorption, leading to a smaller lipid-lowering effect of statin therapy [33]. This problem can be overcome by incorporating a cholesterol-absorbing inhibitor, such as ezetimibe, to complement the statin treatment. As previously noted, ezetimibe monotherapy reduces LDL-C levels by 13–20%. The combination of ezetimibe with statin therapy results in an additional 21–27% decrease in LDL-C levels [34,35]. Several meta-analyses have consistently shown that combination therapy with ezetimibe and a statin is more effective in reducing LDL-C, compared with a twofold increase in the statin dose [36]. In terms of preventing major adverse cardiovascular events (MACEs), most clinical trials focused on secondary prevention among patients with a history of ASCVD (Table 1). Combination therapy demonstrated a superior drop in LDL-C levels and a 17% proportional decrease in MACEs in the Study of Heart and Renal Protection (SHARP) trial, which included individuals with chronic kidney disease who had no history of ASCVD [37]. These results are consistent with previous findings that combination therapy in the vascular surgical setting is protective against MACEs during the first year of follow-up [38]. The IMPROVE-IT study, which is the largest and longest study (enrolling 18,144 participants over 6 yr), showed that the MACE rate in the combined group was lower than the rate in the simvastatin monotherapy group, with absolute risk reduction of 2% (hazard ratio 0.936) [23]. The results concerning MACE risk were inconsistent in Asian patients with ASCVD [39–43]. The Heart Institute of Japan-Proper Level of Lipid Lowering with Pitavastatin and Ezetimibe in Acute Coronary Syndrome (HIJ-PROPER) trial and post hoc analysis of that study demonstrated different results. In HIJ-PROPER, combination therapy did not improve MACE outcomes compared with monotherapy, despite reduction of LDL-C levels [40]. Post hoc analysis of 1,702 patients with ASCVD revealed a significant benefit of combination therapy regarding single vessel disease (hazard ratio 0.72, 95% CI 0.55–0.94) [43]. In a recent large, randomized, open-label study (the randomized comparison of efficacy and safety of lipid lowering with statin monotherapy versus statin–ezetimibe combination for high-risk cardiovascular disease [RACING] trial), combination therapy in patients with high ASCVD risk was non-inferior to high-intensity statin monotherapy (absolute difference −0.78%; 90% CI −2.39 to 0.83) [44]. The previous trials were based on the additive effect of ezetimibe while maintaining a single statin dosage, whereas the RACING trial compared the efficacies of combination therapy comprising moderate-intensity statin with ezetimibe (10 mg rosuvastatin with 10 mg ezetimibe) and monotherapy involving high-intensity statin (20 mg rosuvastatin). Although clinical trials have shown heterogeneous results, pooled analysis of MACEs using a fixed-effects model indicated that combination therapy with ezetimibe resulted in a lower risk of MACEs, compared with the control group. The risk ratio was 0.94 (95% CI 0.90–0.98), indicating a significant benefit [45]. These trial results indicate that patients with higher risk can achieve risk reduction by adding ezetimibe to a statin therapy regimen. Overall, the findings support ezetimibe as a second-line treatment option.
PLEIOTROPIC EFFECTS OF EZETIMIBE IN CLINICAL TRIALS
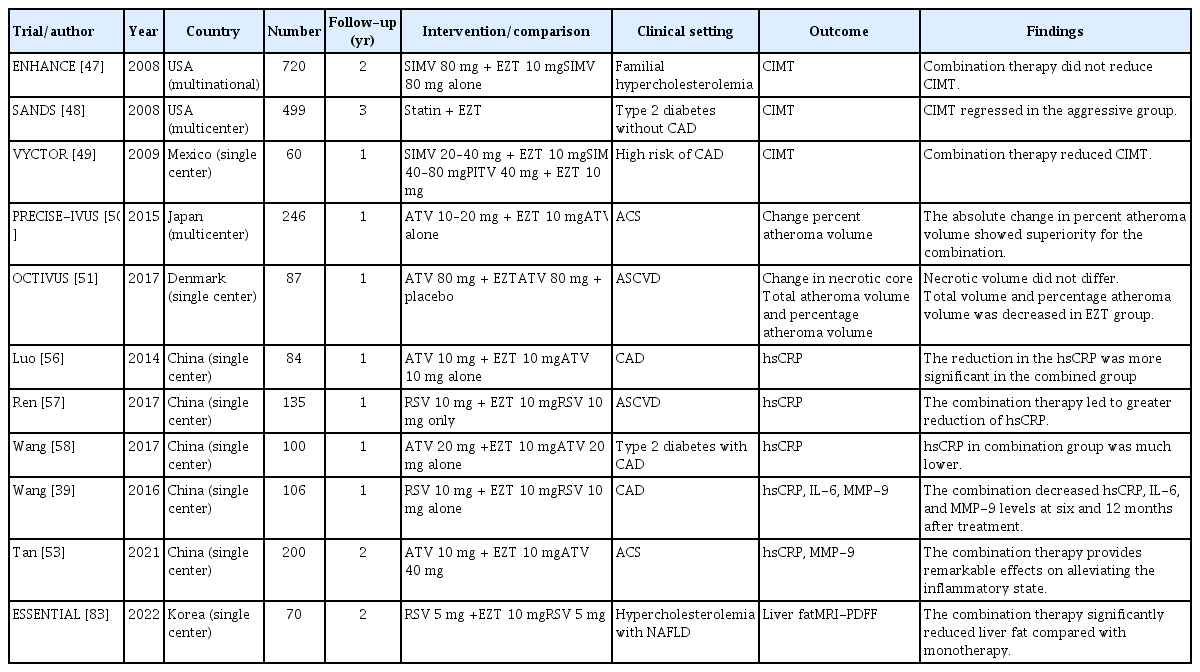
Clinical trials spanning over one year revealed diverse benefits of Ezetimibe, encompassing anti-atherosclerotic effects, anti-inflammation, impact on lipoprotein oxidation, and involvement in glucose metabolism and insulin resistance. These findings provide insights into the multifaceted advantages of Ezetimibe therapy (Table 2).
Anti-atherosclerotic effect based on intima-media thickness or plaque volume
Cholesterol-rich particles infiltrate the walls of arterial blood vessels, causing retention of LDL particles [46]. The ENHANCE trial [47], the Stop Atherosclerosis in Native Diabetics Study (SANDS) [48], and the Vytorin on Carotid Intima-Media Thickness and Overall Arterial Rigidity (VYCTOR) Study [49] evaluated the change in carotid intima-media thickness (CIMT). Despite achieving substantial reduction (16.5%) in LDL-C levels, combination therapy comprising ezetimibe with a statin did not produce a significant difference in CIMT during the ENHANCE trial of patients with familial hypercholesterolemia. In contrast, a benefit with ezetimibe was observed in the SANDS and VYCTOR trials, which were conducted in high-risk patients; a decrease in atherosclerosis was observed upon reduction of the CIMT. In the SANDS, the aggressively treated group exhibited CIMT regression from baseline among patients receiving ezetimibe (−0.025 mm) and non-ezetimibe (−0.012 mm) regimens. Follow-up measurements revealed significant reduction of 0.90–0.93 mm in the CIMT. The mean baseline CIMT values were thicker in these two trials (0.69 and 1.33 mm) than in the ENHANCE study (0.69 mm). The Plaque Regression with Cholesterol Absorption Inhibitor or Synthesis Inhibitor Evaluated by Intravascular Ultrasound (PRECISE-IVUS) trial enrolled participants with high ASCVD risk [50]. In this trial, the combination therapy demonstrated lower LDL-C levels and slower progression of coronary atherosclerosis, with a substantial difference of 20%. Thus, combination therapy involving ezetimibe reversed atherosclerosis in patients with greater baseline CIMT. The Ezetimibe In Addition To Atorvastatin Therapy On The Plaque Composition In Patients With Acute Myocardial Infarction (OCTIVUS) trial investigated the effect of adding ezetimibe to statin therapy on atheroma volume and plaque composition in patients with acute myocardial infarction [51]. The results showed that the combination therapy reduced atheroma volume. The levels of matrix metalloproteinase-9 (MMP-9), an extracellular protein hydrolysate, show similar relationships in human and animal models [52]. Plaque stability is a crucial factor in atherosclerosis development and progression. Wang et al. [39] demonstrated that ezetimibe reduces MMP-9 levels. Consistent with this finding, recent trials in patients with ASCVD showed that MMP-9 levels decrease after 6 months of combination therapy and at the 24-month follow-up [53].
Anti-inflammatory effect
Inflammation is associated with atherosclerosis [54]. High-sensitivity C-reactive protein (hsCRP) is a marker for CVD [55]. In the initial stage of atherosclerosis, hsCRP triggers monocyte attachment to the arterial wall. Several clinical trials have shown that ezetimibe as an add-on to statin therapy decreases inflammatory markers as a primary outcome [56–58]. According to a recent meta-analysis, ezetimibe treatment was more effective in decreasing hsCRP levels, compared with a statin or a PCSK9 inhibitor (mean difference −0.64 mg/L, 95% CI −1.07 to −0.21 mg/dL) [59]. However, cautious interpretation is needed because low levels of hsCRP are not necessarily associated with reduced ASCVD risk. In an animal study regarding ezetimibe treatment, adipocyte size and pro-inflammatory cytokine accumulation decreased [60]. A clinical trial by Wang et al. [39] demonstrated that the levels of interleukin (IL)-6 and hsCRP were lower in the combined group than in the monotherapy group. A recent clinical trial involving patients with ASCVD revealed that phospholipase A2 and IL-1β levels were significantly decreased in response to combined therapy involving ezetimibe [61]. In patients who had isolated dyslipidemia without ASCVD, combination therapy decreased the levels of tumor necrosis factor-α and free fatty acids [62]. Based on a meta-analysis involving 12 studies, significant decreases in IL-6 levels were observed in Asian individuals aged ≥ 60 years, particularly when interventions were maintained for > 3 weeks [63]. The impact of combination therapy on systemic inflammation was greater in individuals with higher LDL-C levels and longer intervention durations.
Lipoprotein oxidation
The oxidation of LDL particles (producing oxidized LDL [ox-LDL]) is widely recognized as the key atherogenic change in LDL and a factor contributing to atherosclerosis onset. A previous study showed that ezetimibe alone or as an add-on to statin therapy prolongs the lag time for LDL-C oxidation [64]. Several clinical trials revealed that oxLDL levels significantly decreased by 8 to 15% after combination treatment [65–67]. Despite the higher rate of LDL-C target achievement with combination therapy, a 12-week treatment regimen did not result in a significant change in oxLDL levels [68]. It remains uncertain whether the reduction in oxLDL levels with combination therapy is beneficial.
Glucose metabolism and insulin resistance
Several randomized trials, observational studies, and meta-analyses have revealed increased risks of new-onset DM or dysglycemia in individuals with DM, particularly during high-dose statin therapy [69–71]. The diabetogenic effects of statin therapy have been linked to the underlying mechanisms, particularly effects on β-cell function and insulin sensitivity [72]. The effect of ezetimibe on glucose metabolism has been sparsely reported, compared with the effects of statins. A study of Korean patients with dyslipidemia showed that ezetimibe could attenuate statin-induced dysglycemia [73]. After an 8-week treatment period, glycated hemoglobin levels increased by 3% in the 20 mg atorvastatin group and 1.2% in the 5 mg rosuvastatin group, whereas they decreased by 0.4% in the 5 mg atorvastatin plus 5 mg ezetimibe group (p = 0.03). However, there was no significant change in the homeostasis model assessment of insulin resistance (HOMA-IR). A Japanese study involving obese patients with dyslipidemia showed that ezetimibe monotherapy significantly decreased HOMA-IR and fasting insulinemia [74,75]. In contrast, ezetimibe therapy in patients with non-alcoholic fatty liver disease (NAFLD) led to increased glycated hemoglobin levels in a small study [76]. Ezetimibe directly inhibits cholesterol absorption, leading to decreased levels of free fatty acids. These reductions in free fatty acids contribute to decreased gluconeogenesis, thereby improving insulin resistance [77]. However, the precise mechanism underlying the ezetimibe-mediated improvement in glucose metabolism is not fully understood.
Non-alcoholic fatty liver disease
NAFLD encompasses a range of histological presentations, beginning with simple steatosis and extending to non-alcoholic steatohepatitis, which can involve varying levels of fibrosis and are accompanied by various metabolic diseases [78]. Ezetimibe targets NPC1L1, which is expressed in the small intestine and liver; it reduces cholesterol by inhibiting NPC1L1 expression. Ezetimibe reduces liver susceptibility to oxidative injury, modulates autophagy, and influences the hepatocyte-driven exosome pathway [79]. In a study evaluating the long-term effects of ezetimibe monotherapy on hepatic steatosis, 45 patients with biopsy-proven NAFLD were treated for 2 years. The study showed a significant decrease in histologically assessed hepatic steatosis and NAFLD activity scores after treatment [80]. Another study investigating the effects of ezetimibe monotherapy revealed improvements in fibrosis stage and ballooning score [76]. In a subanalysis of the IMPROVE-IT study, the combination of ezetimibe and simvastatin led to a substantial decrease in absolute risk (3.7%) and a relative risk reduction of 15% in recurrent cardiovascular events in the high-risk NAFLD sub-group, but the low-risk group did not experience a similar benefit [81]. A meta-analysis demonstrated that ezetimibe significantly decreased the NAFLD activity score compared with statin monotherapy [82]. A more recent clinical trial involving participants with dyslipidemia and NAFLD showed that ezetimibe and rosuvastatin combination therapy significantly decreased liver fat compared with statin monotherapy [83].
EZETIMIBE TOLERABILITY AND SAFETY
The use of high-intensity statins is often linked to statin intolerance, which is frequently associated with muscle-related adverse events. Although various interpretations of statin intolerance have been described in the literature, the definition most widely used by the EAS focuses on the probability of statin-associated muscle symptoms (SAMS) linked to statin usage. This likelihood assessment considers the nature of the muscle symptoms; the elevation of creatine kinase (CK) levels; and temporal relationships with the initiation, discontinuation, and re-initiation of statin therapy [84]. Comprehensive large-scale clinical studies regarding alternatives to statin therapies have demonstrated substantial reduction in statin-associated side effects, including SAMS, accompanied by improvements in clinical outcomes [24,30,85].
In contrast to statins, ezetimibe has not been associated with an increase in muscle toxicity. An analysis of eight RCTs indicated that ezetimibe therapy did not consistently lead to a clinical increase in CK level (≥ 10 times the upper limit of normal); the incidence was < 1% [27]. A comprehensive pooled safety analysis, involving 17 RCTs and 4,558 patients, conclusively showed that combination therapy involving ezetimibe does not worsen SAMS or increase their incidence [86]. Furthermore, a subanalysis from the RACING trial indicated that the rate of SAMS-related discontinuation or therapeutic reduction was lower in the combination therapy group (4.5%) than in the statin monotherapy group (7.9%) [87]. An RCT involving ezetimibe monotherapy demonstrated that myopathy was slightly more common in the ezetimibe group than in the placebo group (5 vs. 4%) without an increase in CK level [88]. However, some case reports of myopathy (muscle pain with increased CK levels) have been linked to the addition of ezetimibe to statin therapy [89,90]. In these cases, ezetimibe withdrawal led to normalization of the CK level and reduction of muscle pain. One plausible mechanism involves glucuronidation. Statins undergo cytochrome P450 hydrolysis and glucuronidation; ezetimibe can also undergo glucuronidation [91]. Another possible underlying mechanism is the obstruction of fatty acid oxidation by ezetimibe [89]. Fatty acids are crucial constituents for energy production and the maintenance of muscle function. Therefore, the effects of ezetimibe on fatty acid oxidation may lead to changes in energy metabolism and function within muscle. The exact mechanisms by which ezetimibe causes muscle-related side effects are currently under investigation.
Ezetimibe is metabolized in the small intestine and liver [91]. Nevertheless, the incidence of increased serum transaminases (≥ 3 times the upper limit of normal) was not affected by ezetimibe monotherapy [27]. Current guidelines indicate that ezetimibe can be used without dose modification in patients with mild hepatic insufficiency. However, these guidelines may not support the use of ezetimibe for individuals with moderate or severe hepatic insufficiency [92].
CONCLUSION
Current guidelines recommend ezetimibe as cost-effective second-line therapy. The addition of ezetimibe to statin therapy produces favorable outcomes in terms of further decreases in LDL-C levels and MACE risks in patients with established ASCVD or high ASCVD risk. The pleiotropic effects of ezetimibe in clinical trials may be linked to improvements in ASCVD outcomes. These effects include protection against atherosclerosis; reduction of inflammation; modulation of lipoprotein oxidation; and potential benefits regarding glucose metabolism, insulin resistance, and NAFLD. However, the long-term pleiotropic effects of ezetimibe remain controversial because clinical trial data have been limited. Further research is needed to explore the benefits of ezetimibe beyond LDL-C reduction.
Notes
CRedit authorship contributions
Jeongmin Lee: conceptualization, writing - original draft; Seung-Hwan Lee: conceptualization, writing - review & editing, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
None