Association between eupatilin and reduction in small bowel bleeding in aspirin users and aspirin plus acid suppressant users
Article information
Abstract
Background/Aims
Capsule endoscopy (CE) has shown that low-dose aspirin occasionally causes small bowel (SB) bleeding. We herein evaluated the protective effects of mucoprotective agents (MPAs) on SB bleeding in aspirin users using the nationwide database of claims data from the National Health Insurance Service (NHIS).
Methods
As CE is an insured procedure, we constructed an aspirin-SB cohort using NHIS claims data, with a maximum follow-up period of 24 months. Patients with anemia, melena, or hematochezia that occurred within 4 weeks before and after performing CE were suspected to have SB bleeding. A Cox proportional hazards regression model was used to determine the risk factors for SB bleeding. Subgroup analyses were conducted among patients who used acid suppressants, such as proton pump inhibitors (PPIs) and histamine-2 receptor antagonists.
Results
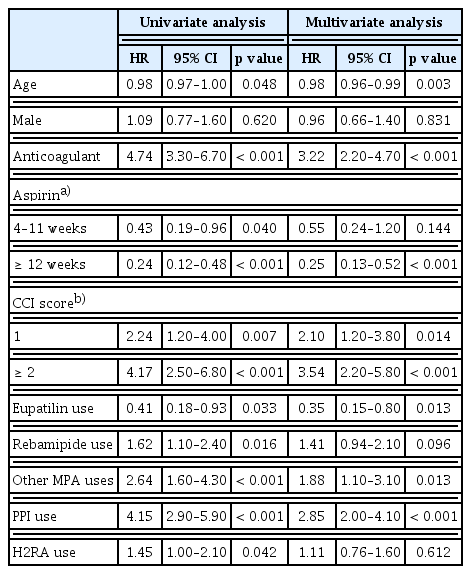
A total of 15,542 aspirin users were included. Anticoagulant use (hazard ratio [HR], 3.22), high Charlson comorbidity index score (≥ 2) (HR, 3.54), and PPI use (HR, 2.85) were significantly associated with SB bleeding, whereas eupatilin use (HR, 0.35) was a preventive factor. SB bleeding occurred more frequently in concurrent users of acid suppressants than in nonusers (1.3% vs. 0.5%). Subgroup analysis revealed that eupatilin significantly reduced the risk of SB bleeding in aspirin users with concurrent use of acid suppressants (HR, 0.23 vs. 2.55).
Conclusions
Eupatilin was associated with a reduced risk of SB bleeding in both aspirin users and those with concomitant use of acid suppressants. Eupatilin use should be considered for aspirin users, especially for those concomitantly taking acid suppressants.
INTRODUCTION
Aspirin, one of the most prescribed oral medications for therapeutic or prophylactic purposes, is often prescribed for long-term use in clinical settings. Large surveys (1980–2009) have shown that low-dose aspirin use for the primary prevention of cardiovascular disease increased from 1% to 12–21% and was highest in patients aged > 65 years [1]. However, the conflict between the benefits and adverse effects of aspirin usage persists. Aspirin may damage the gastrointestinal (GI) tract by disrupting the mucosal barrier function and consequently leading to GI bleeding [2].
GI complications are the most common adverse events in older adults consuming aspirin [3]. Initially, aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) were reported to mainly cause peptic ulcer disease (PUD). However, with the expansion of the clinical applications of capsule endoscopy (CE), aspirin and other NSAIDs have been found to often erode and ulcerate the intestinal mucosa [4], causing small bowel (SB) bleeding [5,6]. According to the Korean CE registry report, the primary reason for CE examinations was obscure GI bleeding (OGIB) (62.7% cases) [7] and drug-induced SB enteropathy, the incidences of which increase with age [8]. A recent hospital-based study also showed that drug-induced enteropathy was the most prevalent etiology of SB bleeding identified in CE [9].
Aspirin irreversibly inhibits cyclooxygenase-1, thereby inhibiting the synthesis of prostaglandins that protect the GI tract. Proton pump inhibitors (PPIs) are widely used for reducing aspirin-induced gastroduodenal complications through potent acid suppression. However, the effects of gastric acid on SB injuries remain unclear. Moreover, a preclinical study reported that PPIs exacerbate NSAID-induced SB injuries by inducing dysbiosis [10]. Mucoprotective agents (MPAs) protect the GI mucosa by promoting mucus secretion, increasing intrinsic prostaglandin synthesis and blood flow, regenerating tissue, and coating the mucosa. Smallscale preclinical studies have demonstrated the protective effects of MPAs on the SB mucosa of aspirin or NSAID users [11–13]. However, relevant evidence for MPAs (except misoprostol) is limited, and no large-scale studies have been conducted. Thus, we aimed to investigate the association between the use of MPAs and reduction in SB bleeding in aspirin users, including concomitant acid suppressant users, using the nationwide database of national claims data from the Korean National Health Insurance Service (NHIS).
METHODS
Participants
This study used the cohort data from the Korean nationwide cohort database NHIS. Since 2002, the NHIS database has been an invaluable resource for patient data, including their residence, income levels (based on salary and property), medical diagnoses, disability or death-related data, and insurance claims. To investigate aspirin-induced SB bleeding, we constructed a cohort of 189,807 aspirin/NSAID-SB users between 2012 and 2019 using the NHIS database. The cohort consisted of 6,035 patients who had undergone CE during the study period and 183,772 age- and sex-matched controls with no history of CEs in a ratio of 1:30 (Fig. 1). We initially wanted to include the data of all patients from the NHIS database, but we could not retrieve all data due to the limited database server capacity. Therefore, we used the sampling data. It was estimated that the number of patients with SB bleeding in the sampling data was small compared with the total number in the database. To secure enough study subjects, we used a 1:30 ratio of controls. Since September 2014, CEs are covered under national insurance for OGIB. Therefore, CE examinations for OGIB have been almost exclusively performed since then. Therefore, patients with a history of CEs before this date were excluded. Patients with inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis, were also excluded to rule out other possible causes of SB bleeding. Patients who died between 2012 and 2019 or who were diagnosed with invasive cancer were excluded.
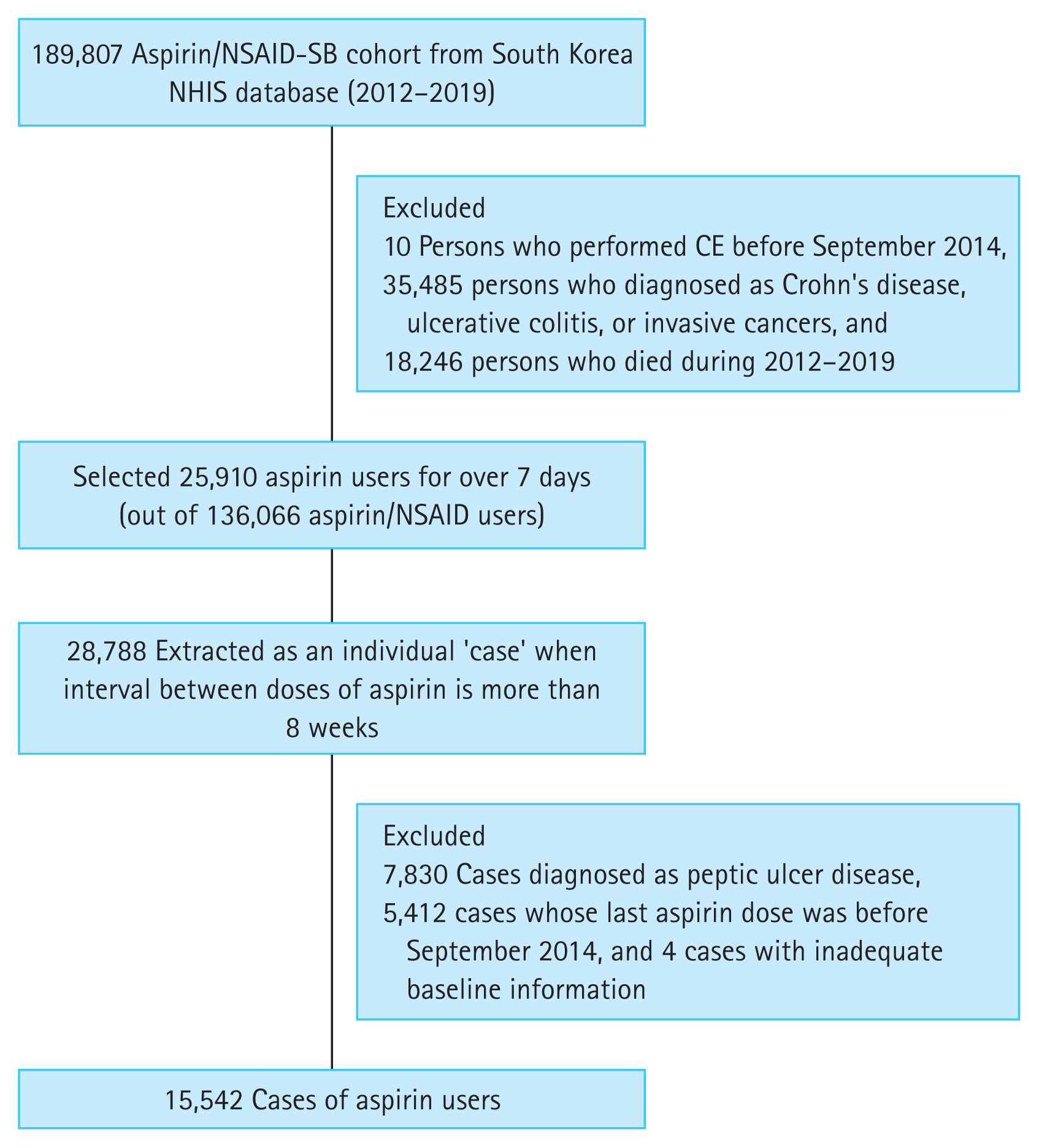
Flow diagram summarizing the general population-based aspirin/NSAID-SB cohort construction to investigate aspirin-induced SB bleeding. NSAID, nonsteroidal anti-inflammatory drug; SB, small bowel; NHIS, National Health Insurance Service; CE, capsule endoscopy.
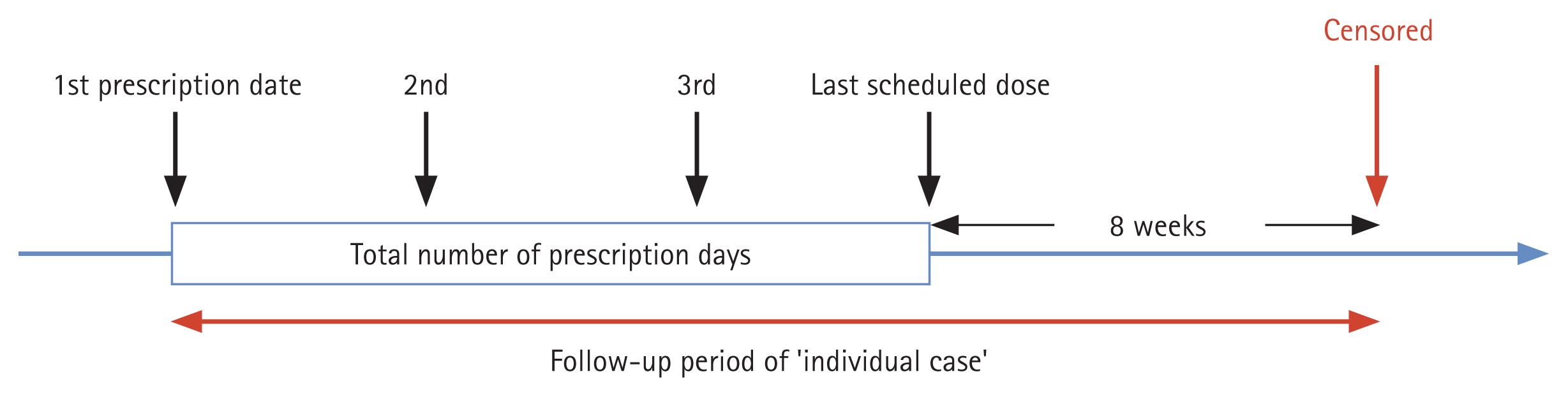
To ensure an exclusive focus on aspirin usage, 25,910 patients prescribed aspirin for more than 7 days were selected from 136,066 aspirin/NSAID users. People stopping aspirin for over 8 weeks and starting again were considered an additional case, resulting in 28,788 cases in total. The follow-up period was a maximum of 24 months, from the first dose of aspirin to 8 weeks after the last dose (Fig. 2). Patients diagnosed with PUD during the follow-up period, who had their last aspirin dose before September 2014, and with inadequate baseline demographics were excluded from the analysis. The event was measured based on suspected SB bleeding and censored at the end of the follow-up period. This study was approved by the Institutional Review Board of the Dongguk University Ilsan Hospital (DUIH 2020-05-010-002), which waived the requirement for informed consent.
Cohort definitions
The diseases were identified using the International Classification of Diseases 10th Revision (ICD-10) codes for PUD (K25–26), IBD (K50–51), and cancers (all C codes) in the NHIS data. Suspected SB bleeding was defined as a case in which CE (Medical Care Benefit code: EZ937) was performed during the follow-up period, and subsequent bleeding-related morbidity, which was anemia (ICD-10 D50–53, D63, D649), melena, or hematochezia (K92.1), occurred within 4 weeks before and after the CE.
The use of aspirin corresponded to the Anatomical Therapeutic Chemical (ATC) code B01AC06 (acetylsalicylic acid). The concomitant use of anticoagulants (warfarin, clopidogrel, cilostazol, prasugrel, ticagrelor, dipyridamole, nonvitamin K antagonist oral anticoagulants, and combination drugs containing these ingredients) was also identified in the ATC. Concurrent prescription of PPIs, histamine-2 receptor antagonists (H2RAs), and MPAs (eupatilin, rebamipide, teprenone, irsogladine, ecabet sodium, polaprezinc, troxipide, sodium alginate, sucralfate, bismuth, sulglycotide, misoprostol, and their combinations) was investigated. Other covariates included age, sex, and Charlson comorbidity index (CCI). The CCI was calculated by scoring the comorbid conditions that could affect the patients’ health outcomes and categorizing them into three groups: 0, 1, 2 or more (high risk).
Statistical analyses
Categorical variables are reported as frequencies and percentages, whereas continuous variables are presented as mean ± standard deviation or median. The endpoint was SB bleeding. Chi-square test was performed to associate the occurrences of SB bleeding with the intake of specific GI drugs. A Cox proportional hazards regression model was used to estimate the hazard ratios (HRs), and 95% confidence intervals were used to determine the SB bleeding risk factors associated with the concomitant use of GI drugs and other covariates. The models were adjusted for sex; age; CCI score; aspirin use duration; and concurrent use of anticoagulants, acid suppressants (PPIs and H2RAs), and MPAs as covariates. Subgroup analyses were performed among users and nonusers of acid suppressants for SB bleeding. In addition, an initial dataset with no follow-up period limits was used for subgroup sensitivity analysis. The cumulative incidence rates of SB bleeding were calculated using the Kaplan-Meier method for different classifications of GI drugs. The statistical significance was set at p < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.0.3 (R studio, PBC).
RESULTS
Baseline characteristics of aspirin users
Overall, 15,542 aspirin users were included in this study (Table 1). The median (Q1, Q3) follow-up duration was 387 days (116, 730). SB bleeding was confirmed in 126 patients (0.8%), with a median follow-up period of 148.5 days (40.8, 328.3). Concomitant use of anticoagulants was noted in 21.9% (3,403/15,542) of patients, with a high rate of SB bleeding observed in this group (2.1%). A higher CCI score was associated with a proportionately higher SB bleeding rate (0.4%, 0.7%, and 1.2% for scores of 0, 1, and ≥ 2, respectively). The concurrent prescription rates were 26.3%, 16.7%, and 27.6% for MPAs, PPIs, and H2RAs, respectively. The SB bleeding rates for MPAs, PPIs, and H2RAs were 1.2%, 2.4%, and 1.2%, respectively. Only 38 misoprostol users were included among the remaining MPAs (38/861).
Factors associated with suspected SB bleeding
Univariate and multivariate analyses were performed on the factors associated with suspected SB bleeding. Univariate analysis showed that anticoagulant use; high CCI scores; and the concurrent use of rebamipide, other MPAs, PPIs, and H2RAs were statistically significantly associated with, SB bleeding whereas the concurrent use of eupatilin was inversely associated. Multivariate analysis demonstrated that the association of SB bleeding with anticoagulant use (HR, 3.22; p < 0.001), high CCI scores (HR, 2.10 and 3.54, respectively, for CCI scores 1 and ≥ 2), the concurrent use of MPAs (except eupatilin and rebamipide) (HR, 1.88; p = 0.013), and PPIs (HR, 2.85; p < 0.001) was statistically significant. In contrast, the concurrent use of eupatilin (HR, 0.35; p = 0.013) was a preventive factor (Table 2).
Comparison of SB bleeding among users and nonusers of acid suppressants based on the concurrent use of MPAs in aspirin users
Overall, 5,801 patients who used acid suppressants, such as PPIs or H2RAs, concurrently with aspirin were included. The rate of SB bleeding was 1.3% (77/5,801) in concurrent acid suppressant users, which was higher than that in nonusers (0.5%; 49/9,741; p < 0.001) (Table 3). The concurrent use of MPAs did not affect the rate of SB bleeding in patients not taking acid suppressants. The rate of SB bleeding was relatively low in patients taking a combination of eupatilin and acid suppressants (0.3%) compared with that in patients taking only acid suppressants (1.3%). In contrast, different combinations of MPAs with acid suppressants did not reduce the SB bleeding rate.
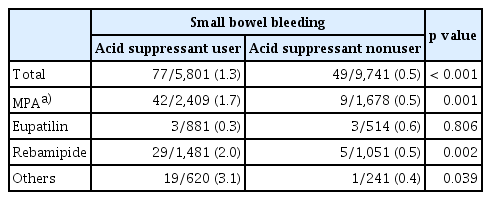
Comparison of small bowel bleeding rates between acid suppressant users and nonusers based on the mucoprotective agent taken by aspirin users
Figure 3 illustrates the Kaplan-Meier curve of cumulative incidence rates of SB bleeding according to the concurrent use of acid suppressants and MPAs in aspirin users. SB bleeding was observed more frequently in the aspirin users with concurrent use of acid suppressants (unaffected by the concurrent use of MPAs, except eupatilin) compared with those without the concurrent prescription of GI drugs (i.e., aspirin only users). When the acid suppressants and eupatilin were prescribed together in aspirin users, the incidence of SB bleeding reduced below the baseline (i.e., the reported SB bleeding in patients taking only aspirin), whereas other MPAs had no effect when used with acid suppressants.
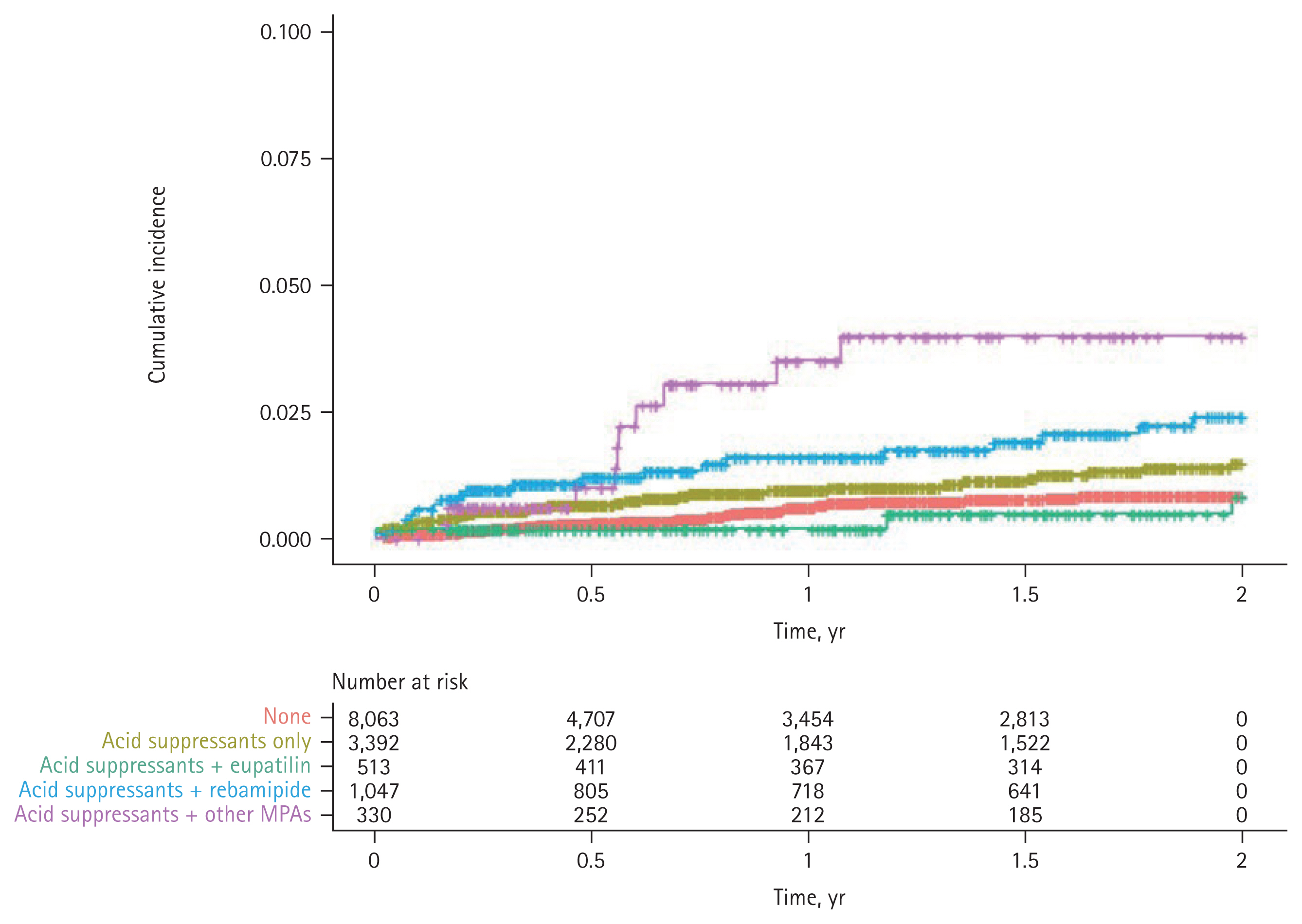
Kaplan-Meier curve illustrating the cumulative incidence of small bowel bleeding based on the concurrent use of acid suppressants (proton pump inhibitor or histamine-2 receptor antagonist) and mucoprotective agents in aspirin users. None, aspirin users without concurrent use of gastrointestinal drugs; Acid suppressants only, aspirin plus acid suppressants; Acid suppressants + eupatilin, aspirin plus acid suppressants and eupatilin; Acid suppressants + rebamipide, aspirin plus acid suppressants and rebamipide; Acid suppressants + other MPAs, aspirin plus acid suppressants and other mucoprotective agents.
Multivariate analyses of the factors influencing SB bleeding were conducted in subgroup analyses among users and nonusers of acid suppressants in aspirin users (Table 4). Concomitant anticoagulant use and high CCI scores were statistically significant in both the acid suppressant user and nonuser subgroups, with more SB bleeding in the acid suppressant user group than in the nonuser group. The concurrent use of rebamipide and other MPAs was statistically significant for SB bleeding, whereas concurrent eupatilin prescription was a preventive factor in the group taking acid suppressants (HR, 1.96 and 2.55 vs. 0.23, respectively). In contrast, there were no differences in the risk of SB bleeding with each MPA type in the acid suppressant nonuser group.
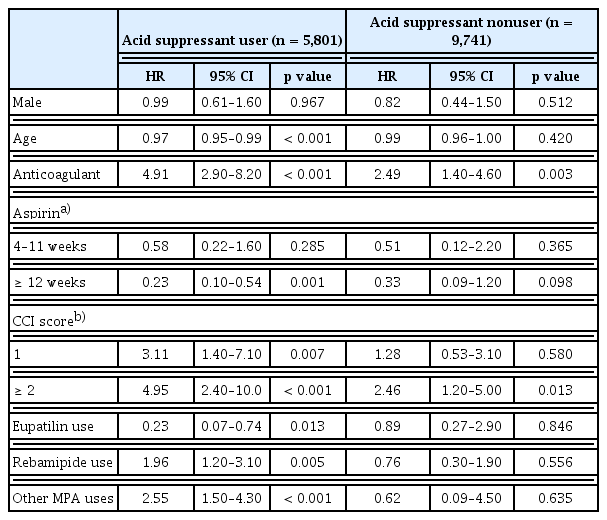
Multivariate analyses of factors associated with SB bleeding in the subgroup analyses of users and nonusers of acid suppressants among aspirin users
SB bleeding was confirmed during the entire study period without limiting the follow-up period to 24 months. In this sensitivity analysis, the risk of SB bleeding based on the concomitant use of GI drugs was comparable to that in the original 24-month follow-up data (Supplementary Table 1).
DISCUSSION
To the best of our knowledge, this is the first study to compare the effects of various MPAs on aspirin-induced SB bleeding using the national claims data. This study represents the general population, as it used data from the Korean NHIS database, which encompasses the entire nation. Other possible risk factors for SB bleeding, such as concomitant anticoagulant use and comorbidities, were statistically analyzed. The results revealed that concomitant anticoagulant use and comorbidities were associated with an increased risk of SB bleeding in aspirin users. Suspected SB bleeding was observed more frequently in PPI users, whereas eupatilin use was inversely associated with SB bleeding. Interestingly, the coadministration of an acid suppressant with eupatilin significantly reduced the incidence of SB bleeding compared with a regimen consisting of acid suppressants alone or in combination with other MPAs. In the subgroup analysis, eupatilin was the only MPA that significantly reduced the risk of SB bleeding in aspirin users concomitantly taking acid suppressants.
At present, CE is the main recommended first-line examination in patients with OGIB, which can be missed by other techniques, such as esophagogastroduodenoscopy (EGD) and colonoscopy [14]. Up to 75% of the patients with OGIB have reported SB bleeding [15]. In the present study, suspected SB bleeding was defined as CE examinations performed in patients with OGIB. In South Korea, CEs were only implemented for OGIB under health insurance for all citizens in September 2014. For CEs to be covered by insurance, there should be no evidence of a bleeding focus from conventional EGDs and colonoscopies. Therefore, if the CE findings could not be identified from the claims data, in this study, the operational definition was sufficient to identify SB bleeding. This was reinforced by the exclusion of 7,830 patients diagnosed with PUD during the follow-up period. Meanwhile, although CE examination for SB assessment has been on the rise with institutional support since 2014, the incidence of SB bleeding remains low compared to PUD. This suggests that conducting hospital-based studies on SB bleeding will remain challenging because of its low incidence. Thus, using a national database for the analysis of SB bleeding based on CE examinations is inevitable for largescale research.
Initially, we constructed an aspirin/NSAID-SB cohort to investigate the aspirin-induced SB bleeding. However, following up all aspirin users to identify a few cases of SB bleeding was inefficient. Consequently, we selected a 1:30 ratio for matching controls. The population-based incidence rates could not be calculated because the claims data are based on prescription codes. The estimation of the drug use duration would not be as clear as hospital-based data. Therefore, we focused on aspirin users as it would be easier to track the total dose and durations, long-term prescriptions of similar doses being the norm. In addition, those who underwent CE examinations before the insurance coverage was implemented or had their last dose of aspirin before September 2014 and those with IBD were excluded from the present study.
In a previous study based on the CE registry, PPI use was found to increase the risk of SB injuries in chronic users of low-dose aspirin [16]. Nevertheless, PPIs are the main drugs for treating or preventing upper GI bleeding in aspirin users. A recent large-scale study found that MPAs and acid suppressants reduced GI bleeding, most likely in the cases of PUD [17]. It has been suggested that PPIs do not offer protection against NSAID-induced SB injuries [18] and may even increase them by altering the gut microbiota [19]. MPAs may be more protective against SB injuries where gastric acid plays a minimal role and the intestinal defense factors are compromised, such as those occurring in older adults. In a recent randomized controlled trial, misoprostol, a prostaglandin analog, significantly healed SB ulcers and reduced bleeding in aspirin users [12]. In previous randomized studies, rebamipide was found to be more effective than placebo in preventing diclofenac-induced SB damage [13] and had a greater healing effect on aspirin- or NSAID-induced enteropathy than placebo [20]. A pilot study showed that polaprezinc is effective against SB mucosal injuries associated with long-term aspirin therapy [21]. Eupatilin was proven to not be inferior to misoprostol in a phase 3 trial for the prevention of NSAID-induced gastroduodenal injuries and had significantly fewer side effects [22]. However, no further studies on SB injuries have been conducted.
In the present study, PPI use was associated with increased SB bleeding in aspirin users. This study had few limitations. First, the intestinal defense system may be disturbed by bacterial overgrowth following prolonged gastric acid suppression by PPIs. Some PPIs were inevitably included in this study. As we used secondary data, the actual drug intake of individuals may have differed from our estimates. However, to minimize this possibility, we included only the GI drugs that were prescribed concurrently with aspirin within the same prescription. Second, because the CE findings could not be identified from the claims data, we used an operational definition as a proxy for SB bleeding. Therefore, we could not differentiate SB bleeding and occult GI bleeding accurately or classify the precise cause of SB bleeding. A proportion of CE examinations in the claims data may not have identified any bleeding origins in SB. Despite these limitations, we believe that the present study is valuable because we evaluated the protective effect of MPAs on SB bleeding in aspirin users based on a nationwide database of claims data.
In conclusion, among the various MPAs, only eupatilin showed preventive effects against SB bleeding in aspirin users. It also reduced the risk of SB bleeding in aspirin users also taking acid suppressants. While acid suppressants are widely used for the prevention of peptic ulcer bleeding in high-risk patients, such as those on anticoagulants or with comorbidities, the coadministration of eupatilin can reduce the risk of SB bleeding. Concurrent eupatilin prescriptions should be considered for aspirin users, particularly in concomitant users of acid suppressants.
KEY MESSAGE
1. We evaluated the protective effect of mucoprotective agents on small bowel bleeding in aspirin users using the nationwide database of claims data from the National Health Insurance Service.
2. Eupatilin showed preventive effects against small bowel bleeding in aspirin users. Moreover, eupatilin reduced the risk of small bowel bleeding in aspirin users concomitantly using acid suppressants.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing of initial manuscript. We would like to thank enago (www.enago.co.kr) for English language editing of final accepted manuscript.
Notes
Conflicts of interest
The authors disclose no conflicts.
CRedit authorship contributions
Hyun Seok Lee: conceptualization, methodology, project administration, visualization, writing - original draft, writing - review & editing; Ji Hyung Nam: conceptualization, methodology, project administration, visualization, writing - original draft, writing - review & editing; Dong Jun Oh: conceptualization, methodology, project administration; Hyun Jung Ahn: data curation, formal analysis, methodology, visualization; Yun Jeong Lim: conceptualization, data curation, funding acquisition, methodology, project administration, visualization, writing - review & editing
Funding
This study was supported by grants from DONG-A ST pharmaceutical research fund and Dongguk University research fund.