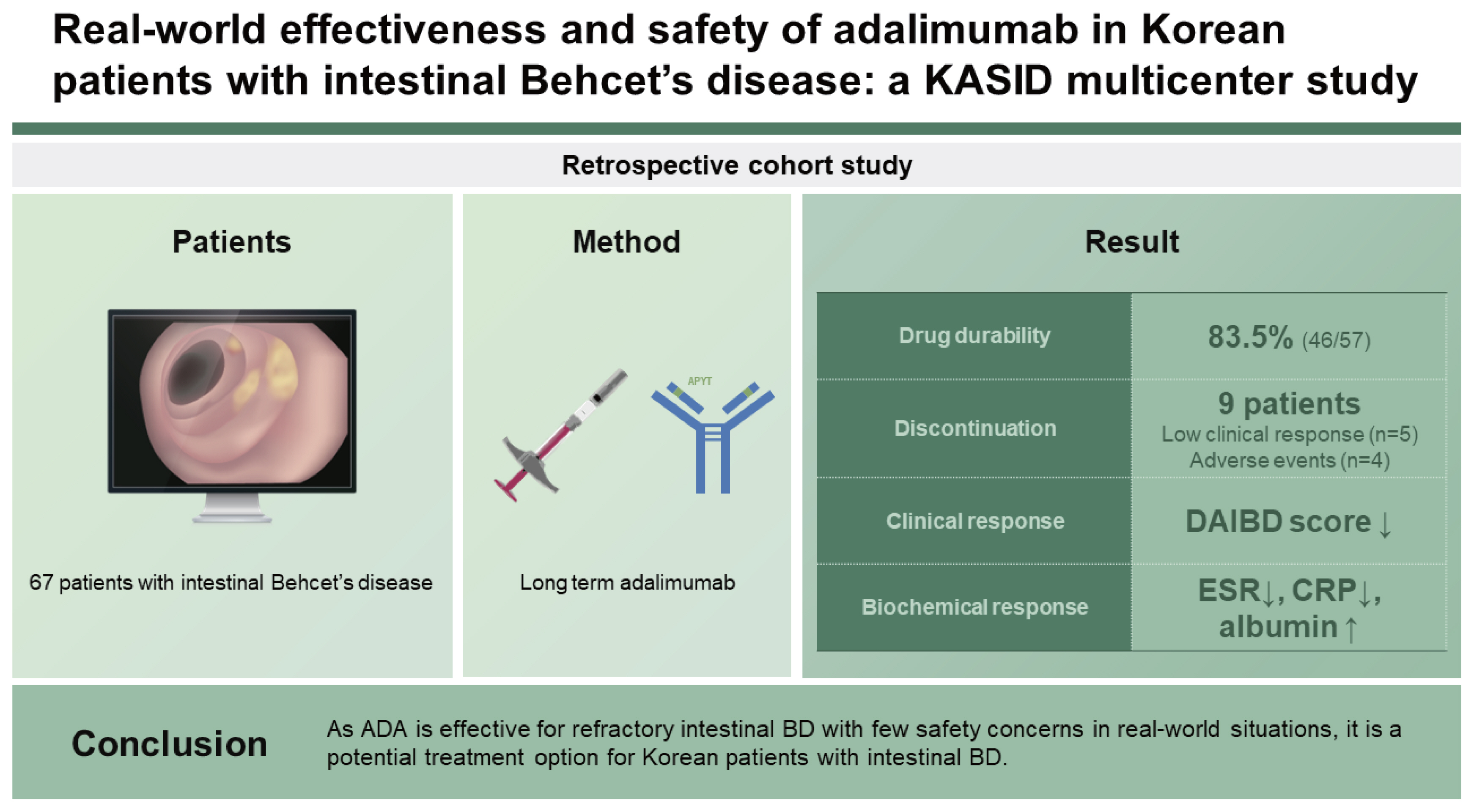
Real-world effectiveness and safety of adalimumab in Korean patients with intestinal Behcet’s disease: a Korean Association for the Study of Intestinal Diseases (KASID) multicenter study
Article information
Abstract
Background/Aims
The short- and long-term effects of adalimumab (ADA) on Korean patients with intestinal Behcet’s disease (BD) for remain unclear. Therefore, a multicenter study was performed to evaluate the efficacy and safety of ADA in Korean patients with intestinal BD in a real-world setting.
Methods
The medical records of 67 patients with BD prescribed ADA between January 2012 and December 2020 at five referral centers in Korea were retrospectively analyzed and the safety and efficacy of ADA within 52 weeks were assessed. To evaluate the clinical efficacy of ADA, the Disease Activity Index for Intestinal BD (DAIBD) and representative blood biochemical markers were compared at 0, 12, 24, and 52 weeks of ADA treatment.
Results
During the follow-up period of 52 weeks, 46 patients continued ADA treatment. The cumulative drug survival rate was 83.5%. The DAIBD score decreased over the study period (p < 0.001). Moreover, the erythrocyte sedimentation rate, serum C-reactive protein levels, and serum albumin levels significantly improved at 12, 24, and 52 weeks of ADA treatment (all, p <0.05).
Conclusions
As ADA is effective for refractory intestinal BD with few safety concerns in real-world situations, it is a potential treatment option for Korean patients with intestinal BD.
INTRODUCTION
Behcet’s disease (BD) is a systemic vasculitis of unknown etiology characterized by recurrent oral aphthous ulcers, uveitis, skin lesions, and genital ulcers. The incidence of BD varies according to geographical location, with the highest prevalence in Turkey (420 per 100,000 people), followed by Iran, northern China, Korea, and Japan [1,2]. Approximately 16–25% of Asian patients with BD may develop intestinal ulcers that are associated with life-threatening complications, including bowel perforation and massive bleeding [3]. Although intestinal BD management has not been well established, BD is traditionally treated with colchicine, corticosteroids, and/or immunomodulators, such as thiopurines [4,5]. Tumor necrosis factor-alpha (TNF-α) has been recently identified as a potential treatment target for BD [6]. Since the 2000s, clinical studies on the use of anti-TNF-α agents, including adalimumab (ADA, a fully human monoclonal antibody for TNF-α) and infliximab (IFX, a chimeric anti-TNF-α), have reported an improved clinical outcome in patients with intestinal BD, primarily from East Asia [7–11]. The revised Japanese consensus guidelines (2014) recommend subcutaneous ADA as the standard treatment for patients with intestinal BD who have severe disease symptoms, with typical deep ulcers confirmed by radiology or ndoscopy [12]. The short- and long-term effects (regarding clinical symptoms and endoscopic findings) of ADA in Japan were reported until 2021, with its safety having been established [13–15]. In 2015, ADA was approved in Korea for the treatment of intestinal BD and has had wide utility; however, data supporting its clinical efficacy and safety in Korean patients are limited owing to the rarity of the disease [16]. Therefore, this study aimed to perform a 52-week analysis of the effects of ADA treatment on Korean patients with intestinal BD.
METHODS
Patients
The medical records of all patients prescribed ADA between January 2012 and December 2020 at five referral centers in Korea were retrospectively evaluated (Fig. 1). Korean patients aged 18–80 years who were diagnosed with intestinal BD and administered ADA treatment for the first time were included in the study. The indications for ADA treatment include being refractory to stable treatment with corticosteroids or immunomodulators, or failure to taper corticosteroids. Patients prescribed ADA for reasons other than intestinal BD were excluded from the analysis. Patients with latent tuberculosis (TB) received approximately three weeks of anti-TB medication before treatment with ADA. Data regarding the demographic characteristics of patients, including sex; age at diagnosis; age at initiation of ADA treatment; disease duration from diagnosis to initiation of ADA treatment; baseline disease characteristics of BD such as concomitant oral and genital ulcers, uveitis, skin lesions, and joint involvement; prior bowel resection history; and prior medication history including other anti-TNF-α treatments, were obtained. We classified the patients as follows: ‘complete,’ ‘incomplete,’ ‘suspected,’ and ‘others’ according to the criteria of Behcet’s Disease Research Committee of Japan, 1987. Baseline colonoscopy was used to evaluate the status of ileocecal ulcer, and data on the number of ulcerative lesions and the maximum diameter of the ulcer were obtained. The Disease Activity Score for Intestinal BD (DAIBD) score, the most commonly used scoring system in Korea, was used to assess disease activity [17]. The DAIBD score of each patient was routinely evaluated at each visit. The clinical data of patients, including the DAIBD score, laboratory data, and all adverse events (AEs) observed within 52 weeks of ADA treatment, were obtained. AEs included skin problems, injection site reactions, headaches, any infections, and newly occurring malignancies.
Outcomes
The primary outcome was the drug durability of ADA within 52 weeks of treatment. The clinical response was evaluated based on the clinical scoring system, DAIBD, including the general well-being of the patients, fever, extra-intestinal manifestations, abdominal pain/tenderness or mass, intestinal complications, and stool quality [17]. The DAIBD score was estimated serially every four weeks in available patients for 24 weeks of ADA treatment, and then approximately every 8–12 weeks until week 52. Biochemical responses were assessed based on changes in blood markers, including the erythrocyte sedimentation rate (ESR), serum C-reactive protein (CRP), and serum albumin. To analyze the factors related to the biochemical response, the response was determined when each item was restored to the normal range: ESR ≤ 20 mm/h, serum CRP ≤ 0.5 mg/dL, and serum albumin ≥ 4.0 g/dL at week 52. In addition, to further evaluate the clinical response, patients who did not discontinue steroid administration even after 52 weeks of ADA administration were investigated and classified as the ‘sustained systemic steroid use’ group.
Statistical analysis
In the descriptive analysis, categorical variables are expressed as numbers with percentages, and continuous variables are expressed as medians with interquartile ranges (IQRs). The drug survival for ADA was analyzed using the Kaplan–Meier method. To investigate the factors associated with drug durability, Cox’s regression tests were performed. The least squares mean (LS mean) values were compared using a covariance pattern model, in order to compare changes in representative biochemical markers in repeated measurements. The binary logistic analysis was used to evaluate the factors associated with the biochemical response and sustained systemic steroid use at week 52. All results were considered statistically significant at two-sided p values of < 0.05. Variables were selected for inclusion in the multivariate model, based on univariate p values of < 0.2. Statistical analyses were performed using SPSS (version 24.0, IBM Corp., Armonk, NY, USA) and SAS (version 9.4, SAS Institute, Cary, NC, USA).
Ethics statement
This study was approved by the institutional review board of all participating institutions including Asan Medical Center (IRB number: 2021-1099) and Ulsan University Hospital (IRB number: 2022-04-003). The requirement for informed consent was waived given its observational nature.
RESULTS
Patient characteristics
A total of 67 patients administered ADA between 2015 and 2020 were included in this study (Fig. 1). Demographic data and the disease-related characteristics of the study population are summarized in Table 1. The median age at diagnosis and initiation of ADA treatment was 46 years (IQR: 35–58 yr) and 52 years (IQR: 40–62 yr), respectively. The median disease duration at the initiation of ADA treatment was 20 months (IQR: 5–102 mo). Fifteen patients had a history of bowel surgeries. The median baseline DAIBD score was 80 (IQR: 60–115). Regarding non-intestinal BD manifestations, 54 (80.6%) and 26 (38.8%) patients had oral and genital ulcers, respectively. Most patients (66/67) had a history of prior corticosteroid use, and 52 (77.6%) used a concomitant immunomodulator at the initiation of ADA treatment. Baseline colonoscopy results were available for all patients. Single ulcers with a diameter of 10–30 mm were the most prevalent; however, multiple ulcers were not uncommon. Some patients had suspected small intestinal lesions based on computed tomography; however, such lesions were not confirmed by colonoscopy.
Drug durability of ADA treatment and associated factors
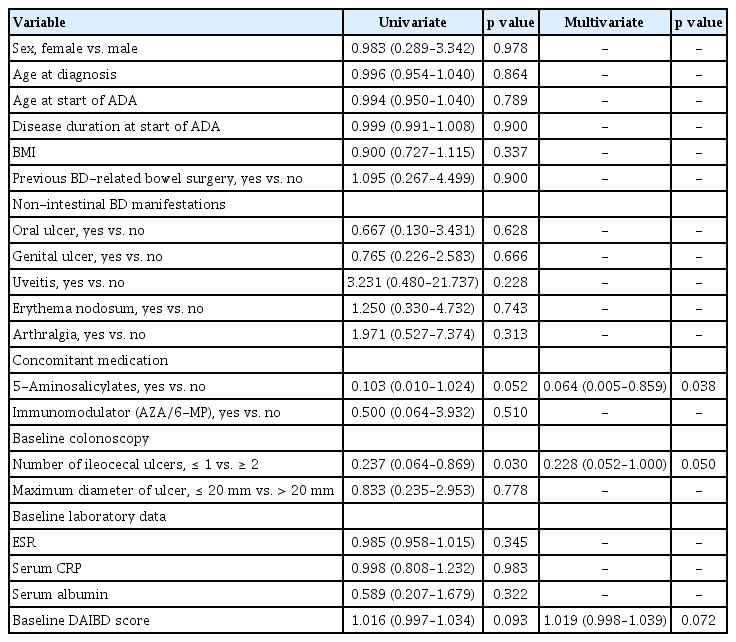
Long-term durability data on ADA treatments were collected until December 2021. The drug durability for all patients is shown in Figure 2. During the follow-up period (52 wk), 46 patients continued ADA therapy. The cumulative drug survival rate was 83.5%. Nine patients in the study population did not complete the 52-week follow-up period, but continued ADA treatment until the last follow-up. Nine patients discontinued ADA within the study period. Five patients did not show an appropriate clinical response to ADA and discontinued the treatment after a median of 29 weeks (range 6–42 wk). Four patients discontinued medication due to AEs; one (1.9%) patient had an infectious disease (tuberculous peritonitis) at 39 weeks, two (3.7%) had progression of comorbid hematologic disorders, and one (1.9%) developed a malignancy (tonsil cancer). Factors associated with drug durability are shown in Table 2. Concomitant use of 5-aminosalicylic acid (5-ASA), systemic steroid, or immunomodulatory drugs at the start of ADA administration was found to be related to drug durability (all p <0.05). Another significant factor was the co-occurrence of genital ulcer. However, no statistically significant factors were identified after multivariate analysis.
Clinical and biochemical response during ADA treatment
Blood inflammatory markers, namely ESR, serum CRP, and serum albumin, exhibited significant changes during the 52-week ADA treatment (Fig. 3). ESR and serum CRP levels significantly decreased (all p values < 0.05). The LS mean values of ESR at baseline and week 52 were 30.2 and 21.7 mm/h, respectively. The LS mean values of CRP levels at baseline and week 52 were 2.6 and 0.8 mg/dL, respectively. The serum albumin levels significantly increased over the 52-week treatment period (all p values were < 0.05). The LS mean value serum albumin levels at baseline and week 52 were 3.6 and 3.9 g/dL, respectively. Additionally, the DAIBD scores significantly decreased during the follow-up period (all p values < 0.01). Colonoscopy was performed in a total of 25 of the 46 patients with a 52-week follow-up. Complete healing of ulcers was observed in 15 patients (60.0%) and incomplete healing was observed in four patients, with the diameter of the ulcers reducing to at least half the original size. In a total of six cases, the condition of the ulcers either showed no improvement, i.e., no reduction in ulcer size, or had worsened.
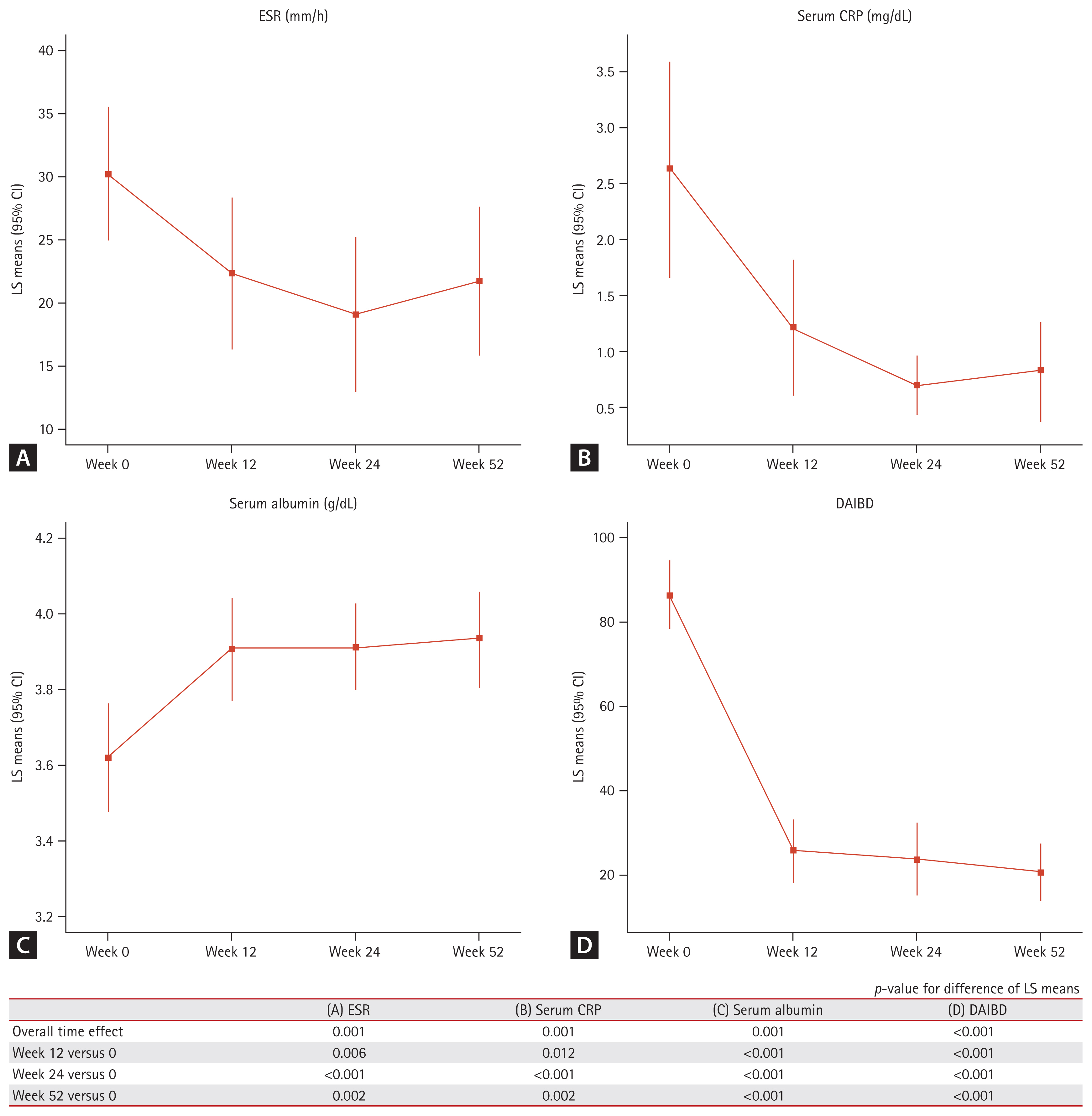
Changes in the biochemical markers and clinical index over 52 weeks of adalimumab treatment for intestinal BD. (A) ESR, (B) serum CRP, (C) serum albumin, and (D) DAIBD score. BD, Behcet’s disease; CI, confidence interval; CRP, C-reactive protein; DAIBD, Disease Activity Score for Intestinal BD; ESR, erythrocyte sedimentation rate; LS mean, least squares mean.
Factors associated with biochemical responses and sustained systemic steroid use
To evaluate the efficacy of ADA treatments, factors associated with the biochemical response and sustained systemic steroid use at week 52 were investigated. Among 46 patients who completed follow-up until 52 weeks, biochemical responses were shown in 60.0% (27/45) of ESR, 75.6% (34/45) of serum CRP, and 54.3% (25/46) of serum albumin, respectively. In analyses for the factors related to the biochemical response in 46 patients, no significant factors were found to be simultaneously associated with all three parameters – ESR, serum CRP, and serum albumin (Table 3). When patients were classified according to the criteria of the Behcet’s Disease Research Committee of Japan, no difference in ADA response was observed according to each diagnostic category. Systemic steroids were used prior to ADA administration in 46 patients; steroid use was continued in 16 patients (34.8%) at 52 weeks. In the multivariate analysis, concomitant use of 5-ASA was found to be a significant protective factor. In addition, the presence of two or more ulcers during baseline colonoscopy was a statistically significant risk factor (Table 4).
DISCUSSION
The results of this study revealed that ADA steadily improved the clinical index and representative biochemical markers during the 52-week treatment period, in patients with intestinal BD who were resistant to conventional treatment. The cumulative drug survival rate reached 83.5%, and was similar to that obtained from long-term follow-up data from clinical trials in Japan; approximately 75% of the study patients showed long-term durability of ADA for intestinal BD at week 52 [15]. This result was also similar to that shown in the ADA real-world data for Crohn’s disease (CD) [18]. Out of five patients with an inadequate clinical response (at 6–42 wk), three had worsening fever and abdominal pain, and the remaining two had bowel perforation and intra-abdominal abscess. Of the five patients who developed an inadequate response, four could not discontinue steroids during ADA administration.
We intended to investigate characteristics related to ADA durability but did not find any statistically significant factors after the multivariate analysis, likely due to the small sample size. According to a previous Korean multicenter study on IFX administration in patients with intestinal BD, the following results were presented as factors predicting a sustained response: age at diagnosis ≥ 40 years; female sex; disease duration ≥ 5 years; achievement of remission at week 4; and concomitant immunomodulator use [19]. However, since the number of study subjects was small (n = 28) and a multivariate analysis was not performed, a comparison with the results of this study does not seem appropriate. Future large-scale and prospective design studies are thus needed to validate our findings.
The increased risk of TB in patients who were administered anti-TNF-α agents may be attributed to TNF-α-mediated immune mechanisms. These are involved in the pathological changes in latent TB infection (LTBI), particularly the maintenance of the formation and function of granulomas, which prevent mycobacteria from disseminating into the blood [20]. In Korea, a country with a high TB prevalence, an LTBI test is recommended before the use of an anti-TNF-α agent. For an LTBI-positive tuberculin skin test or a positive interferon-gamma release assay (IGRA) test, the standard treatment guideline necessitates starting an anti-TNF-α treatment after administering anti-TB treatment for approximately three weeks. In this study, one patient with TB peritonitis was identified at week 39, leading to the discontinuation of ADA treatment. The patient showed a positive result in the IGRA test and ADA was started after 3 weeks of anti-TB therapy. Despite screening, the occurrence of TB in Korean IBD patients treated with anti-TNF-α agents has been previously reported [21]. Therefore, caution must be exercised for the use of anti-TNF-α in regions with high TB prevalence, such as Korea.
In this study, one case of tonsil cancer was confirmed at 52 weeks after ADA administration; however, no other solid organ malignancies were observed. In a previous study on ADA safety, the rate of malignancies among patients treated with ADA, except for lymphoma and non-melanoma skin cancer, did not differ from the expected rate in the general population [22]. According to two representative studies on ADA for CD patients, the probability of malignancy occurrence with ADA treatment did not significantly increase compared with placebo treatment during the study period of 52 or 56 weeks [23,24]. As tonsil cancer is difficult to detect through screening tests, in the present study, we could not ascertain its occurrence after the initiation of ADA treatment in the patients. Although there was no evidence in previous reports that ADA increased the incidence of head and neck cancers, including tonsil cancer, ADA treatment was discontinued for this patient. Two patients in this study had exacerbated myelodysplastic syndrome (MDS), a pre-existing hematologic disorder; thus, allogeneic hematopoietic stem cell transplantation (allo-HSCT) was performed and ADA was discontinued. Of note, these patients were diagnosed with MDS prior to the initiation of ADA treatment, as MDS has been reported to concomitantly occur with BD in some patients [25]. The prevalence of concurrent BD-MDS ranges from 0.4–3.1% in all patients with BD and has been mainly reported in Korea, Japan, and China [26–28]. According to a Chinese study, the prevalence of gastrointestinal involvement in patients with concurrent BD-MDS was higher than that in patients with BD without MDS (62.5 versus 11.9%) [29]. In this study, it was difficult to confirm the effect of ADA on the hematological deterioration of the two patients with MDS. Furthermore, there are no previous reports of the association between ADA and hematologic disorders, such as MDS or acute leukemia.
In our study, the clinical response evaluated using DAIBD and the biochemical response of ESR, serum CRP, and serum albumin displayed consistent improvement after starting ADA treatment. For the results in which the DAIBD and laboratory data were compared at 0, 12, 24, and 52 weeks after ADA treatment, a statistically significant improvement was observed, highlighting the effects of ADA treatment on intestinal BD. This finding was also confirmed by the most recent Asian study in Japan (n = 462) [15]; thus, the current study reinforces that ADA can be an effective treatment strategy for intestinal BD refractory to traditional treatment in Korea. In our study, we attempted to examine the baseline characteristics related to the biochemical and clinical responses. However, factors related to all changes in ESR, serum CRP, and serum albumin were not identified.
It should be noted that 66 of the 67 patients were using concomitant corticosteroids at the time of ADA initiation in the study. Among 46 patients who completed follow-up until 52 weeks, sustained systemic steroid use was reported in 16 (34.8%) patients. To further evaluate the clinical response, factors associated with sustained systemic steroid use at week 52 were also investigated. As a result, concomitant use of 5-ASA and the number of ulcers on the baseline colonoscopy may be related, and additional studies are expected to be conducted.
This study had a few limitations. First, the study had a relatively small sample size and a retrospective design; these may potentially distort the findings. In particular, analysis of factors related to drug durability or biochemical response did not yield meaningful results. Second, endoscopic and radiologic data were unavailable during follow-up for approximately half of the patients; therefore, the effectiveness of ADA in mucosal healing could not be evaluated. Third, analysis of the changes in the levels of fecal calprotectin, an important parameter for evaluating responses in inflammatory bowel disease, could not be performed due to a lack of follow-up data. Nevertheless, the treatment response was assessed using other representative biomarkers, such as ESR, serum CRP, and serum albumin. Fourth, considering the biochemical response, not all patients underwent laboratory tests every four weeks; hence, the number of patients evaluated at each time point was different. An attempt to overcome these shortcomings was made using paired tests. Finally, as patients who discontinued ADA were excluded, the possibility of selection bias in the evaluation of biochemical responses was revealed.
Despite these limitations, to our knowledge, this is the first study in Korea to evaluate the use of ADA for patients with intestinal BD using a meaningful number of samples. Our study confirmed that despite its intolerability in some patients, ADA could be a treatment option for Korean patients with intestinal BD. Overall, ADA was demonstrated to be safe and effective for treating intestinal BD in patients refractory to conventional therapies.
KEY MESSAGE
1. Adalimumab showed improvement of clinical index and biochemical markers during the 52-week with few safety conserns in patients with intestinal Behcet's disease who were resistant to conventional treatment.
2. The cumulative drug survival rate was 83.5%, and 4 patients discontinued medication due to adverse events such as tuberculous peritonitis, progression of comorbid myelodysplastic syndrome, and tonsil cancer.
Notes
CRedit authorship contributions
Seung Bum Lee: conceptualization, data curation, formal analysis, writing - original draft; Hee Seung Hong: conceptualization, data curation; Chang Kyun Lee: data curation; Bo-In Lee: data curation; Sol Kim: data curation; Seong-Joon Koh: data curation; Hosun Yu: data curation; Jung-Bin Park: writing - review & editing; Sung Wook Hwang: writing - review & editing; Byong Duk Ye: writing - review & editing; Suk-Kyun Yang: writing - review & editing; Sang Hyoung Park: conceptualization, formal analysis, writing - original draft
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR21C0198) and the National Research Foundation of Korea (NRF) grant, funded by the Korean government (MSIT), awarded to Sang Hyoung Park (No. 2021R1G1A1094252).