Clinical safety and effectiveness of the Genoss drug-eluting stent in real-world clinical practice
Article information
Abstract
Background/Aims
The Genoss DES™ is a novel, biodegradable, polymer-coated, sirolimus-eluting stent with a cobalt-chromium stent platform and thin strut. Although the safety and effectiveness of this stent have been previously investigated, real-world clinical outcomes data are lacking. Therefore, the aim of this prospective, multicenter trial was to evaluate the clinical safety and effectiveness of the Genoss DES™ in all-comer patients undergoing percutaneous coronary intervention.
Methods
The Genoss DES registry is a prospective, single-arm, observational trial for evaluation of clinical outcomes after Genoss DES™ implantation in all-comer patients undergoing percutaneous coronary intervention from 17 sites in South Korea. The primary endpoint was a device-oriented composite outcome of cardiac death, target vessel-related myocardial infarction (MI), and clinically driven target lesion revascularization (TLR) at 12 months.
Results
A total of 1,999 patients (66.4 ± 11.1 years of age; 72.8% male) were analyzed. At baseline, 62.8% and 36.7% of patients had hypertension and diabetes, respectively. The implanted stent number, diameter, and length per patient were 1.5 ± 0.8, 3.1 ± 0.5 mm, and 37.0 ± 25.0 mm, respectively. The primary endpoint occurred in 1.8% patients, with a cardiac death rate of 1.1%, target vessel-related MI rate of 0.2%, and clinically driven TLR rate of 0.8%.
Conclusions
In this real-world registry, the Genoss DES™ demonstrated excellent safety and effectiveness at 12 months among all-comer patients undergoing percutaneous coronary intervention. These findings suggest that the Genoss DES™ may be a viable treatment option for patients with coronary artery disease.
INTRODUCTION
Coronary artery disease (CAD) is commonly treated with percutaneous coronary intervention (PCI) using metallic stents. New-generation drug-eluting stents (DESs) have improved clinical outcomes compared with first-generation DESs by using newer or reduced doses of active drugs, biocompatible durable polymer, or biodegradable polymers, and thinner struts [1–4]. However, adverse outcomes such as stent thrombosis (ST) and restenosis remain a concern, leading to the development of newer stent technologies aimed at reducing these events [5,6].
The Genoss DES™ (Genoss Company Limited, Suwon, Korea) is a novel sirolimus-eluting stent designed to improve vascular healing after implantation with its thin strut and abluminal biodegradable polymer. Previous first-in-man trials have shown similar angiographic and clinical outcomes to the Promus Element stent (Boston Scientific, Natick, MA, USA) at 9 months and 5 years of follow-up [7,8]. However, the sample size of those trials was too small to provide robust evidence for the safety and efficacy of the Genoss DES™.
To address this gap, we conducted a prospective Genoss DES registry study to evaluate the clinical safety and effectiveness of Genoss DES™ in unselected patients, reflecting real-world practice. We previously reported on the interim analysis of this registry [9], and here we present the final analysis of the Genoss DES registry.
METHODS
Genoss DES™
The Genoss DES™ is a sirolimus-eluting stent with an abluminal biodegradable polymer and a cobalt-chromium platform, designed to release nearly 70% of its initial drug payload (1.15 mg/mm2) within the first 30 days after implantation [7]. The stent features an open-cell design with a uniform architecture for enhanced conformability and resistance to shortening. The strut thickness is 70 and 78 μm for stents with diameters of 2.25–2.50 mm and 2.75–5.00 mm, respectively. A 3-μm ultrathin coating is applied only to the abluminal side of the stent to achieve the desired drug release profile and minimize the amount of polymer used. The biodegradable coating comprises a proprietary blend of poly(lactic-co-glycolic acid) and poly(L-lactic acid) and is almost completely degraded within nine months. The Genoss DES™ was approved by the Korean Food and Drug Administration in 2016. It has also received the Conformité Européenne Mark and is available in certain Asian and Latin American countries.
Genoss DES registry
The Genoss DES registry (ClinicalTrials.gov identifier: NCT03045913) is a prospective, single-arm, observational, multicenter, sponsor-initiated trial to enroll 2,000 patients from 17 sites in South Korea. The trial aimed to collect baseline clinical characteristics and angiographic and procedural data of consecutive patients with CAD treated with Genoss DES™ for at least one significant coronary stenosis. Patients aged ≥ 19 years presented with stable angina or acute coronary syndrome were eligible. The exclusion criteria included intolerance to medication, allergy to stent components, surgery planned within 12 months of the index PCI, cardiogenic shock during the index PCI, and life expectancy < 12 months. Clinical follow-up visits were scheduled for 1, 6, and 12 months after the index PCI. All patients were followed up either via office visits or via telephone, as necessary. Data quality was assessed via independent monitoring, and all events were adjudicated by an independent clinical event committee. All patients provided written informed consent for participation. The Institutional Review Boards of Wonju Severance Christian Hospital (CR216010) and each participating center approved this study. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Procedure
Standard interventional techniques for PCI were employed. The number, diameter, and length of the stents used were not restricted. The use of various agents, devices, and techniques such as glycoprotein IIb/IIIa inhibitors, heparin, thrombectomy devices, intravascular ultrasound (IVUS), optical coherence tomography, and pressure wires were at the operator’s discretion. Plain old balloon angioplasty (POBA) with or without a drug-coated balloon (DCB) in conjunction with Genoss DES™ implantation was permitted in cases of multivessel or bifurcation PCI. Lesion success was defined as a residual stenosis ≤ 20% and a final Thrombolysis in Myocardial Infarction flow grade of 3 by visual estimation. The clinicians were encouraged to determine the selection, dose, and duration of dual antiplatelet therapy (DAPT) with aspirin plus P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) according to the current guidelines [10,11].
Clinical endpoints
The primary endpoint was a 12-month device-oriented composite outcome (DOCO) comprising cardiac death, target vessel-related myocardial infarction (MI), and clinically indicated target lesion revascularization (TLR). The secondary endpoints were a patient-oriented composite outcome (POCO) comprising any death, any MI, and any revascularization, each component of the DOCO and the POCO, and Academic Research Consortium-defined ST, all determined at the 12-month clinical follow-up. All definitions of the above endpoints are provided elsewhere [12]. Periprocedural MI was not included in this endpoint because periprocedural cardiac biomarkers are not routinely collected. Lesion and procedural characteristics were reported according to visual estimation. Core laboratory–adjudicated quantitative coronary angiography was not conducted.
Statistical analysis
We expressed continuous variables as means ± standard deviations and categorical variables as numbers (percentages). Event-free survival was assessed using the Kaplan–Meier method, with patients being censored at 12 months or at the time of death, whichever occurred first. Patients lost to follow-up were censored at the time of last contact. The patient population was stratified into subgroups based on age, sex, acute MI, hypertension, diabetes, chronic kidney disease, smoking, prior MI, PCI for any in-stent restenosis (ISR) lesion, PCI for any chronic total occlusion lesion (CTO), PCI for any bifurcation lesion, use of IVUS, concomitant use of a DCB or POBA, use of any stent with a diameter ≤ 2.5 mm, and use of any stent with a length > 30 mm. In each subgroup, the event-free survival of DOCO and TLR at 12 months of follow-up was assessed using the Kaplan–Meier method. We considered p values less than 0.05 as clinically significant. Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA).
RESULTS
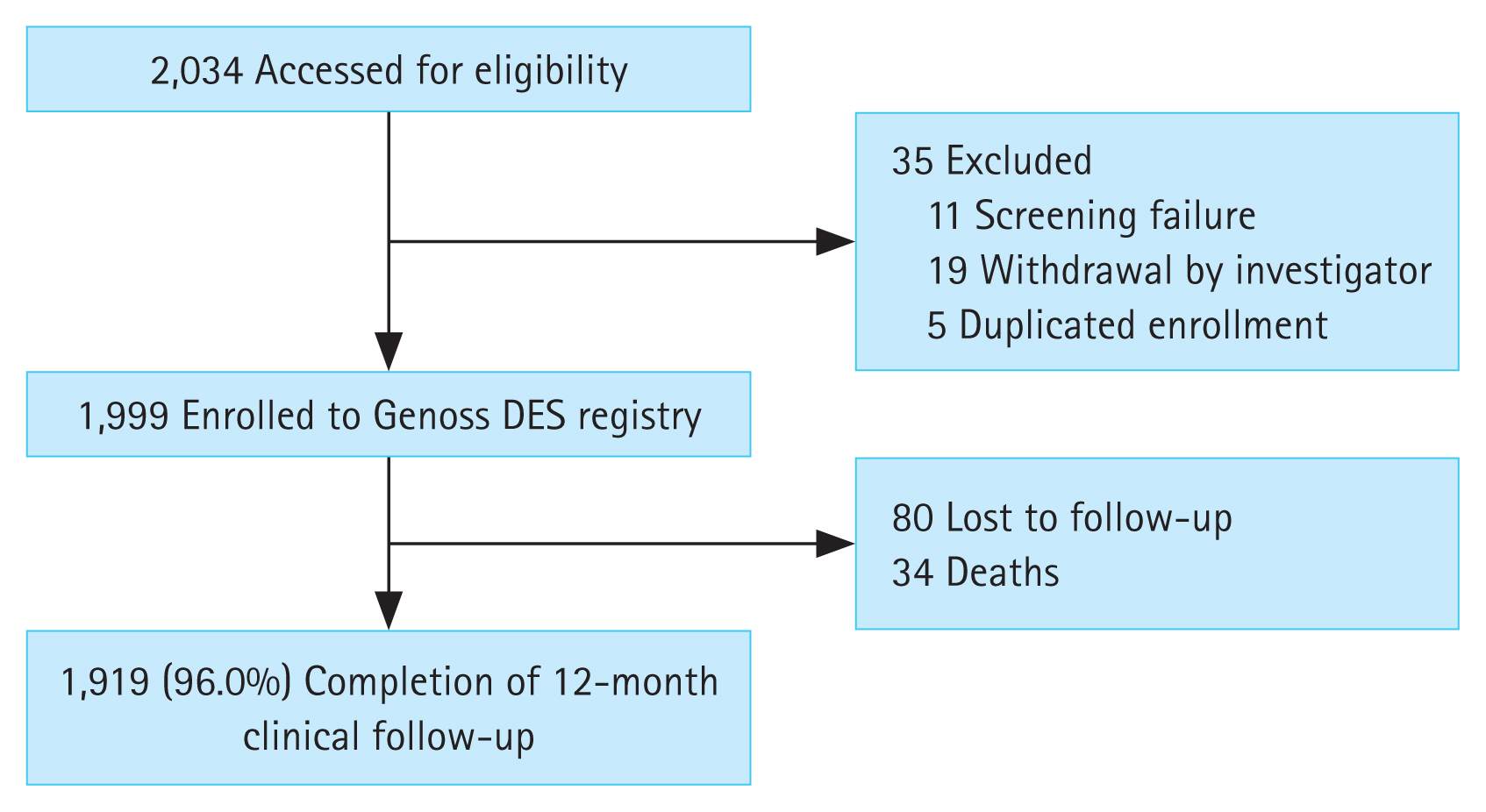
Among the 2,034 patients screened from November 2016 to November 2021, 1,999 were analysed for clinical outcomes after 35 were excluded (Fig. 1).
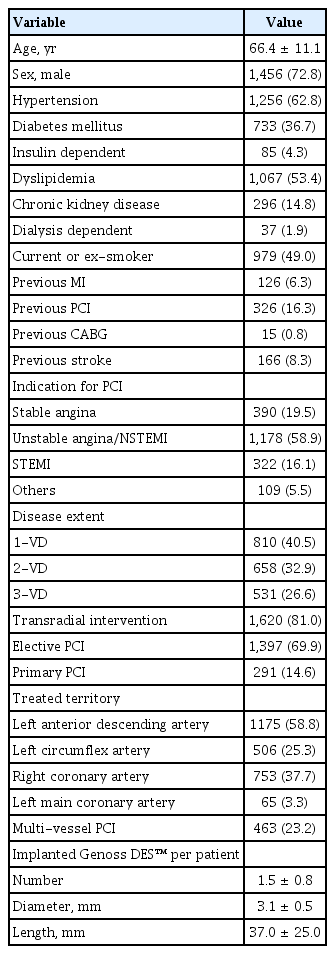
The baseline clinical, angiographic, and procedural characteristics of the patients are summarized in Table 1. The mean age of the patients was 66.4 ± 11.1 years, and the majority (72.8%) were male. Common comorbidities included hypertension (62.8%), dyslipidemia (53.4%), and diabetes mellitus (36.7%). The majority of patients underwent PCI for unstable angina or non-ST-segment elevation MI (NSTEMI) (58.9%), followed by stable angina (19.5%) and STEMI (16.1%). The mean number of Genoss DES™ stents implanted per patient was 1.5 ± 0.8, with a mean diameter of 3.1 ± 0.5 mm and a mean length of 37.0 ± 25.0 mm. Transradial intervention was used in 81.0% of cases.
The angiographic and procedural characteristics of the lesions are summarized in Table 2. A total of 2,544 lesions were treated, with the majority being ACC/AHA type B2/C lesions (78.5%) and located in the left anterior descending artery (47.1%). The use of IVUS was reported in 27.6% of cases, and thrombectomy was performed in 5.3%. The majority of lesions were treated with Genoss DES™ only (95.5%). The mean implanted Genoss DES™ per lesion was 1.2 ± 0.5, with a mean diameter of 3.0 ± 0.5 mm and mean length of 29.7 ± 16.2 mm. Lesion success was achieved in 97.2% of cases, and post-procedure TIMI flow grade 3 was reported in 98.1%. The mean diameter stenosis post-procedure was 7.9 ± 7.8%.
Medications at discharge and antithrombotic agents at the 12-month follow-up among the survivors at discharge (n = 1,985) are presented in Supplementary Table 1. The majority of patients (98.1%) were discharged on DAPT, with aspirin and clopidogrel being the most commonly used combination (62.9%). Other medications prescribed at discharge included statins (96.3%), renin-angiotensin-system blockers (61.5%), and nitrates or nicorandil (62.3%). At 12-month follow-up, 56.0% of patients were still on DAPT, while 17.1% were on a P2Y12 inhibitor only and 15.8% were on aspirin only. A small proportion of patients were on oral anticoagulant therapy in combination with either DAPT or a P2Y12 inhibitor, or on oral anticoagulant therapy alone.
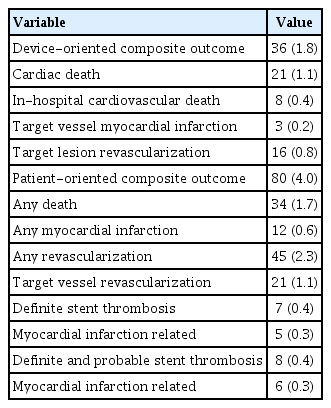
Clinical outcomes at the 12-month follow-up are presented in Table 3. A 12-month follow-up was completed in 96.0% of patients. The DOCO occurred in 36 (1.8%) of patients, with 21 (1.1%) cardiac deaths, 3 (0.2%) target vessel-related MI, and 16 (0.8%) TLR. The POCO occurred in 4.0% of patients, with 34 (1.7%) any deaths, 12 (0.6%) any MI, and 45 (2.3%) any revascularizations. Definite and probable ST occurred in 8 (0.4%) of patients, with 6 (0.3%) being MI-related. The Kaplan–Meier survival plots of the DOCO, POCO, TLR, and target vessel-related MI are illustrated in Figure 2.
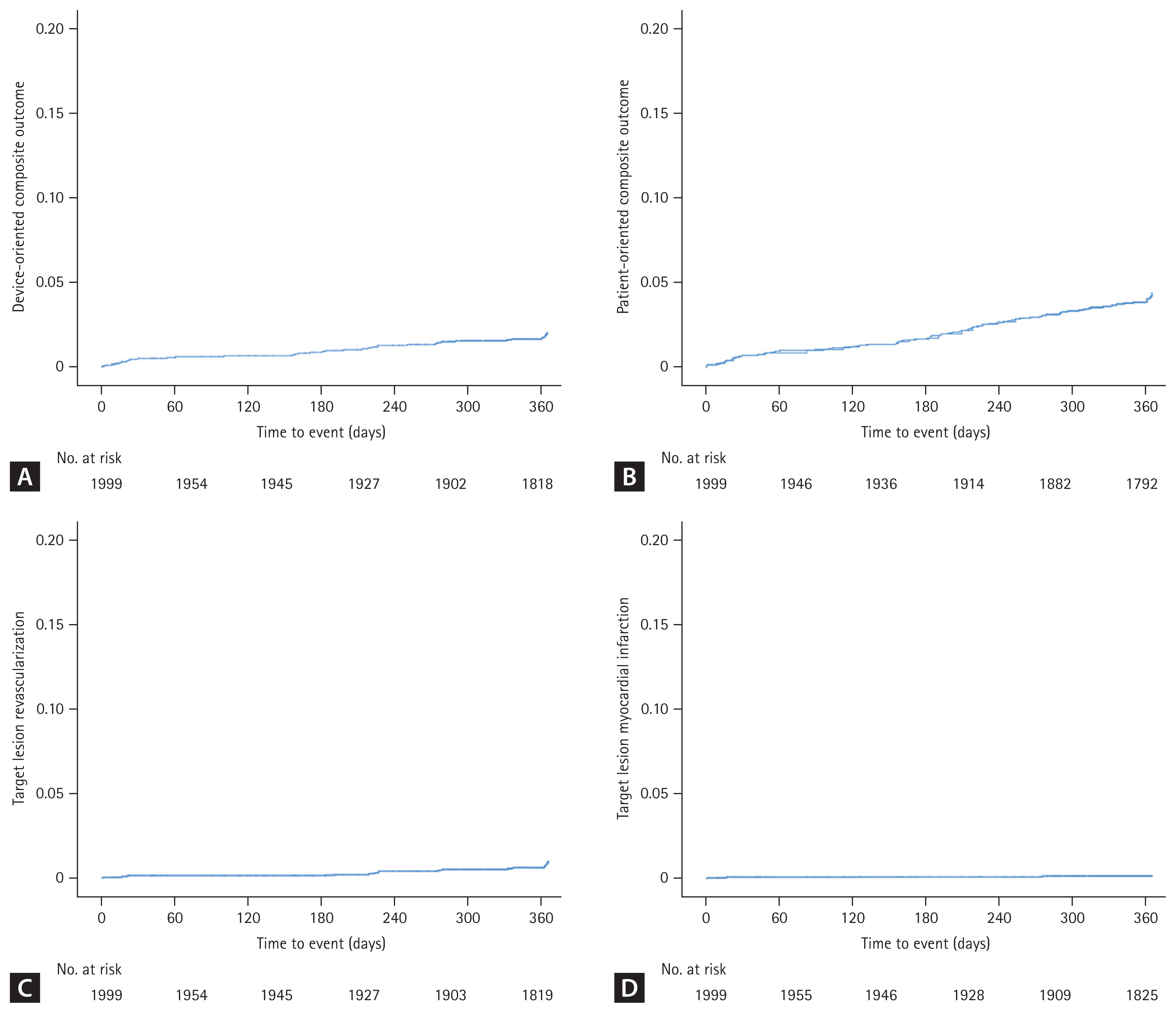
Kaplan–Meier survival curves. Event rates were estimated based on the unadjusted Kaplan–Meier method. (A) Device-oriented composite outcome, (B) patient-oriented composite outcome, (C) target lesion revascularization, and (D) target lesion myocardial infarction.
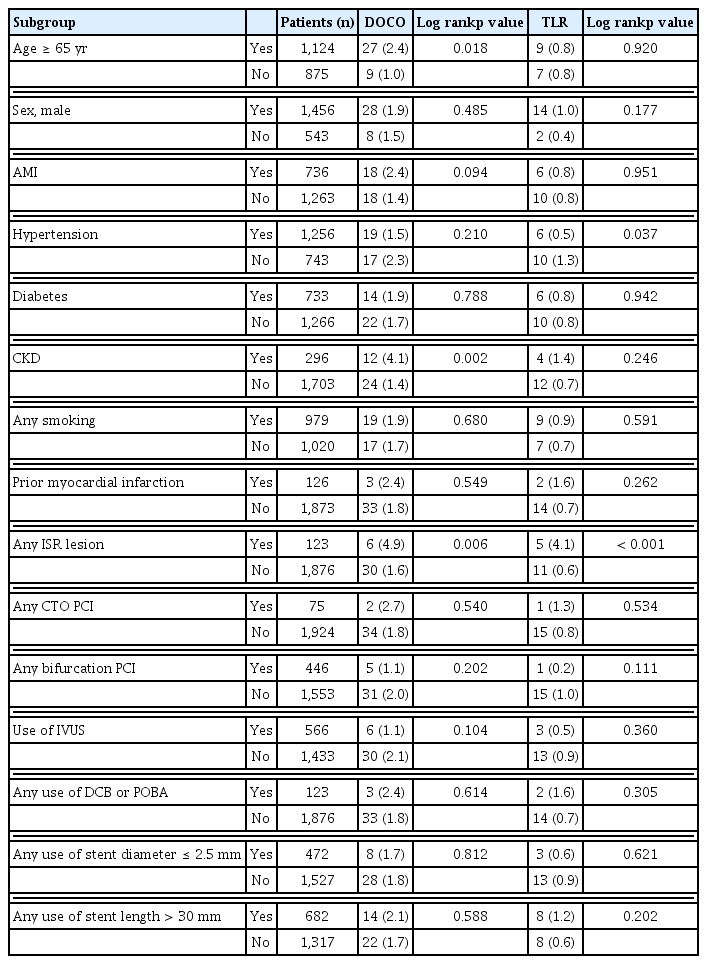
The results of the subgroup analysis for the DOCO and TLR at the 12-month follow-up are presented in Table 4. Some subgroups showed significant differences in the incidence of DOCO and TLR, including age ≥ 65 years, CKD, and any ISR lesion. However, most subgroups did not show significant differences in the incidence of DOCO and TLR.
DISCUSSION
The study presented here reports on the safety and effectiveness of the Genoss DES™ in real-world practice, with low clinical event rates observed at the 12-month follow-up. The primary results were as follows: 1) treatment of patients with CAD with the Genoss DES™ resulted in low clinical event rates, with a DOCO rate of 1.8% and a definite and probable ST rate of 0.4% at the 12-month follow-up; 2) the rates of target vessel-related MI and clinically driven TLR were 0.2% and 0.8%, respectively, at the 12-month follow-up.
In the first-in-man random trial, patients treated with the Genoss DES™ (n = 38) were compared to those treated with the Promus Element stent (n = 39). At the 9-month follow-up, the in-stent late lumen loss did not differ significantly between the groups (0.11 ± 0.25 mm vs. 0.16 ± 0.43 mm, p = 0.567), nor did the rates of death, MI, TLR, and target vessel revascularization [7]. After 5 years, the Genoss DES™ yielded comparably low rates of major adverse cardiac events (5.3% vs. 12.8%, p = 0.431) and TLR (2.6% vs 2.6%, p > 0.999) to the Promus Element stent [8]. The Genoss DES registry includes high-risk patients with acute MI, CTO lesions, ISR lesions, left main disease, or diffuse long lesions. Nevertheless, the 12-month adverse event rate was low. Thus, with this study, we confirmed the results of the first-in-man trial in the real-world setting. Currently, a few Korea-made DES, such as D+Storm™ stent (CG Bio Co., Ltd., Seoul, Korea) and Centum™ stent (Osstem Cardiotec Co., Seoul, Korea), are available. However, there is limited evidence of the safety and efficacy of these DESs [13,14]. Genoss DES registry provides the most extensive evidence of the Genoss DES™.
The favorable results of this study may be associated with the biodegradability of the polymer and the thinness of the strut. In a meta-analysis of 10 randomized controlled trials, ultrathin-strut DESs (strut thickness < 70 μm) reduced the target lesion failure (relative risk [RR] 0.84; 95% confidence interval [CI]: 0.72–0.99) and MI (RR 0.80; 95% CI: 0.65–0.99) rates compared with thicker-strut second-generation DESs [15]. In another meta-analysis of 9 randomized controlled trials, biodegradable-polymer DESs showed a trend of the low incidence of definite or probable ST (RR 0.78; 95% CI: 0.59–1.01) and similar clinical outcomes compared with durable-polymer DES [16].
Despite the all-comer nature of the study population, event rates in this trial were low, which may be explained as follows: First, patients with periprocedural MI were excluded, which may have reduced the rate of MI compared with previous studies. Second, transradial intervention was used in 81% of patients. Transradial access is well-known to be associated with a lower risk of adverse clinical events than femoral access, particularly in patients with ST-segment elevation MI [17]. Third, ethnic or genetic protective factors may affect the clinical event rate [18]. In the recently published BIODEGRADE trial, in which Korean patients were enrolled, Orsiro stents (Biotronik, Bülach, Switzerland) were non-inferior to the BioMatrix stent (Biosensors, Singapore) in terms of target lesion failure (2.1% vs. 2.9%) at the 18-month follow-up [19]. Considering the similar properties between the Genoss DES™ and Orsiro stents, the event rate in this study was in line with that of the BIODEGRADE trial.
Limitations
This study has several limitations. One major limitation is the lack of a control group for a direct comparison, which may affect the interpretation of the results. Another limitation is that only patients from Korea were enrolled in the registry, which may limit the generalizability of the findings to other ethnicities and regions. Additionally, the follow-up period was relatively short (12 months), and the potential long-term benefits of the biodegradable polymer should be investigated in future studies. The use of operator-reported angiographic data instead of adjudicated data by an independent core laboratory is also a limitation. However, we provided detailed instructions to the participating hospitals on how to measure and record the angiographic data. We believe that this minimized the interobserver variability in angiographic measurements. Finally, the authors did not collect data on cases in which the stent failed to pass the lesion, which may underestimate the adverse events related to the stent. We believe that the delivery of the Genoss DES is generally acceptable.
Conclusions
The results of this study demonstrate the safety and efficacy of the Genoss DES™ in real-world practice among a diverse patient population with complex lesions. The low rates of adverse events and the consistent outcomes across different subgroups suggest that the biodegradable polymer and ultrathin strut design of the Genoss DES™ are associated with favorable clinical outcomes. However, further randomized controlled studies with larger sample sizes and longer follow-up periods are needed to confirm these findings and to evaluate the potential benefits of the biodegradable polymer over an extended period.
KEY MESSAGE
1. The Genoss DES™ is a novel sirolimus-eluting stent with an abluminal biodegradable polymer and a cobalt-chromium platform with thin strut.
2. The Genoss DES registry is a prospective, single-arm, observational, multicenter trial to enroll 2,000 consecutive patients with coronary artery disease treated with the Genoss DES™ with minimal exclusion criteria from 17 sites in South Korea.
3. In this real-world registry, the Genoss DES™ demonstrated excellent safety and effectiveness at 12 months among all-comer patients undergoing percutaneous coronary intervention.
Notes
Conflicts of interest
Junghan Yoon received a research grant from Genoss Company Limited; none of the other investigators had any financial relationships or any other biases or conflicts of interest related to this study.
CRedit authorship contributions
Young Jin Youn: writing - original draft; Jun-Won Lee: writing - review & editing; Sung Gyun Ahn: writing - review & editing; Seung-Hwan Lee: writing - review & editing; Junghan Yoon: conceptualization, writing - review & editing, funding acquisition; Jae Hyoung Park: investigation; Sang-Yong Yoo: investigation; Woong Chol Kang: investigation; Nam Ho Lee: investigation; Ki Hwan Kwon: investigation; Joon Hyung Doh: investigation; Sang-Wook Lim: investigation; Yangsoo Jang: investigation; Dong Woon Jeon: investigation; Jung Ho Heo: investigation; Woong Gil Choi: investigation; Sungsoo Cho: investigation; Bong-Ki Lee: investigation; Hyeonju Jeong: investigation; Bum-Kee Hong: investigation; Hyun-Hee Choi: investigation
Funding
This study was funded by Genoss Company Limited, Suwon, South Korea.