Optimization of guideline-directed medical treatment for heart failure patients with reduced ejection fraction
Article information
Abstract
With the increasing number of medications demonstrating mortality benefits in heart failure with reduced ejection fraction (HFrEF), the pharmacological treatment of HFrEF is entering a new phase. To enhance outcomes in heart failure patients through medical treatment, the choice of appropriate medications and simultaneous and rapid uptitration are critical. However, there are several challenges encountered during this medication uptitration, including issues like hypotension, fatigue, worsening renal function, and hyperkalemia. This paper addresses strategies for effectively managing these challenges to successfully reach the maximum tolerated dose in patients. Additionally, it will discuss the management of comorbidities often associated with heart failure, the importance of exercise and rehabilitation, and the significance of proper nutrition intake, in addition to guideline-directed medical therapy.
INTRODUCTION
The prevalence of heart failure (HF) in Korea has progressively increased over time: it was 0.77% in 2002, 1.48% in 2013, and 2.24% in 2018. This increase is attributed to an aging population, heightened cardiovascular risk factors, and improved survival rates after acute coronary events [1,2]. Managing HF presents significant challenges due to its complex and diverse nature [3,4].
HF can be categorized into three groups based on the left ventricular ejection fraction (LVEF): HF with reduced EF (HFrEF, EF ≤ 40%), HF with mildly reduced EF (HFmrEF, EF 41–49%), and HF with preserved EF (HFpEF, EF ≥ 50%) [5-7]. Previous criteria for HFrEF were based on medical treatment trials enrolling patients with LVEF below 40 or 35%. Cases with LVEF between 41 and 49% were initially considered as mid-range LVEF resembling HFpEF. However, subsequent investigations revealed similar efficacy of HFrEF medications in HFmrEF patients, leading to the renaming of this group as HF with mildly reduced LVEF [7-11]. In the Korean HF registry, based on LVEF status, 57.6% of HF patients had HFrEF, 17.3% had HFmrEF, and 25.1% had HFpEF [7].
With an expanding array of medical interventions and therapeutic devices, HF treatment has gained significant attention. Despite improved prognostic outcomes through treatment advances, HF remains associated with significant morbidity and mortality [12,13]. This review aims to clarify the rationale behind the use of key HF medications, provide guidelines for their judicious application, and address the treatment of underlying conditions specific to HF patients.
RECENT CLINICAL TRIALS IN THE MEDICAL TREATMENT OF HFrEF
Beta-blockers and angiotensin-converting enzyme (ACE) inhibitors have long been standard therapies for HF patients. However, the introduction of angiotensin receptor-neprilysin inhibitors (ARNIs) in 2014, followed by their adoption in Korea in 2017, marked a significant milestone in HF treatment. ARNIs demonstrated impressive efficacy for HFrEF management in the PARADIGM-HF trial, causing a substantial shift in the treatment paradigm [14-17]. In the Paradigm-HF trial, the primary endpoint was cardiovascular death or hospitalization for HF, with ARNI treatment achieving a reduction in primary events with a hazard ratio of 0.8. Additionally, sodium-glucose cotransporter-2 (SGLT2) inhibitors have exhibited mortality benefits in HFrEF patients in trials like DAPA-HF and EMPEROR-Reduced, meriting a class I indication for their usage [18]. Both the DAPA-HF and EMPEROR-Reduced trials employed a primary outcome of cardiovascular death or deteriorating HF, resulting in fewer events with hazard ratios of 0.74 and 0.75, respectively. SGLT2 inhibitors are believed to indirectly impact the renin-angiotensin-aldosterone system (RAAS) by decreasing the intraglomerular pressure rather than directly influencing it (Table 1, Fig. 1 [19]).
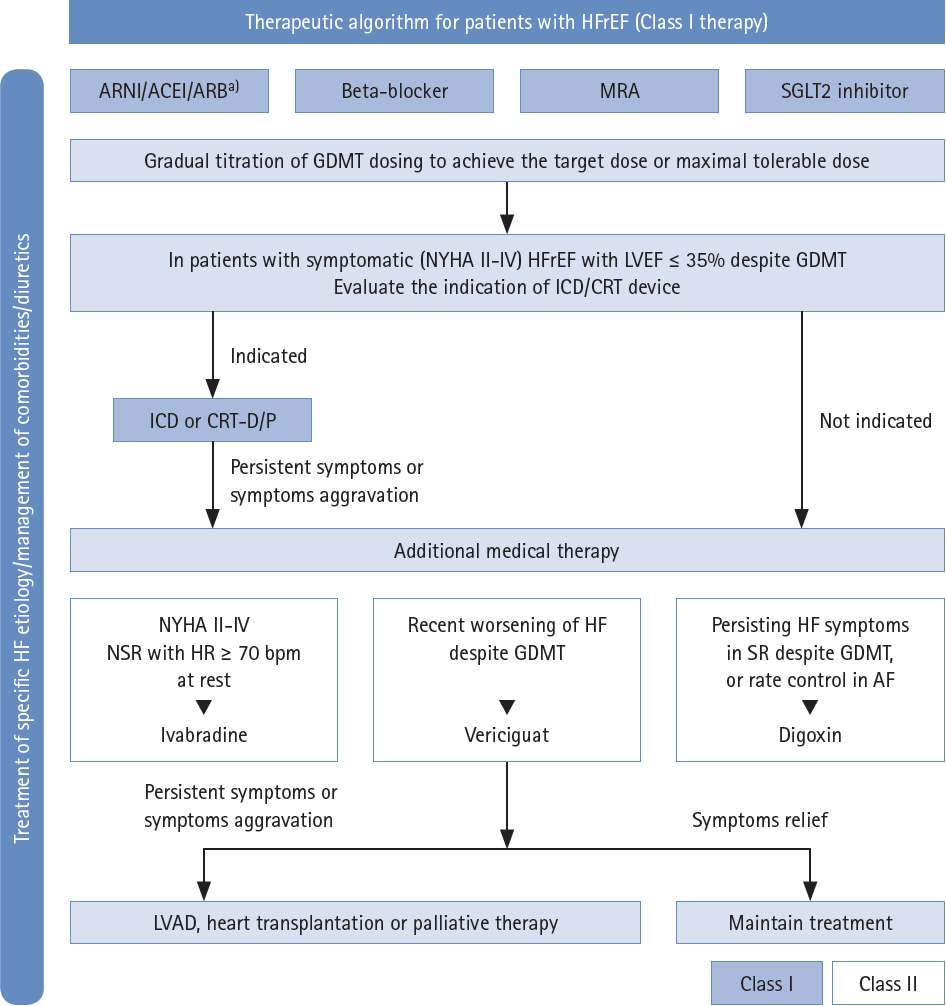
Therapeutic algorithm for HFrEF based on the Korean Society of Heart Failure (KSHF) guidelines. ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor-neprilysin inhibitor; CRT, cardiac resynchronization therapy; GDMT, guideline-directed medical therapy; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; ICD, Implantable cardioverter-defibrillators; LVAD, Left Ventricular Assist Devices; MRA, mineralocorticoid receptor antagonist; NSR, normal sinus rhythm; NYHA, New York Heart Association; SR, sinus rhythm. a)If patients with chronic HFrEF are intolerant to ACEI because of cough or angioedema and when the use of ARNI is not feasible, the use of ARB is recommended to reduce morbidity and mortality. Addapted from Youn et al. [19].
OPTIMIZATION OF MEDICAL TREATMENT FOR HFrEF
Importance of rapid up-titration to the target dose
Following the initiation of effective medical treatment for HF, many physicians opt for initial doses considerably lower than the target dose due to concerns about adverse effects, including hypotension and dizziness. While numerous landmark trials have sought to determine whether HF medication should be employed, limited attention has been given to its optimal utilization.
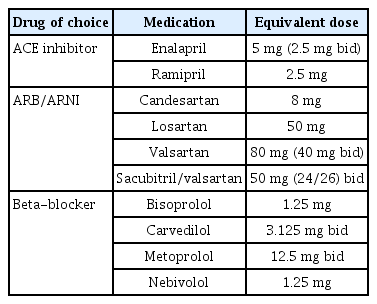
The Assessment of Treatment with Lisinopril and Survival (ATLAS) randomized controlled trial (RCT) addressed ACE inhibitor dosages, revealing a notable (15%) reduction in the combined outcome of HF readmission or death (p < 0.001) with higher ACE inhibitor doses, alongside increased rates of dizziness and renal dysfunction [20]. In the Heart Failure Endpoint Evaluation of Angiotensin II Antagonist Losartan (HEAAL) study, 3,846 HFrEF patients (LVEF < 40%) were administered high (150 mg) or low (50 mg) doses of losartan. The high-dose group experienced a 13% lower incidence of HF hospitalization (p = 0.03) without a significant improvement in mortality [21]. A similar small RCT evaluated beta-blocker dosages, with carvedilol target doses of 6.25, 12.5, and 25 mg, and demonstrated a dose-related enhancement in EF coupled with lower cardiovascular hospitalization and mortality rates [22]. Regarding mineralocorticoid receptor antagonists (MRAs), higher doses were associated with an increased risk of hyperkalemia but clinical outcomes were not dose dependent [23]. Apart from these RCTs conducted for guideline-directed medical therapies (GDMTs), the STRONG HF trial highlights the significance of rapid up-titration in HF patients. This trial compared a high-intensity care group, which titrated up to the target dose within 2 weeks after hospital discharge, with a usual care group and demonstrated a 34% reduction in HF readmission or all-cause death events in the high-intensity care group [24].
The sequence of drug administration in HF treatment has no significant impact on prognosis. The CIBIS III trial demonstrated that the prognosis was unaffected by the order of administration of beta-blockers and ACE inhibitors [25]. Rather than focusing solely on increasing the dose of a single medication, targeting all key pathways involved in HF pathophysiology is of paramount importance.
In spite of robust evidence supporting dose up-titration, real-world data indicate a disappointing rate of achievement of appropriate drug dosages [26]. The CHAMP-HF data, spanning the period 2015–2017, revealed that only 1% of the study population achieved the recommended dosage for all ACE inhibitors/angiotensin receptor blockers (ARBs)/ARNIs, beta-blockers, and MRAs [27]. A Korean multicenter retrospective study also documented that 40% of the patients initially prescribed low-to-moderate ARNI doses did not undergo drug up-titration within 1 year [28]. Surprisingly, titration to the target dose is not often achieved even with systolic blood pressure (BP) exceeding 110 mmHg [29]. To overcome these limitations, simultaneous administration of appropriate medications upon diagnosis and increasing the frequency of outpatient visits are now favored.
During the mid-2010s, guidelines recommended a sequential approach to initiating therapy for HFrEF. However, this approach brought challenges, including potential underutilization of drugs due to hesitancy in reaching target dosages. To address these concerns, recent guidelines now advocate for the simultaneous use of four key drugs from the initial diagnosis and endorse early and rapid up-titration whenever possible. Safety and efficacy data support the administration of ACE inhibitors/ARBs, SGLT2 inhibitors, and ARNIs before discharge [30-32]. Pre-discharge checklist completion can be beneficial for efficient early up-titration. Patients whose physicians completed a checklist at discharge exhibited high adherence to GDMT and experienced a low incidence of all-cause mortality and HF-related rehospitalizations within 2 months after discharge [33]. Standardizing drug administration protocols and implementing frequent hospital visits for swift up-titration led to most patients achieving target doses, resulting in improved clinical outcomes [24].
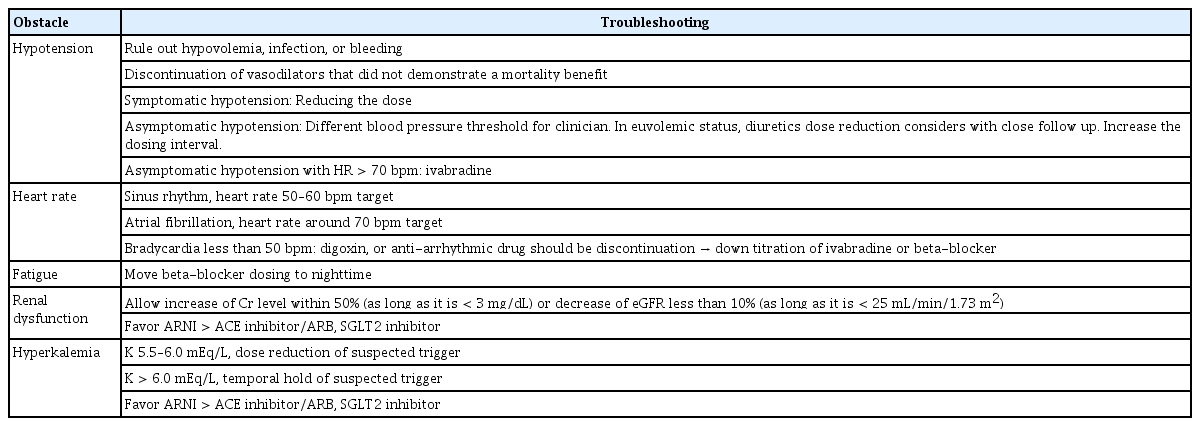
Despite the collaborative efforts of the HF community, several significant obstacles impede the up-titration process, including hypotension, fatigue, deteriorating renal function, and hyperkalemia. Real-world HF patients are often older, have a heavier burden of comorbidities, and exhibit greater frailty compared to those enrolled in RCTs [34,35]. The following section explores the measures necessary for the up-titration of HF medications (Table 2-5).
Concern and consensus about hypotension during titration
Patients with HFrEF often exhibit lower BP compared to the general population, which presents a notable challenge in titrating HF medications. There is a lack of consensus on the ideal BP threshold for HF patients during medication titration. While the Paradigm-HF trial necessitated a systolic BP > 100 mmHg for enrollment, individual clinicians may employ different thresholds for dose adjustment.
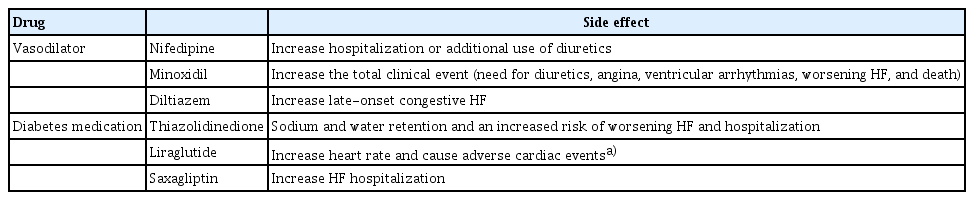
When BP falls to a level hindering the addition of new medications, it becomes imperative to assess patients for hypovolemia, infection, and bleeding. The use of BP-lowering drugs, such as nitrates, calcium channel blockers, and other vasodilators, which lack significant outcome benefits, should be reevaluated. If symptomatic hypotension persists even after discontinuing such drugs, the HF medication dose should be reduced.
For euvolemic patients, diuretic therapy can be prudently reduced or modified, with vigilant monitoring for signs of fluid retention. In cases where maintaining multiple GDMTs proves challenging due to low BP, prioritizing beta-blockers over renin angiotensin system blockers might be advisable, given the more robust dose-response data for beta-blockers [36]. Adjusting administration times may also be considered [36]. The COPERNICUS trial demonstrated that among patients with a systolic BP of 85–95 mmHg without hypotensive symptoms, beta-blocker treatment did not lead to further systolic BP reduction [37].
SGLT2 inhibitors and MRAs exert relatively minimal effects on BP. Ivabradine could be a suitable treatment option in cases of low BP, as it does not impact systemic BP.
Appropriate heart rate target
Among the pharmacological agents within the category of GDMT, beta-blockers and ivabradine reduce the heart rate (HR). Elevated baseline HR is linked to poor prognosis in HF patients [38]. Rigorous HR control in HFrEF patients in sinus rhythm, targeting a HR < 70 bpm, correlates with lower rates of all-cause death, cardiovascular death, and HF hospitalization [38-40]. For HFrEF patients in sinus rhythm, maintaining an HR around 60 bpm seems most suitable [34]. By managing HR with beta-blockers and ivabradine, neurohormonal activation and stress response can be reduced, simultaneously enhancing coronary artery blood flow by prolonging the diastolic phase [38,41].
According to the guidelines, if the maximal beta-blocker dosage fails to lower the HR below 70 bpm, the addition of ivabradine should be considered. In the CARVIVA HF trial, the combination of ivabradine and beta-blockers enabled patients to reach higher doses compared to beta-blocker monotherapy [42]. If beta-blocker-induced fatigue arises, evening administration can be considered.
Conversely, the clinical benefit of HR reduction in HF patients with atrial fibrillation (AF) remains unclear. While maintaining an HR around 80 bpm in HF patients with AF yields favorable outcomes, titrating beta-blockers to the maximum dose in HF patients with AF < 70 bpm could prove detrimental [39]. When low BP coincides with AF, digoxin may be favored over beta-blockers for HR control.
If HR falls below 50 bpm or bradycardia-related symptoms manifest, discontinuation of medications, such as diltiazem, verapamil, digoxin, and anti-arrhythmic drugs, should be considered if possible. In fact, diltiazem and verapamil contribute to pulmonary edema and negative inotropic effects, rendering them unsuitable for HF patients. Subsequent down-titration of ivabradine or beta-blockers may be needed if bradycardia persists even after discontinuing these medications.
Concern and consensus about worsening renal function and hyperkalemia
The presence of chronic kidney disease (CKD) in HF patients is linked to increased mortality and morbidity [43-45]. HF itself can contribute to renal dysfunction through mechanisms such as reduced perfusion and venous congestion, which are key for cardio-renal syndrome [43]. The decline in estimated glomerular filtration rate (eGFR) in HF patients is more significant compared to individuals without HF, even when considering other risk factors [46].
Physicians treating HF patients with pre-existing renal impairment face the challenge of titrating HF medications, which can potentially worsen renal function due to hypotension and RAAS inhibition during medication up-titration. However, a meta-analysis of five RCTs demonstrated that the reduction in mortality risk associated with RAAS inhibitors, including ACE inhibitors, ARBs, and MRAs, was more pronounced in patients with worsening renal function compared to those without it [47]. This suggests that clinicians should not discontinue RAAS inhibitors solely based on declining renal function [48]. While RAAS inhibitors and SGLT2 inhibitors can reduce eGFR, this does not necessarily indicate glomerular loss. With RAAS inhibitors, there may be an initial eGFR decrease that stabilizes after several weeks. SGLT2 inhibitors can lead to an early eGFR decline around week 4 of treatment, followed by a slower reduction. After 1 year, the eGFR may be higher in the SGLT2 inhibitor group compared to the placebo group [36].
Among HF medications, ARNIs and SGLT2 inhibitors are known to slow the rate of eGFR decline in HF patients [49,50]. SGLT2 inhibitors are also associated with a lower incidence of hyperkalemia compared to controls [51]. When ARNIs and ACE inhibitors were compared, patients in the ARNI group exhibited better renal outcomes and a lower incidence of hyperkalemia in both HFrEF and HFpEF [9,15,52]. Compared to ACE inhibitors, the increase in proteinuria observed with ARNIs is attributed to changes in the glomerular capillary ultrafiltration coefficient and tubular protein reabsorption rather than glomerular loss [49]. Therefore, ARNIs and SGLT2 inhibitors can be considered for the treatment of patients with underlying CKD and frequent hyperkalemia.
However, there is limited evidence supporting the use of both RAAS inhibitors and SGLT2 inhibitors in HF patients with an eGFR < 30 mL/min/1.73 m2 compared to those with an eGFR > 30 mL/min/1.73 m2. Therefore, their use requires caution in this population. While small retrospective studies have reported safe and effective use of ARNIs even in end-stage renal disease (ESRD) patients [53], the safety and efficacy of SGLT2 inhibitors in HF patients with CKD stage 5 or ESRD have not been established. In contrast to RAAS and SGLT2 inhibitors, beta-blockers do not significantly impact eGFR [54]. Beta-blockers have demonstrated clinical benefits in patients with CKD stage 4–5 and ESRD [55]. The recommended guidelines for managing changes in creatinine or eGFR levels during HF medication titration are as follows: a moderate increase in creatinine level (within 50%, as long as it remains < 3 mg/dL) or a decrease in eGFR of < 10% (as long as it remains < 25 mL/min/1.73 m2) after initiating GDMT is generally tolerated without interruption. Hyperkalemia, with an incidence of 0.4–10%, is a common concern associated with the use of ACE inhibitors, ARBs, ARNIs, and MRAs. Hyperkalemia is the second most common cause of medication discontinuation after renal dysfunction. Typically, a reduction in the dose of these agents is advised if potassium levels are in the range of 5.5–6.0 mEq/L, and temporary discontinuation is recommended if the potassium level exceeds 6.0 mEq/L, with reinitiation of the drug only when potassium levels drop below 5.5 mEq/L [36]. The use of new potassium binders, such as patiromer, may enable the continued use of RAAS inhibitors in patients with hyperkalemia. However, their use has not been associated with improved mortality [56].
SPECIAL CONSIDERATIONS IN SPECIFIC CARDIOMYOPATHY PHENOTYPES
Amyloidosis
In recent years, there has been a growing awareness of amyloidosis because of the development of new treatment options, such as bortezomib and tafamidis [57]. However, when it comes to HF caused by cardiac amyloidosis, treatment options remain limited. Traditional HF medications, including RAAS inhibitors and beta-blockers, are generally not recommended in amyloidosis patients due to the risk of hypotension and their limited efficacy.
Digoxin, a medication commonly used for HFrEF, is also contraindicated in amyloidosis because it can deposit on amyloid fibrils and potentially cause toxicity. Regarding arrhythmia control, amiodarone can be used in cardiac amyloidosis. However, since patients with amyloidosis often have reduced stroke volumes, it is crucial to carefully adjust the amiodarone dose to optimize HR while maintaining cardiac output and simultaneously suppressing arrhythmias.
Hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM), characterized by cardiac hypertrophy resulting from sarcomere mutations, may manifest with overt dysfunction in 5–10% of cases [58]. In patients with HCM and preserved EF, the primary therapeutic objectives include alleviating LV outflow tract obstruction, maintaining optimal volume status, and preventing sudden cardiac death [59]. In cases of burnout-phase HCM, the initiation of HF medications such as ARNIs, ACE inhibitors, ARBs, beta-blockers, and MRAs may be considered [60]. Calcium channel blockers should be discontinued or avoided due to their negative inotropic effects. Anti-arrhythmic drugs, such as amiodarone and sotalol, can be used to manage arrhythmias in HCM. While amiodarone has demonstrated efficacy in reducing non-sustained ventricular tachycardia in HCM patients, its effectiveness in preventing sudden cardiac death is inadequate. Anticoagulation therapy is indicated when AF is clinically or subclinically detected in HCM patients, with direct oral anticoagulants being the preferred choice for anticoagulation.
Acute decompensated heart failure in HFrEF
In HF patients, a decrease in cardiac output or an increase in filling pressure can lead to the development of ADHF, which is often associated with a worsening prognosis and reduced quality of life. Acute decompensated heart failure (ADHF) can occur regardless of the LVEF and is commonly accompanied by signs of congestion, necessitating the prompt initiation of diuretic therapy. Loop diuretics are the most effective agents for decongestion in ADHF patients. However, their use may result in electrolyte imbalances and renal dysfunction, making concurrent administration with other agents advisable. Notably, the use of SGLT2 inhibitors for ADHF has demonstrated tolerability and beneficial effects [61]. In cases of elevated or normal BP, vasodilators may be considered; however, caution should be exercised when the systolic BP is < 90 mmHg [62]. Among vasodilators, diltiazem and verapamil should be avoided due to their negative inotropic effects. While the PIONEER-HF trial has established the safety and efficacy of ARNIs in hemodynamically stable acute HF, there is currently a lack of evidence supporting their use in cardiogenic shock.
SPECIAL CONSIDERATION FOR SPECIFIC COMORBIDITIES
Iron deficiency
In HF, nutritional imbalances and chronic inflammatory conditions can disrupt iron absorption and metabolism, leading to the development of anemia due to iron deficiency. Correcting iron deficiency in these patients improves functional capacity and reduces HF-related hospitalizations, even in patients without anemia [63,64]. It is crucial to distinguish anemia-related fatigue from medication-induced fatigue (such as that caused by beta-blockers) in HF patients. Resolving iron deficiency, with or without anemia, can also help optimize GDMT from this perspective. Therefore, current guidelines recommend intravenous iron replacement as a class 2A recommendation for HF patients with LVEF < 50%. It is worth noting that while intravenous iron preparations have demonstrated this effect, oral iron preparations have not shown similar efficacy, likely due to inadequate iron absorption in HF patients [65]. SGLT2 inhibitors, which are part of the four pillar drugs for HF, have been shown to promote erythropoiesis and increase iron utilization, leading to a reduction in the occurrence of anemia in HF patients [66].
Diabetes
The prevalence of comorbid diabetes mellitus (DM) in HF patients is 20–36% in Korea and 25–45% in Western countries [3,67]. Managing DM is crucial in HF patients [68]. SGLT2 inhibitors, one of the four cornerstone drugs, are recommended as first-line therapy for diabetic patients with HF due to their renal protection, cardiovascular outcome-improving effects, and mortality benefits in HFrEF regardless of DM status. However, clinical benefits in patients with lower eGFR have not been established, as studies on SGLT2 inhibitors have primarily included patients with eGFR ≥ 30 mL/min/1.73 m2.
Another class of drugs that can be used for HF patients with DM is glucagon-like peptide-1 (GLP-1) agonists [69]. GLP-1 agonists are known to improve cardiovascular outcomes in DM patients, but there is still limited evidence regarding their use in HF patients. Among GLP-1 agonists, caution should be exercised in the use of liraglutide in HF patients. The LIVE study, an RCT investigating the effects of liraglutide in HFrEF patients, showed an increase in HR and adverse cardiac events with the use of this agent, while the FIGHT study demonstrated neutral results [70,71]. Therefore, additional research is needed to confirm the safety of liraglutide in HF patients. Dipeptidyl peptidase 4 inhibitors are generally considered to have a neutral effect, although saxagliptin should be avoided due to its association with an increased risk of HF hospitalization [72].
Atrial fibrillation
The combination of HF and AF has a synergistic effect, negatively impacting quality of life, increasing the risk of HF hospitalization, and reducing exercise capacity [73-75]. Therefore, current guidelines recommend specific interventions for AF management in HFrEF patients. If symptoms persist despite rate control measures, pulmonary vein isolation therapy is recommended as a class 2A recommendation, while amiodarone and cardioversion are recommended as class 2B recommendations [6,76]. Given the increased risk of thromboembolism associated with the coexistence of HF and AF, anticoagulant therapy is recommended for AF patients with HF, regardless of the HF phenotype [6,77,78].
The goal of rate control in HF is to alleviate symptoms, optimize functional capacity, and prevent tachycardia-induced cardiomyopathy [79]. While beta-blockers have shown mortality benefits in sinus rhythm, their mortality benefits in AF have not been established. Therefore, beta-blockers should be prescribed in a manner that does not compromise the patient’s functional capacity in the presence of AF. Diltiazem and verapamil are not recommended for rate control in HF patients with AF due to their potential to cause pulmonary edema. In HF, beta-blockers are typically considered as the first-line treatment for rate control. Digoxin may be added if a beta-blocker is ineffective, contraindicated, or not tolerated.
DIET AND LIFESTYLE MODIFICATION
Salt intake and fluid restriction
Excessive salt intake can lead to fluid retention in HF patients. The 2021 European Society of Cardiology guidelines discourage the consumption of > 5 g of salt per day to help manage HF. While there is a consensus on avoiding excessive salt intake, the evidence regarding the benefits of strict salt restriction (< 2 g/d) for reducing death or hospitalization in HF patients is not consistent [80-82]. In fact, one RCT showed that a group consuming 2–3 g of salt per day had a numerically lower risk of death compared to one consuming < 2 g [80].
Both the European Society of Cardiology and American Heart Association guidelines recommend water restriction to < 1.5–2 L per day in cases of advanced HF with hyponatremia. However, there is a lack of appropriate RCTs to support this recommendation. Only a small pilot study reported that a fluid restriction group experienced fewer typical HF symptoms, greater thirst distress, and stable heart-related quality of life [83]. To further investigate the effects of fluid restriction in HF patients, a planned RCT called Fluid REStriction in HF vs. liberal fluid UPtake (FRESH-UP) aims to determine whether fluid restriction to < 1.5 L contributes to symptom improvement in New York Heart Association class II or III symptomatic HF patients [81,84].
Exercise
Guidelines emphasize the importance of exercise for patients who can engage in physical activity to improve exercise capacity, enhance quality of life, and reduce the risk of HF hospitalization. Exercise can decrease mortality and hospitalization while increasing exercise capacity in HF patients [85]. An exercise prescriber is recommended as part of a multidisciplinary team to provide appropriate exercise guidance and supervision for HF patients [85]. Additionally, cardiac rehabilitation should be considered for frail patients, as it can offer tailored exercise programs and comprehensive support to optimize outcomes [86].
CONCLUSION
Recent medical treatments for HFrEF have made significant progress. An essential component of the medical management of HFrEF is timely and effective uptitration of medications to reach the highest tolerated dose. Coupled with the management of underlying comorbidities and lifestyle adjustments, this medical treatment has the potential to further improve patient prognosis.
Notes
CRedit authorship contributions
Minjung Bak: conceptualization, investigation, writing - original draft, writing - review & editing; Jin-Oh Choi: conceptualization, writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
None