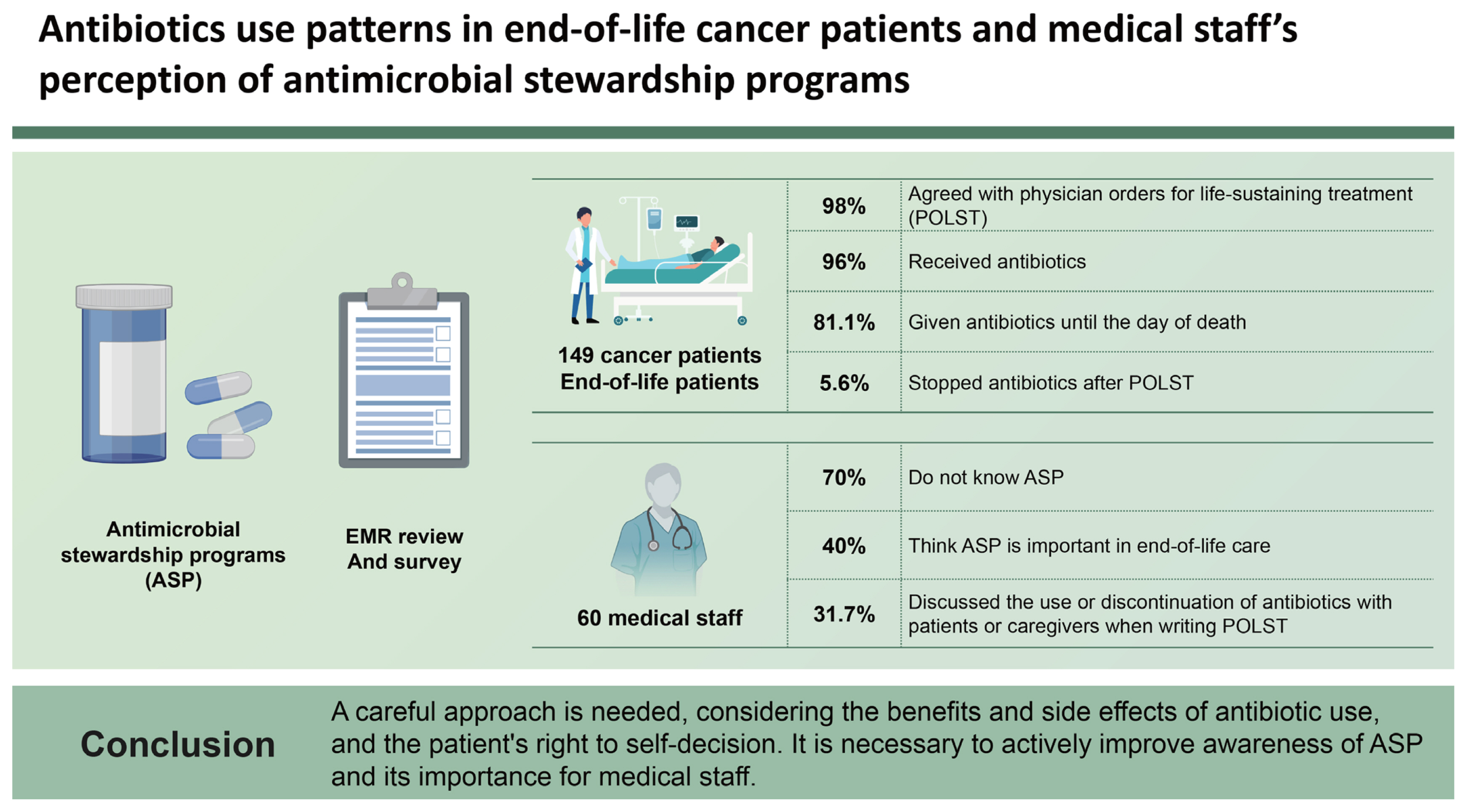
Antibiotics use patterns in end-of-life cancer patients and medical staff’s perception of antimicrobial stewardship programs
Article information
Abstract
Background/Aims
While most cancer patients with end-of-life (EOL) care receive antibiotic treatments, antibiotic use should be decided appropriately considering the benefits, side effects, resistance, and cost effects. Antimicrobial stewardship programs (ASP) are important for patients with EOL care, but there is limited study analyzing actual antibiotic use in EOL care and the perceptions of Korean medical staff.
Methods
Electronic medical records of 149 deceased cancer patients hospitalized in the medical hospitalist units at Asan Medical Center in Seoul from May 2019 to September 2021 were reviewed. Basic information, antibiotic use, duration, and changes were investigated. We surveyed medical staff’s perceptions of antibiotics in cancer patients with EOL.
Results
Of the 149 cancer patients with EOL care, 146 (98.0%) agreed with physician orders for life-sustaining treatment (POLST). In total, 143 (95.9%) received antibiotics, 110 (76.9%) received combination antibiotic treatment, and 116 (81.1%) were given antibiotics until the day of death. In a survey of 60 medical staff, 42 (70.0%) did not know about ASP, and 24 (40.0%) thought ASP was important in EOL care. Nineteen doctors (31.7%) discussed the use or discontinuation of antibiotics with patients or caregivers when writing POLST, but only 8 patients (5.6%) stopped antibiotics after POLST.
Conclusions
Most cancer patients with EOL care continue to receive antibiotics until just before their death. A careful approach is needed, considering the benefits and side effects of antibiotic use, and the patient’s right to self-decision. It is necessary to actively improve awareness of ASP and its importance for medical staff.
INTRODUCTION
Terminal cancer patients often experience infectious complications toward the end of their lives. Consequently, many end-of-life (EOL) cancer patients who are hospitalized continue to receive antibiotics until their passing [1–3]. Even when physician orders for life-sustaining treatment (POLST) dictate minimal treatment, antibiotics are frequently administered continuously as an exception [4]. The common reasons for the ongoing administration of antibiotics to EOL cancer patients include: (1) presence of an actual infection in the patient; (2) difficulty in determining whether patients are infected because typical symptoms of infection such as fever or elevated inflammatory markers can also manifest in non-infected cancer patients; and (3) medical staff may feel guilty if a patient deteriorates or dies after discontinuing antibiotics. In addition to these three reasons, various factors contribute to the continued use of antibiotics, such as caregivers’ requests [5].
Antibiotic use is associated with adverse drug effects, drug interactions, injection site inflammation, intravenous catheter-related infections, Clostridioides difficile infection, and the emergence of multidrug-resistant organisms (MDRO) [1,2,6]; therefore, antibiotics should be administered cautiously in EOL care, which prioritizes patient comfort over cure. Accordingly, the Infectious Diseases Society of America has recommended implementing antimicrobial stewardship programs (ASP) for EOL patients [7].
There are limited studies on antibiotic use in patients nearing death in Korea. In 2006, one study reported that 84.4% of terminal cancer patients received antibiotics within a month of their death, with 63.8% continuing to receive antibiotics until their last day [1]. Another study found that 90.6% of patients continued to receive antibiotics after their POLST decision and 60.1% received antibiotic combination treatment [8]. A recent nationwide multicenter cohort study found that 88.9% of terminally ill patients received antimicrobial agents during the last two weeks of their lives [9]. Of these patients, 63.6% received inappropriate antibiotic treatment [9]. However, no studies to date have assessed the medical staff’s perception of antibiotic use in EOL patients, particularly among internal medicine residents, specialists, and hospitalists who primarily manage hospitalized patients.
This study aimed to evaluate the pattern of antibiotic use in hospitalized cancer patients receiving EOL care and determine whether there were changes in antibiotic use patterns before and after POLST decisions. Additionally, we investigated the perceptions of medical staff who prescribe and administer antibiotics to EOL cancer patients.
METHODS
Patients and study design
We identified patients with cancer who passed away in the internal medicine hospitalist units at Asan Medical Center (Seoul, Korea) between May 2019 and September 2021. Internal medicine hospitalist units, in comparison to oncology wards, tend to admit cancer patients who are in an acute and severe disease state, typically via the emergency room. Conversely, oncology wards tend to admit a higher number of cancer patients who are scheduled for chemotherapy and are discharged after treatment. Cancer patients under the care of internal medicine hospitalist units have a higher mortality rate due to the severity of their illness and higher likelihood of complications. Patients were excluded if they (1) died within 48 hours of admission, (2) were hospitalized for more than 30 days, or (3) had hematologic malignancies. We retrospectively reviewed the electronic medical records to gather their basic information, evidence of infection, antibiotic use, and patterns of antibiotic use before and after the POLST decision. From September 12 to 23, 2022, we conduced a web-based, self-administered questionnaire to assess medical staff’s perceptions regarding antibiotic administration and use in EOL cancer patients. The questionnaire was modified based on a previous relevant study [5]. We targeted internal medicine residents in Asan Medical Center and internal medicine specialists (hospiatalists and non-hospitalists), and total number was 114. We sent an online-based survey link to them via text messages and e-mails. The responders were anonymized, and only one response from each participant was accepted. There was no compensation provided for participating in the survey. In order to increase response rates, we sent a reminder to each participant on the seventh day following the initial request to complete the questionnaire (Supplementary Table 1).
Definition
A patient in the process of EOL was defined as a person who had no chance of survival, did not recover despite treatment, was on the verge of death due to rapid progression of symptoms, and was medically judged by their doctor and an expert in a specific disease field [10]. A terminal patient was defined as someone who has no possibility of recovery from underlying diseases and whose symptoms gradually deteriorate despite active treatment, which is expected to result in death within several months according to their doctor and an expert in a specific field [10]. Life-sustaining treatment referred to extending only the period of the dying process without treatment effect, such as cardiopulmonary resuscitation, hemodialysis, chemotherapy for malignancy, ventilator care, and other medical procedures (e.g., extracorporeal membrane oxygenation, blood transfusion, inotropics and chronotropics administration, and other procedures deemed necessary to be withheld and discontinued) [10]. POLST was defined as documentation of the patient’s decision to discontinue life-sustaining treatment and hospice, depending on the patient’s intentions [10].
Among the terms related to antibiotic use, escalation was defined as changing to a broader spectrum of antibiotics or combination therapy. Conversely, de-escalation was defined as changing to a narrower spectrum of antibiotics or discontinuing one or more antibiotics during combination [11]. Stop was defined as the cessation of all antibiotics. Compared to existing antibiotics, it was judged that the antibiotics were changed to a broad spectrum if antimicrobial range was wide, and the antibiotics were changed to a narrow spectrum if antimicrobial range was narrow.
Outcomes
The primary outcome was antibiotic use pattern in cancer patients with EOL in Korea. The secondary outcome was perceptions of Korean medical staff regarding antibiotic administration to cancer patients with EOL.
Statistical analysis
Categorical variables were compared using chi-squared tests or Fisher’s exact tests, while continuous variables were compared using Student’s t-test or Mann–Whitney U-test. All significance tests were two-tailed and p values < 0.05 were considered statistically significant. Statistical analysis was performed using the IBM SPSS software package version 24 for Windows (IBM Corp., Armonk, NY, USA).
Ethics statements
The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2021-1520). Identifying information in an electronic database was encrypted to protect personal privacy. Informed consent was waived by the Institutional Review Board considering the retrospective nature of the study.
RESULTS
Baseline characteristics of EOL cancer patients
A total of 212 cancer patients expired during the study period. Of them, 149 EOL patients were included in this study and their baseline characteristics are shown in Table 1. The median age of the patients was 66 years and 87 were male (58.4%). Hepatobiliary cancer and lung cancer were the most common type of malignancy (n=39 [26.2%]), followed by gastrointestinal cancer (n=37 [24.8%]). A total of 55 (36.9%) patients were hospitalized for infection, with pneumonia being the most common cause. During hospitalization, 91 (61.1%) patients had a fever, and bacteremia was present in 24 (16.1%). Enterobacteriaceae gram-negative bacteria such as Escherichia coli and Klebsiella species were the most common type of pathogen (Supplementary Table 2). Out of 149 patients, 143 (96.0%) were given antibiotics. Most patients (98.0%) agreed to POLST, and the median time to death after obtaining POLST was 3 days.
Characteristics of EOL status in cancer patients with antibiotics use
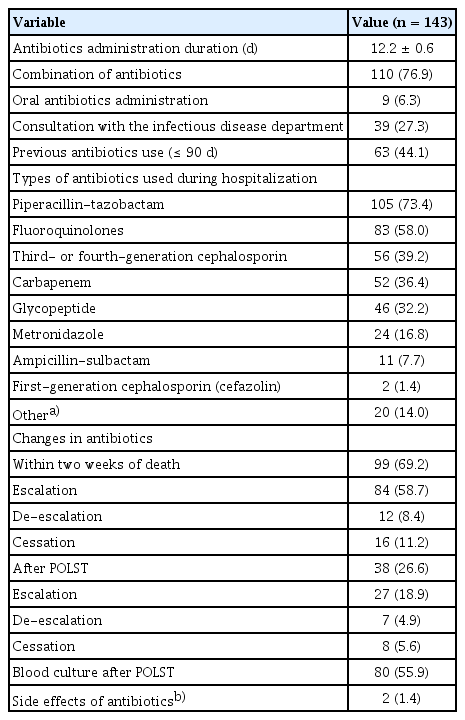
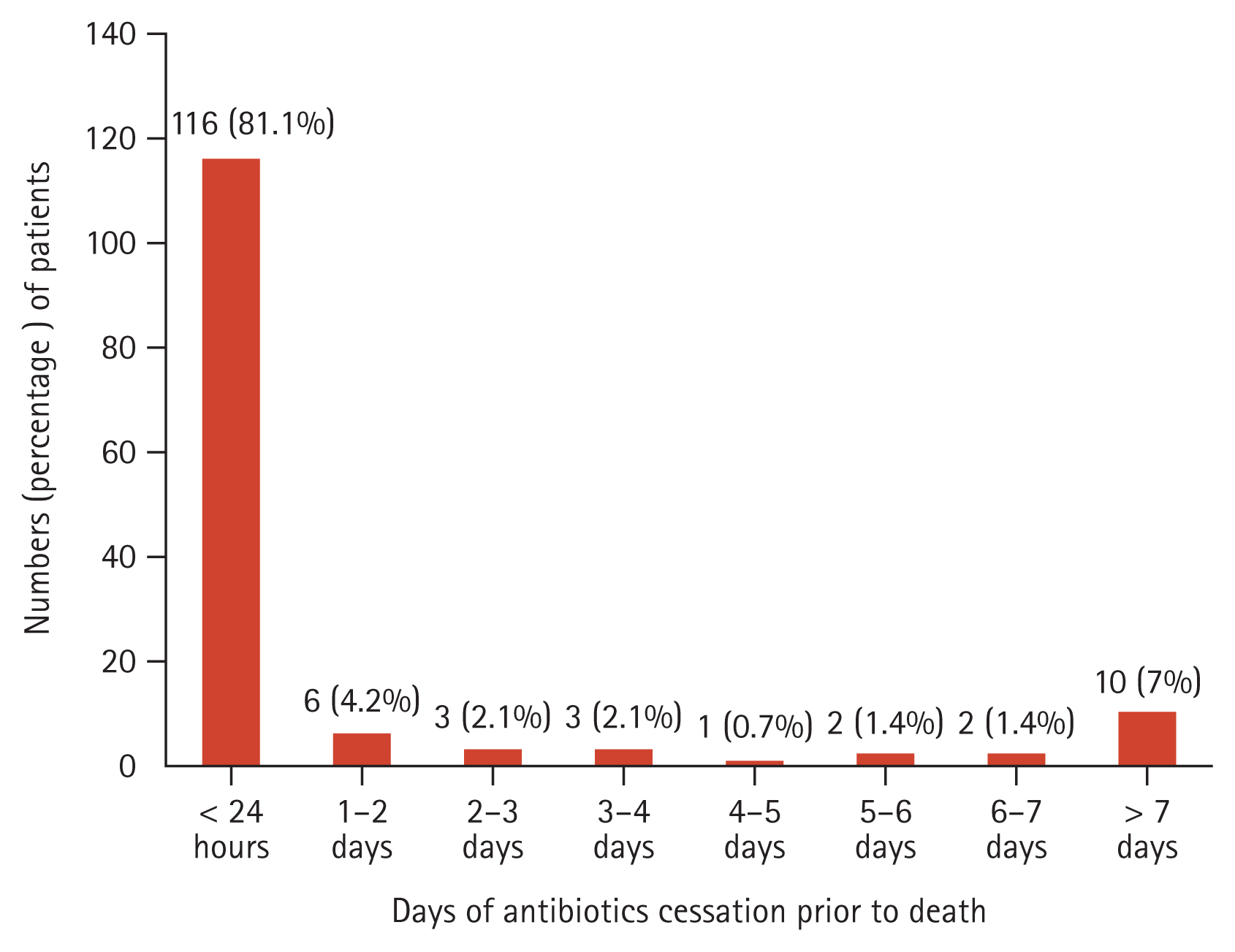
Table 2 presents the details of antibiotic use patterns in hospitalized EOL cancer patients. Among the 143 patients who received antibiotics, the mean duration of antibiotic administration was 12.2 ± 0.6 days, and 110 patients (76.9%) were administered antibiotics as combination therapy. A total of 27.3% requested consultation with the Department of Infectious Diseases regarding the appropriate use of antibiotics. The most commonly used antibiotic was piperacillin-tazobactam (n=105 [73.4%]), followed by fluoroquinolones (n=83 [58.0%]) and third or fourth-generation cephalosporins (n=56 [39.2%]). After obtaining POLST, antibiotic escalation was carried out in 27 (18.9%) patients, and de-escalation or discontinuation was carried out in 15 (10.5%) patients. Blood cultures were performed in 80 (55.9%) patients even after completing the POLST. Body temperature, white blood cell, and C-reactive protein levels did not change significantly during 4–7 days of antibiotic treatment (Supplementary Table 3). Figure 1 shows the time prior to death when antibiotics ceased in cancer patients with EOL. Out of the 143 patients who used antibiotics, 116 (81.1%) received antibiotics until within 24 hours of death. A total of 89.5% of patients received antibiotics within 4 days of death.
Medical staff perception of antibiotic use in EOL care
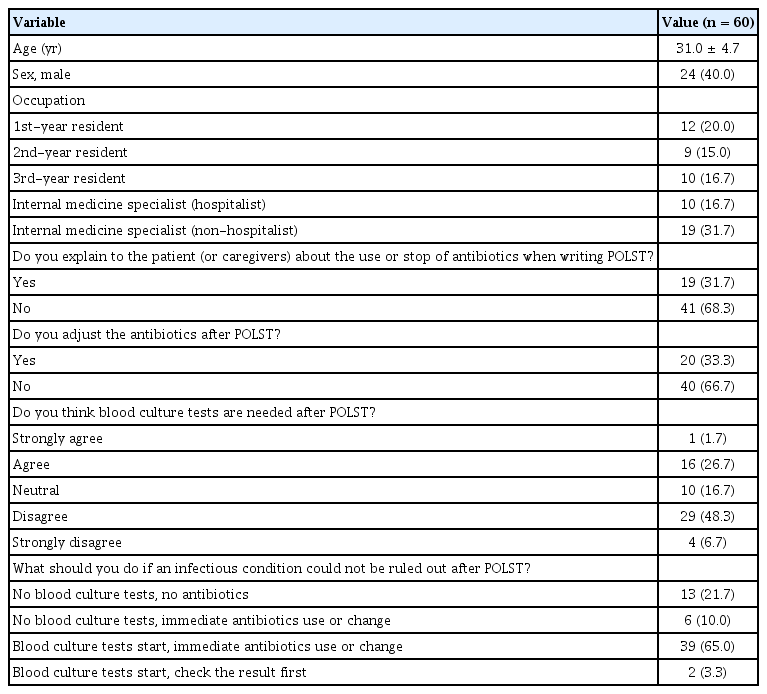
A questionnaire was distributed to 77 internal medicine residents and 37 internal medicine specialists; internal medicine specialists consisted of 4 infectious disease specialists, 9 oncologists, 19 hospitalists, and 5 other internal medicine specialists. A total of 60 doctors (52.6%) responded to the questionnaire. Figure 2 shows the medical staff’s perception of ASP in EOL care. Forty-two doctors (70.0%) were not familiar with ASP. Even when informed about the advantages of ASP, only 40.0% considered ASP important in EOL care.

Medical staff’s perception of ASP in EOL care (n = 60). (A) How much do you know about the ASP? (B) Do you think ASP is important in patients with EOL? (C) Who has the most significant impact on determining antibiotic use after POLST? (D) Do you consider use or cessation of antibiotics for patients with EOL when writing POLST? ASP, antimicrobial stewardship programs; EOL, end-of-life; POLST, physician orders for life-sustaining treatment.
When asked who had a significant impact on antibiotic use after POLST, 90% responded that the doctor in charge had the greatest influence on the decision to use antibiotics after POLST. Nineteen doctors (31.7%) discussed the use or discontinuation of antibiotics with their patients or caregivers when completing POLST. Seventeen (28.3%) believed that blood culture tests were needed even after POLST, and 45 (75.0%) adjusted antibiotics if an infectious condition could not be ruled out, even after obtaining POLST (Table 3).
Reasons for adjusting antibiotics after the acquisition of POLST
Figure 3 shows the distribution of the reasons for maintaining or replacing broad-spectrum antibiotics and the reasons for stopping or de-escalating antibiotics after POLST. Among the reasons for maintaining or replacing broad-spectrum antibiotics after POLST, the most common responses were “I’m not 100% sure about the prognosis of the dying phase” and “I want to give the impression of “healing as much as possible” instead of the impression of “giving up,” each with 36 votes (60.0%). Following this, 16 votes (26.7%) stated, “I think antibiotics can relieve the pain due to infection”. Among the reasons for stopping or de-escalating antibiotics after POLST, the most common response was “It was judged that antibiotics could no longer treat infections in POLST situations”, which received 33 votes (55.0%). Next, 25 votes (41.7%) said, “Antibiotic treatment burden is excessive (e.g., frequency and route of administration, measurement of drug concentration)”.
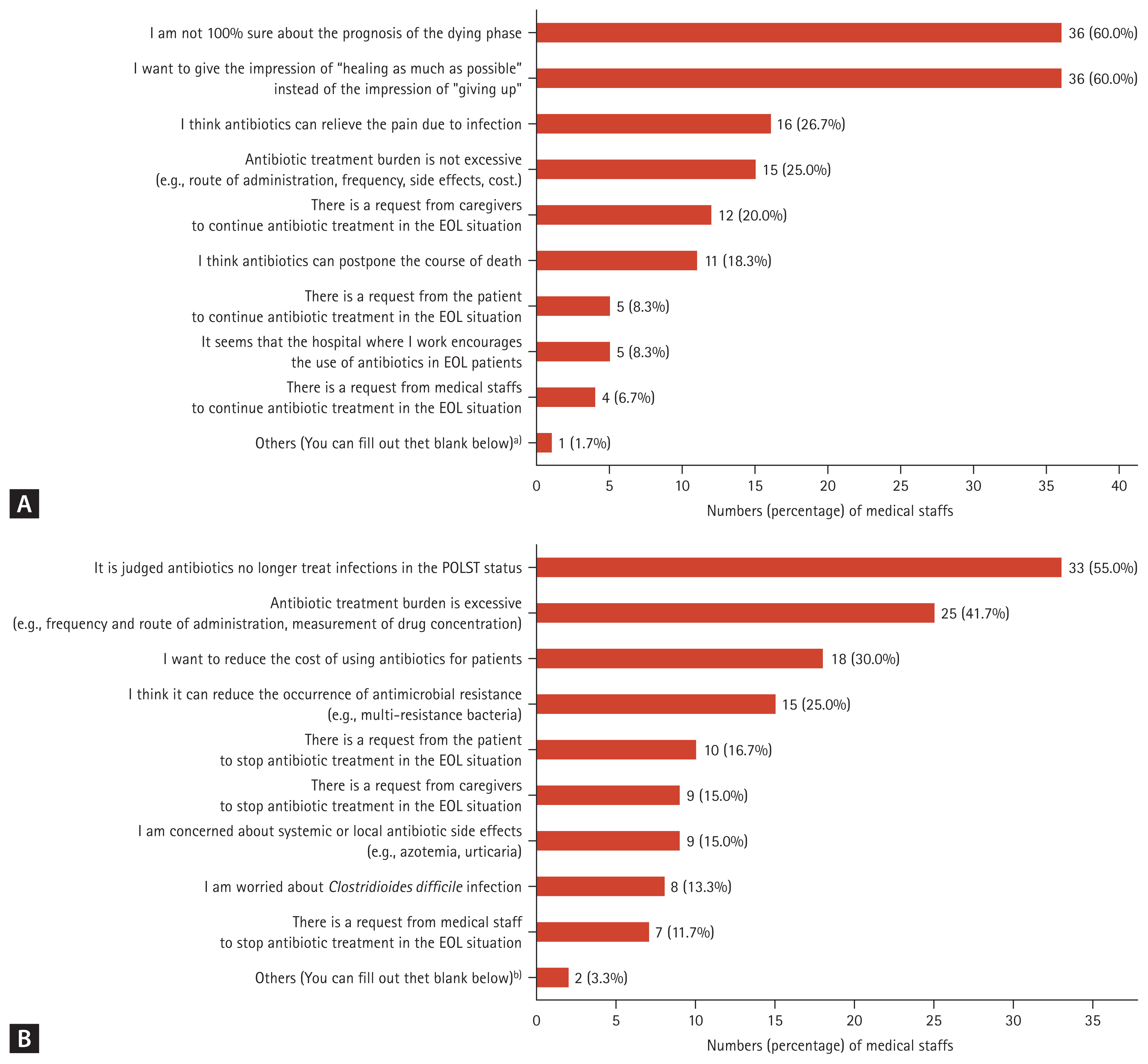
Medical staff’s perception of antibiotics use after POLST status (n = 60). (A) Why do you maintain using antibiotics or replace them with broad spectrum antibiotics after POLST? (B) Why would you stop or de-escalation antibiotics after POLST? ASP, antimicrobial stewardship programs; EOL, end-of-life; POLST, physician orders for life-sustaining treatment. a)It is unkown about main doctor’s intention to use antibiotics when I am on night duty, so I tended to maintain or escalate antibiotics in spite of EOL course. b)I want to reduce the workload of nurses on antibiotics.
DISCUSSION
This study is to examine the patterns of antimicrobial use and perceptions of medical staff in cancer patients with EOL care in Korea, and is the first study on perception of antibiotics in Korean medical staff. In our study, 96.0% of EOL cancer patients received antibiotics, and 81.1% were administered antibiotics until within 24 hours of death. A total of 77% were treated with combination antibiotics. The decision on the administration of antibiotics in EOL cancer patients was mainly determined by the medical staff. The results of a questionnaire-based survey showed that many medical staff were not aware of ASP, and even those who thought ASP was important did not properly utilize it in the actual clinical setting. We believe that our findings may provide valuable data for considering how ASP can be applied to EOL cancer patients in South Korea.
Infection and fever are the most common acute complications experienced by terminally ill patients. It is challenging to distinguish between normal parts of the dying process and aspects that are reversible or clinically treatable [6]. For this reason, antibiotics are often administered until the day of death for EOL cancer patients [1,2,9]. Antibiotics use in EOL care could have some benefits for improving infection related symptoms (e.g., pain, dysuria), signs (e.g., fever), inflammatory marker [12,13]. However, in our study, no improvement in fever or inflammatory markers was observed even if antibiotics were administered during the EOL period, and a previous study reported that there was no significant difference in time to mortality if antibiotics were stopped in terminally ill patients [14]. Since EOL patients are often cared for with the goal of “comfort” rather than “cure”, it is necessary to make a decision to properly use or stop antibiotics, considering various benefits (e.g., long-term survival effects, symptom improvement) and risks (e.g., drug side effects, occurrence of MDRO).
Excessive antibiotic use in patients with EOL care could increase the incidence of antibiotic resistance, which leads to increases in hospitalization days, mortality, and medical costs [15–17]. One study reported that antibiotics were continued to be administered even after Do-Not-Resuscitate requests, and more MDRO were isolated from those who died [18]. As MDRO can spread to other patients, this can ultimately lead to increased global healthcare costs. C. difficile infection can occur during or after antibiotic administration, which is also on the rise worldwide, causing problems such as extended hospitalization, increased re-admission rates, and overall medical costs [19–21]. Additionally, antibiotic injections can cause side effects, such as injection site inflammation, phlebitis, and catheter-related bloodstream infection [22–24]. There are also side effects of antibiotics themselves, such as drug fevers, skin rash, urticaria, hypersensitivity reactions, and gastrointestinal upsets [25–28]. Although patients may not be able to complain of discomfort because they are in the EOL course, they could still experience various side effects. Moreover, antibiotics may interact with the drugs being administered at the EOL care, leading to additional problems such as increased workload for nurses who perform antibiotic administration and increased decision-making for doctors. Considering these downsides of antibiotics use, antibiotics should be properly administered, de-escalated, or stopped altogether in patients with EOL. Determining the use of antibiotics can result in better treatment services for patients and benefit both medical institutions and the nation.
In our study, only 31.7% of medical staff discussed the use of antibiotics with patients and caregivers when writing POLST, and decision-making on the use of antibiotics in EOL cancer patients was largely determined by the attending physician. The situation regarding antibiotic use in EOL cancer patients is complex and can be a source of ethical and practical challenges for healthcare professionals. In some patients, continuing to use antibiotics might be seen as prolonging the dying process rather than providing comfort and dignity in the final stages of life. In the POLST state, it is necessary to consider the life dignity of patients in EOL care and give patients or caregivers the right to decide on the use of antibiotics. To this end, it is important to provide accurate information to patients and caregivers first, and to fully explain the scenarios that may occur regarding antibiotics use or cessation [2]. Ethical judgment is a very complicated and difficult issue when it comes to the use of antibiotics in patients who are rapidly progressing to their death. We think that it is necessary to allow cancer patients to decide on the use of antibiotics based on their dignity when writing POLST. It is time to clarify that antimicrobial therapy is considered as life-sustaining treatment which can be withdrawn or withheld in EOL cancer patients [6]. We also suggest that the context about benefits and risks of antibiotics should be included in POLST documentation. In addition, the use of antibiotics in EOL patients depends on a combination of factors, such as ambiguity of therapeutic effectiveness, physician perception, and attitudes and opinions of patients and caregivers. Therefore, it should be recorded when writing POLST if the decision is made not to use antibiotics during EOL care [3], which would help reduce antibiotic use in the EOL phase. A study revealed that indicating a preference for limited antimicrobials on a POLST form ≥ 30 days before death may lead to less inpatient antimicrobial use in the last 30 days of life [3]. Therefore, we think that the medical law revision are required, including discussions on the use or discontinuation of antibiotics when writing POLST.
There have been studies on medical staff’s perceptions of antibiotic stewardship in EOL patients. One survey study found that many doctors who provide palliative care tend to initiate and maintain or extend antibiotics use in EOL patients, even in cases where antibiotics might not be appropriate or where the risks might outweigh the benefits [4]. Another study found that physicians have divergent attitudes toward the management of infectious diseases in terminally ill patients with cancer [29]. Our survey reveals a significant lack of awareness about ASP among internal medicine residents and specialists, and that doctors found it difficult to use antibiotics appropriately in EOL patients. We believe the reasons why antibiotics are prescribed so frequently, used for long periods, and not easily discontinued are likely due to doctors’ personal concerns of stopping antibiotics and the unclear benefits and risks of antibiotics cessation. In particular, regular education on palliative care is still insufficient before and after POLST implementation. In South Korea, doctors face many practical difficulties in the clinical field before and after POLST [30]. There is limited education related to antibiotic use guidelines for EOL patients in medical schools and clinical settings. Although a few tertiary centers have ASP support teams or hospice palliative care teams, there are no practical guidelines on appropriate antibiotic use in EOL patients. It is necessary for physicians to develop the ability to use antibiotics appropriately in cancer patients with EOL and make informed decisions regarding the proper use or discontinuation of antibiotics, considering the benefits and risks of antibiotics for hospitalized cancer patients with EOL aiming for “comfort” and not “cure”. We believe this ability could be developed through appropriate ASP activities [9]. Moreover, proper education on the use of appropriate antibiotics is needed from the time of medical school and throughout residency training, and clear guidance for ASP in EOL care is essential.
We acknowledge some limitations to our study. First, there is potential selection bias as this study is a retrospective study conducted at a single institution. A more accurate analysis and results would be achieved through a multicenter study. Second, this study focused on hospitalized patients with solid organ cancer and internal medicine doctors. Additional research is needed on EOL cancer patients with hemaologic malignancy and medical staff belonging to departments other than internal medicine. Third, this study excluded patients who showed improvement after receiving antibiotics during the EOL stage, and therefore cannot determine the effectiveness of antibiotic use during this period or provide guidance on whether to initiate or discontinue their use.
Despite these limitations, we were able to determine the status of antibiotic use and medical staff perception among hospitalized EOL cancer patients. This study could provide hospital antibiotic management specialists with a direction for ASP development and serve as valuable data to enhance the ability of residents, specialists, and hospitalists who primarily manage inpatients to use antibiotics appropriately. Additionally, there is a need to improve medical staff’s awareness of ASP, which may be achieved by actively implementing ASP, adding antibiotic education in EOL situations to medical school curricula, or actively guiding the use of antibiotics in patients with EOL in hospitals.
In conclusion, most cancer patients with EOL continued to receive antibiotics until just before death, and antibiotic use in these patients should be carefully determined by comprehensively considering the benefits, potential problems, and the patient’s right to self-decision. During EOL care discussions for hospitalized patients with terminal cancer, antibiotics usage should be taken into consideration, and efforts should be made to increase medical staff awareness on appropriate usage of antibiotics.
KEY MESSAGE
1. Most cancer patients with EOL care continue to receive antibiotics until just before their death.
2. Antibiotics use in EOL patients should be carefully determined by comprehensively considering the benefits, potential problems, and the patient’s right to self-decision.
3. It is necessary to actively improve awareness of ASP and their importance for medical staff.
Notes
CRedit authorship contributions
Min Kwan Kwon: conceptualization, methodology, resources, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, supervision, project administration; Kyung Hwa Jung: writing - review & editing; Sungim Choi: writing - review & editing; Hyeonjeong Kim: writing - review & editing; Chang-Yun Woo: writing - review & editing; Mingee Lee: writing - review & editing; Jeong Geun Ji: writing - review & editing; Hyo-Ju Son: conceptualization, methodology, resources, investigation, data curation, formal analysis, writing - original draft, writing - review & editing, supervision, project administration
Conflicts of interest
The authors disclose no conflicts.
Funding
None