Treatment pattern of chronic lymphocytic leukemia/small lymphocytic lymphoma in Korea: a multicenter retrospective study (KCSG LY20-06)
Article information
Abstract
Background/Aims
Little attention is paid to chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in Korea due to the rarity of the disease. With its rising incidence, we aimed to evaluate recent changes in treatment patterns and survival outcomes of patients with CLL/SLL.
Methods
A total of 141 patients diagnosed with CLL/SLL between January 2010 and March 2020 who received systemic therapy were analyzed in this multicenter retrospective study.
Results
The median patient age was 66 years at diagnosis, and 68.1% were male. The median interval from diagnosis to initial treatment was 0.9 months (range: 0–77.6 months), and the most common treatment indication was progressive marrow failure (50.4%). Regarding first-line therapy, 46.8% received fludarabine, cyclophosphamide, plus rituximab (FCR), followed by chlorambucil (19.9%), and obinutuzumab plus chlorambucil (GC) (12.1%). The median progression-free survival (PFS) was 49.3 months (95% confidence interval [CI], 32.7–61.4), and median overall survival was not reached (95% CI, 98.4 mo–not reached). Multivariable analysis revealed younger age (≤ 65 yr) (hazard ratio [HR], 0.46; p < 0.001) and first-line therapy with FCR (HR, 0.64; p = 0.019) were independently associated with improved PFS. TP53 aberrations were observed in 7.0% (4/57) of evaluable patients. Following reimbursement, GC became the most common therapy among patients over 65 years and second in the overall population after 2017.
Conclusions
Age and reimbursement mainly influenced treatment strategies. Greater effort to apply risk stratifications into practice and clinical trials for novel agents could help improve treatment outcomes in Korean patients.
INTRODUCTION
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is the most common leukemia in Western countries. Accordingly, there have been numerous achievements in this field, such as novel targeted agents [1], prognostication tools [2,3], ideal sequence and duration of treatment [4], and management of minimal residual disease [5]. Consequently, survival has improved substantially with increasing disease incidence. However, CLL/SLL remains an incurable disease without allogeneic stem cell transplantation; therefore, building a research-based treatment plan is crucial [6].
A recent study revealed that the overall incidence of CLL/SLL is increasing, most rapidly in East Asia [7]. Additionally, CLL/SLL has a heterogeneous clinical course influenced by cytogenetic aberrations [8,9] and partly by ethnicity [10,11]. CLL/SLL incidence is at least a 20-fold lower in Asian countries than in Western regions, therefore, real-world data is limited, especially in Korea [12,13]. Thus, further research is needed to determine the disease features in an Asian population.
In this study, we evaluated real-world data regarding recent changes in therapeutic patterns and survival outcomes of CLL/SLL in Korea to expand our insight into the disease and establish an effective management plan.
METHODS
Study design
This is a multicenter retrospective observational study of patients with CLL/SLL. We reviewed electronic medical records of patients meeting the criteria from ten institutions in Korea. Eligible criteria were: patients 1) diagnosed with CLL/SLL based on International Workshop on CLL (iwCLL) criteria [14] between January 2010 and March 2020; 2) who received systemic therapy for CLL/SLL; and 3) who had reliable medical records.
Data collection and definitions
We collected data on baseline demographics, laboratory findings, CLL/SLL diagnosis date, Rai and Binet stage, cytogenetic and mutational status, treatment indications, treatment regimens, drug administration dates, and progression and death dates. Treatment regimens were classified as follows: chlorambucil, fludarabine, bendamustine, a combination of fludarabine, cyclophosphamide, and rituximab (FCR), a combination of obinutuzumab and chlorambucil (GC), ibrutinib, and others.
The objective response rate (ORR) was defined as the combined proportion of patients with the best response of complete response (CR) and partial response (PR). We calculated progression-free survival (PFS) from the treatment initiation date to disease progression or death, whichever occurred first. Data from patients who were free of disease progression or lost to follow-up were censored at the date of the last follow-up visit. Overall survival (OS) was calculated from the date of treatment initiation to death from any cause.
Statistical analyses
Patient demographics were summarized with frequencies, percentages, medians, and value ranges. Additionally, we analyzed treatment indications and responses based on iw-CLL criteria [14] and survival outcomes.
We used Kaplan–Meier methods to calculate PFS and OS and the log-rank test for comparison. Hazard ratios (HRs) were calculated using the multivariable Cox proportional hazard model with stepwise backward selection. Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. We considered a two-sided p value less than 0.05 to be statistically significant. Statistical analyses and graphics were generated using STATA version 16.1 (StataCorp LP, College Station, TX, USA) and R version 4.2.1 (Vienna, Austria).
Ethics statement
The Institutional Review Boards (IRBs) of the Hanyang University Guri Hospital (IRB No. GURI 2020-06-022-001) and each participating center (IRB No. H-2107-132-1236, Seoul National University Hospital) reviewed and approved the protocol. This study was conducted in accordance with the Principles of the Declaration of Helsinki. The requirement for informed consent was waived by the IRBs owing to the retrospective analysis. Data were anonymized and de-identified before analysis.
RESULTS
Patient characteristics
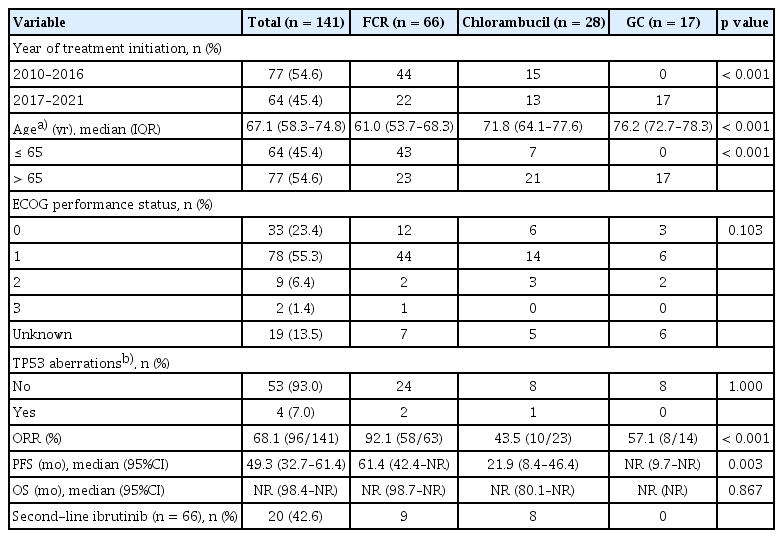
A total of 141 patients treated for CLL/SLL at participating institutions as of November 31, 2021 (data cutoff) were included in the analysis. The median age was 66 years at diagnosis (range: 38–95 yr) and 67 years first-line therapy initiation (range: 40–95 yr). The median interval from diagnosis to initial treatment was 0.9 months (range: 0–77.6 mo). Table 1 shows the baseline characteristics at initial diagnosis. Ninety-six (68.1%) patients were male, and 111 (78.7%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Seventy patients (49.6%) were at Rai stage 3 or 4, and 63 patients (44.7%) were at Binet stage C. Of 57 evaluable patients, 7.0% (4/57) had TP53 aberrations (i.e., deletion of the TP53 locus on chromosome 17p13.1 [del(17p)] or TP53 gene mutations).
Indications for systemic therapy
With multiple choices allowed, the most common indication for initial treatment was progressive marrow failure (50.4%), followed by progressive lymphocytosis (31.2%), splenomegaly (17.7%), symptomatic lymphadenopathy (17.0%), disease-related symptoms (i.e., weight loss, fatigue, fever, and night sweats) (13.5%), symptomatic extra-nodal involvement (4.3%), and autoimmune complications (0.7%) (Fig. 1).

Treatment indications for first-line therapy based on the International Workshop on Chronic Lymphocytic Leukemia. FCR, fludarabine, cyclophosphamide, and rituximab.
Common indications for second-line therapy were progressive marrow failure (42.6%, 20/47), followed by progressive lymphocytosis (29.8%) and symptomatic lymphadenopathy (27.7%). In the third line, symptomatic lymphadenopathy (50.0%, 8/16) was the most common (data not shown).
Distribution of treatment regimens
For first-line therapy, 46.8% (66/141) of patients received FCR, followed by chlorambucil (19.9%) and GC (12.1%). Since 2017 when GC gained reimbursement, GC has become the second most common regimen (26.6%) after FCR (34.4%) (Table 2) and was the most common (42.5%) among patients over 65 years (data not shown). Chlorambucil was a consistent choice for first-line therapy during the study period (Fig. 2A). Though small in proportion, five patients received bendamustine plus anti-CD20 monoclonal antibody (i.e., rituximab or obinutuzumab), and two patients received acalabrutinib as a frontline treatment through clinical trials.
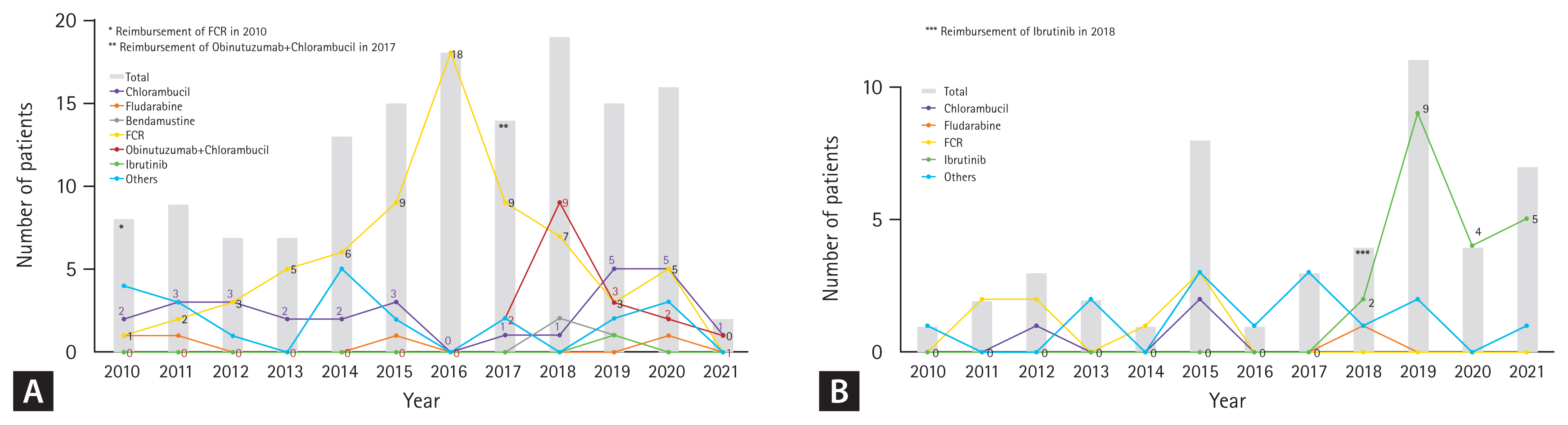
Treatment patterns by year in the (A) first- and (B) second-line settings (from 2010 to 2021). FCR, fludarabine, cyclophosphamide, and rituximab.
In the second-line setting, ibrutinib was the most common regimen, comprising 76.9% (20/26) of treatments after reimbursement in 2018 (Fig. 2B). Among patients treated with FCR or chlorambucil as first-line therapy, 13.6% (9/66) and 28.6% (8/28) received ibrutinib in the second-line setting, respectively (Table 2).
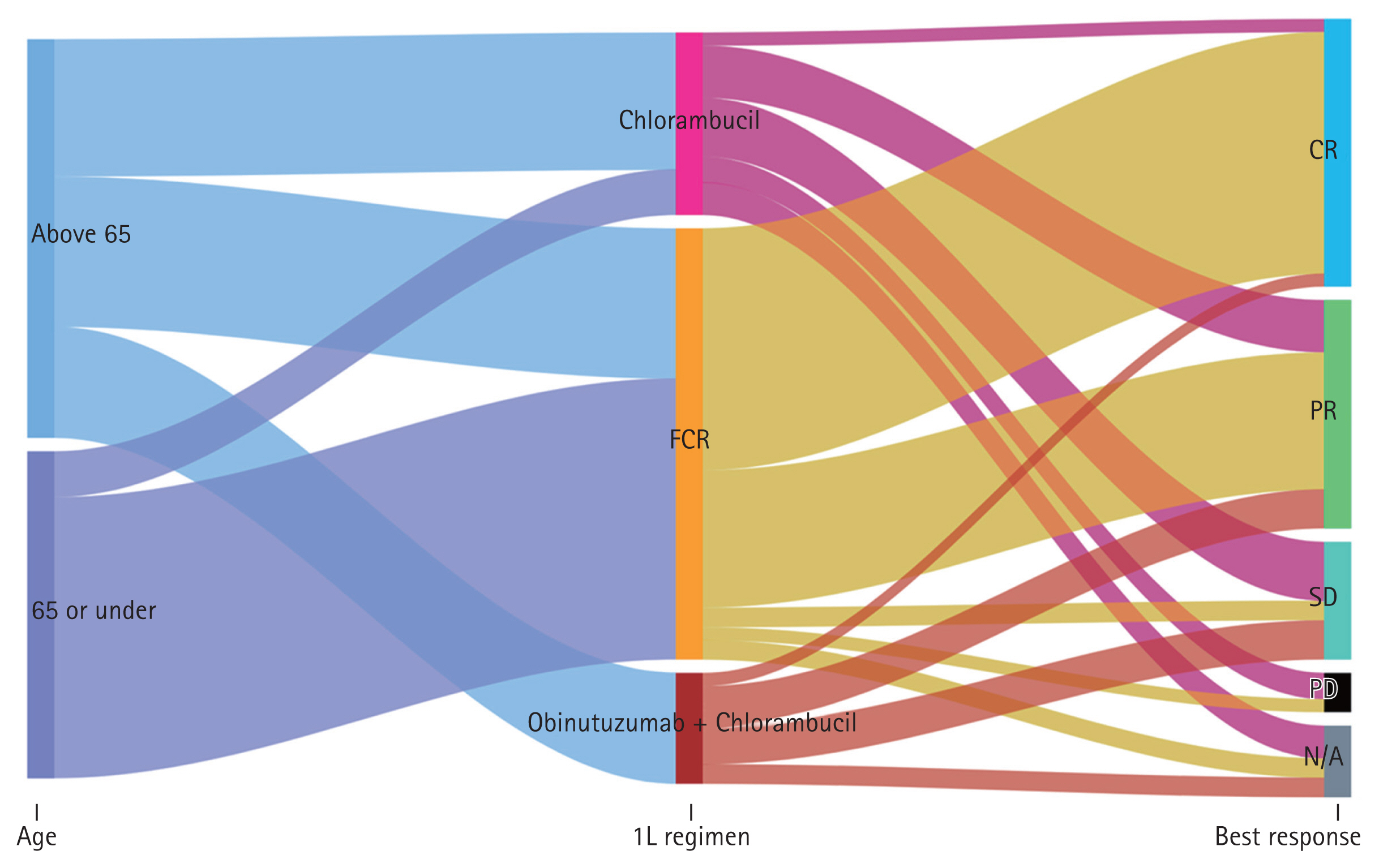
Regarding age groups and first-line therapy, patients aged over 65 tended to receive GC or single agents rather than FCR, while those under 65 received FCR (Fig. 3).
Treatment outcomes
A total of 124 patients were evaluable for first-line therapy. Among these patients, FCR ORR (92.1%, 58/63) was superior to GC (57.1%, 8/14) and chlorambucil (43.5%, 10/23) (p < 0.001) (Table 2).
After a median follow-up of 62.5 months (95% confidence interval [CI], 53.4–70.4), median PFS was 49.3 months (95% CI, 32.7–61.4), and median OS was not reached (NR) (95% CI, 98.4–NR) (Fig. 4A, B). Patients treated with FCR showed significantly increased median PFS compared with non-FCR (31.7 vs. 61.4 months, p = 0.001), but there was no significant difference in median OS (NR vs. NR, p = 0.686) (Fig. 4C, D). Of those who started first-line FCR treatment, younger patients (≤ 65 years) had significantly improved PFS (NR vs. 39.2 mo, p = 0.0011) and OS (NR vs. NR, p = 0.0083) (Supplementary Fig. 1).
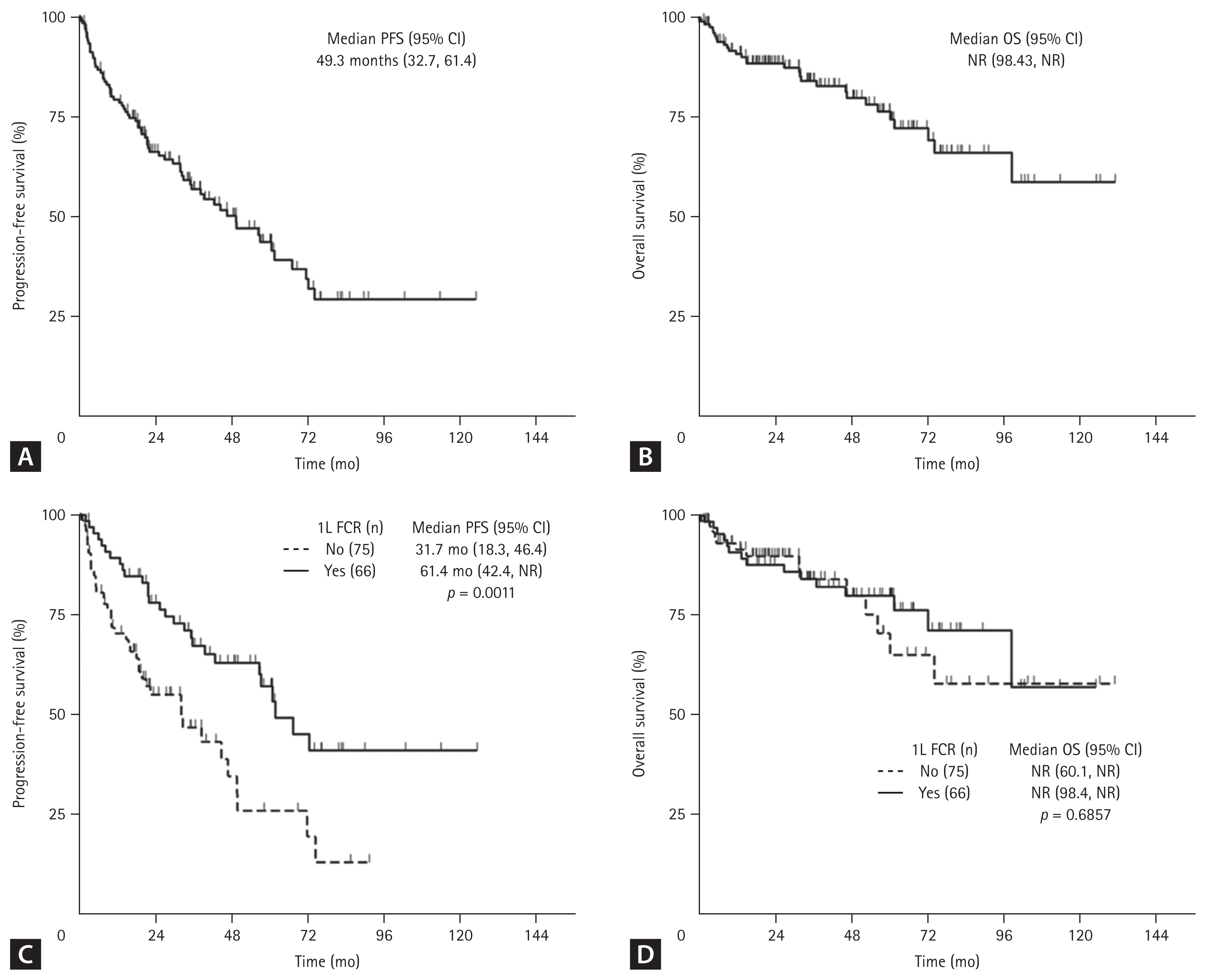
Survival outcomes from first-line (1L) therapy. (A) Progression-free survival (PFS), (B) overall survival (OS) in the overall population, (C) PFS, and (D) OS by regimen (FCR vs. non-FCR). CI, confidence interval; FCR, fludarabine, cyclophosphamide, and rituximab; n, number; NR, not reached.
By regimen, median PFS for FCR, chlorambucil, and GC was 61.4 months (95% CI, 42.4–NR), 21.9 months (95% CI, 8.4–46.4), and NR (95% CI, 9.7–NR), respectively (p = 0.003). However, median OS was NR and not significantly different (p = 0.867) (Table 2). In those patients over 65 years, treatment with GC resulted in better tendency of median PFS (NR vs. 39.2 mo, p = 0.395) and median OS (NR vs. NR, p = 0.333) than FCR (Supplementary Fig. 2).
When viewed from the time patients started the first-line therapy (2010–2016 vs. 2017–2021), treatment periods showed no statistically significant difference in median PFS (46.4 vs. 61.4 mo, p = 0.735) and OS (108.9 vs. NR, p = 0.102) (Fig. 5). Similarly, median PFS (31.7 vs. 44.3 mo, p = 0.626) and OS (74.1 mo vs. NR, p = 0.298) in patients over 65 years differed numerically by treatment period (Supplementary Fig. 3).

Survival outcomes from the first-line therapy by initiation period (2010–2016 vs. 2017–2021). (A) Progression-free survival (PFS) and (B) overall survival (OS). CI, confidence interval; n, number; NR, not reached.
Of 57 patients who underwent a test for TP53 status, 4 (7.0%) had aberrations. Patients with TP53 aberrations had significantly worse PFS (4.0 vs. 60.5 mo, p = 0.0022) and OS (46.1 mo vs. NR, p < 0.0001) than wild type (Supplementary Fig. 4). However, there was no significant difference in PFS and OS according to first-line regimens in patients with TP53 aberrations (Supplementary Fig. 5). Additionally, the patient treated with chlorambucil was under second-line ibrutinib for more than 15 months at the data cutoff.
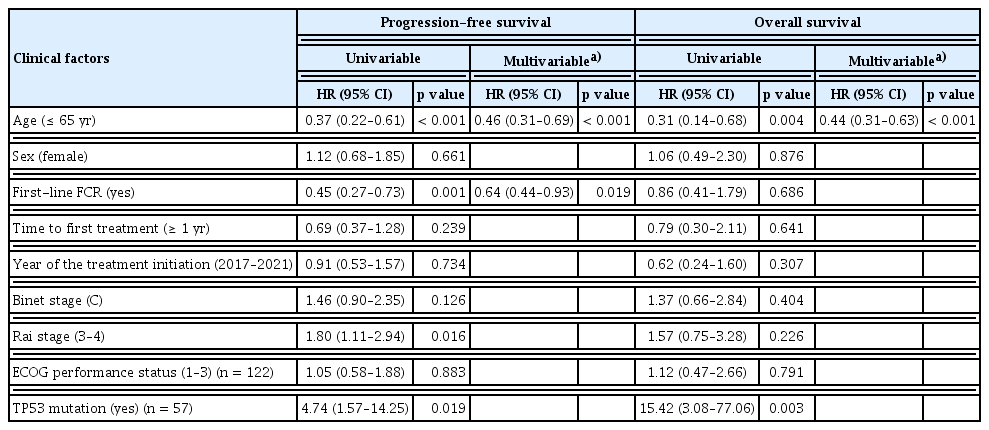
Multivariable analysis revealed that younger age (≤ 65 yr) (HR, 0.46; 95% CI, 0.31–0.69; p < 0.001) and adopting FCR as the first-line regimen (HR, 0.64; 95% CI, 0.44–0.93; p = 0.019) were independently associated with improved PFS. Moreover, younger age was also associated with improved OS (HR, 0.44; 95% CI, 0.31–0.63; p < 0.001) (Table 3). Three patients experienced Richter’s transformation (2.1%, 3/141).
Safety
Among 66 patients who received FCR as initial systemic treatment, 48.5% (32/66) and 7.6% (5/66) had grade 3/4 neutropenia and thrombocytopenia, respectively, according to CTCAE version 5.0. Moreover, 12.1% (8/66) and 4.6% (3/66) experienced pneumonia and other types of infections. There were no statistically significant differences between age groups (≤ 65 vs. > 65 yr) (Supplementary Table 1).
DISCUSSION
Our study found that treatment patterns were affected mainly by age and reimbursement system. While younger patients mainly received aggressive treatment, elderly patients tended to receive agents with modest efficacy and less toxicity. Younger age and first-line FCR were independently associated with improved PFS.
CLL and SLL only manifest differently by primarily affecting peripheral blood or lymphoid tissues, respectively, and are managed similarly [14,15]. The median age at initial systemic treatment in our study was younger than in Western regions, but the male-to-female ratio similarly approximated 2:1 [16]. The relatively short interval between diagnosis and initial systemic treatment compared with a prospective European study [17] may stem from our study design. The fact that patients who received systemic therapy were included regardless of prognostic factors and CLL in Asians tends to show more aggressive features than in white patients may have led to the shorter interval [11,18]. Conversely, this supports another study performed in another East Asian country [19]. Consistent with previous studies, nearly half of patients who received treatment were diagnosed at later stages (i.e., Rai stage III/IV or Binet stage C) [13,20]. However, Richter’s transformation occurred in a lower proportion (2.1%) than expected [21], and the occurrence of secondary malignancies following chemoimmunotherapy (CIT) [22] was not detected.
As we gained more insight into the CLL/SLL biology, genetic aberrations were found to play an important role. Particularly, aberrations in tumor suppressor gene TP53 and unmutated immunoglobulin heavy-chain variable region (IGHV) are associated with inferior response to CIT [23,24]. Accordingly, those aberrations were recently incorporated into novel prognostic models for risk stratification [2,3], besides conventional Rai and Binet staging systems. Intriguingly, numerous attractive targeted agents, such as Bruton tyrosine kinase inhibitors (ibrutinib, acalabrutinib), phosphoinositide 3-kinase inhibitors (idelalisib, duvelisib), or B-cell lymphoma 2 inhibitor (venetoclax) improved survival in high-risk patients [6]. Thus, these novel agents are recommended in the presence of del(17p) or TP53 mutation [25], revolutionizing the CLL/SLL treatment landscape.
Despite its importance in treatment decisions and prognostication [25,26], only 40.4% of our study population underwent analyses for TP53 status. The primary reason we inferred was that test results did not affect the treatment plan within the current reimbursement system. The abovementioned targeted agents are covered by insurance only in relapsed/refractory settings in Korea, and CIT is still the backbone of first-line therapy, irrespective of TP53 status. Although a patient’s age, per se, may not be a decisive factor in the era of targeted agents [27], the choice of regimen seems to be influenced by age rather than risk stratification in this context. Meanwhile, survival outcomes are still comparable to those of Western regions [28,29], even when a fifth of patients were treated with conventional chlorambucil. It is possible that survival outcomes would have been improved by second- or later-line ibrutinib or access to clinical trials. Additionally, ethnic differences may have partly been involved in response to chlorambucil or CITs [30].
As CLL/SLL incidence is expected to grow, we need procedures that consider realistic problems like the government’s financial capacity. Furthermore, we should pay close attention to clinical trials to secure access to novel, effective agents, which will help overcome the time lag in adopting them. Additionally, to expand knowledge of ethnic differences and fill in knowledge gaps regarding CLL/SLL biology, efforts to accumulate cytogenetic information about Asian patients are needed. Moreover, establishing an optimal treatment strategy for CLL/SLL with redefined high-risk populations would enable more effective patient care, proceeding toward precision medicine.
Our study has some limitations. First, owing to the nature of the retrospective observational study, we could not report detailed data on treatment indications, side effects, and causes of follow-up loss. Second, as decisions were made in a multicenter setting, the threshold for treatment initiation would have been different, resulting in a heterogeneous population. Third, we could not prognosticate uniformly because cytogenetic evaluation methods varied with missing data. Fourth, our study focused on patients who received treatment for CLL/SLL, so we should carefully interpret these results because they do not cover patients under a watch-and-wait strategy. Lastly, the follow-up duration was relatively short, considering the natural course of the disease. Thus, clinical outcomes, such as the incidence of Richter’s transformation and secondary malignancies, could not be analyzed thoroughly.
In conclusion, age and reimbursement largely influenced treatment strategies in patients with CLL/SLL. Hopefully, more interest and adherence to prognostic or predictive indices and clinical trials will improve survival for Korean patients.
KEY MESSAGE
1. Age and reimbursement mainly influenced treatment strategies in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in Korea.
2. Greater effort to apply risk stratifications and more clinical trials for novel agents could help improve treatment outcomes in Korean patients with CLL/SLL.
Notes
CRedit authorship contributions
Jung Sun Kim: data curation, formal analysis, methodology, visualization, writing - original draft, writing - review & editing; Tae Min Kim: conceptualization, formal analysis, visualization, writing - review & editing; Myoung Joo Kang: writing - review & editing; Sung Ae Koh: writing - review & editing; Hyunkyung Park: writing - review & editing; Seung-Hyun Nam: writing - review & editing; Jae Joon Han: writing - review & editing; Gyeong-Won Lee: writing - review & editing; Young Jin Yuh: writing - review & editing; Hee Jeong Lee: writing - review & editing; Jung Hye Choi: conceptualization, data curation, formal analysis, methodology, project administration, visualization, writing - original draft, writing - review & editing
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Funding
This study was supported by the National R&D Program for Cancer Control through the National Cancer Center (NCC) funded by the Ministry of Health & Welfare, Republic of Korea (HA22C0012).