Role of echocardiography in acute pulmonary embolism
Article information
Abstract
Although pulmonary embolism (PE)-related mortality rate has decreased because of prompt diagnosis and effective therapy use, acute PE remains a potentially lethal disease. Due to its increasing prevalence, clinicians should pay attention to diagnosing and managing patients with acute PE. Echocardiography is the most commonly used method for diagnosing and managing acute PE; it also provides clues about hemodynamic instability in an emergency situation. It has been validated in the early risk stratification and impacts management strategies for treating acute PE. In hemodynamically unstable patients with acute PE, echocardiographic detection of right ventricular dysfunction is an indication for administering thrombolytics. In this review article, we discuss the role of echocardiography in the diagnosis and management of patients with acute PE.
INTRODUCTION
Pulmonary embolism (PE), a form of venous thromboembolism (VTE), is the third leading cause of cardiovascular death in Western countries, and its prevalence has increased over time [1–4]. Approximately 75 to 269 cases of VTE occur per 100,000 individuals yearly in Western Europe, North America, Australia, and southern Latin America [5]. In Korea, the annual incidence of VTE was 53.7 cases per 100,000 person-years in 2018, increasing from 32.8 in 2014, exhibiting a tendency to gradually increase over years [6]. Additionally, the annual incidence rate of PE also increased with age, with an approximately 26-fold higher rate in individuals aged ≥ 75 years than in those aged < 35 years. These data demonstrate the importance of PE in the aging population of Korea as well as Europe, further suggesting that PE will increasingly burden healthcare systems globally in the coming years [7]. PE contributes to approximately one-third of this VTE burden. Although prompt diagnosis and appropriate treatment have reduced all-cause and PE-related mortality, acute PE remains a potentially lethal disease, with a current mortality of approximately 5% to 7% [8,9].
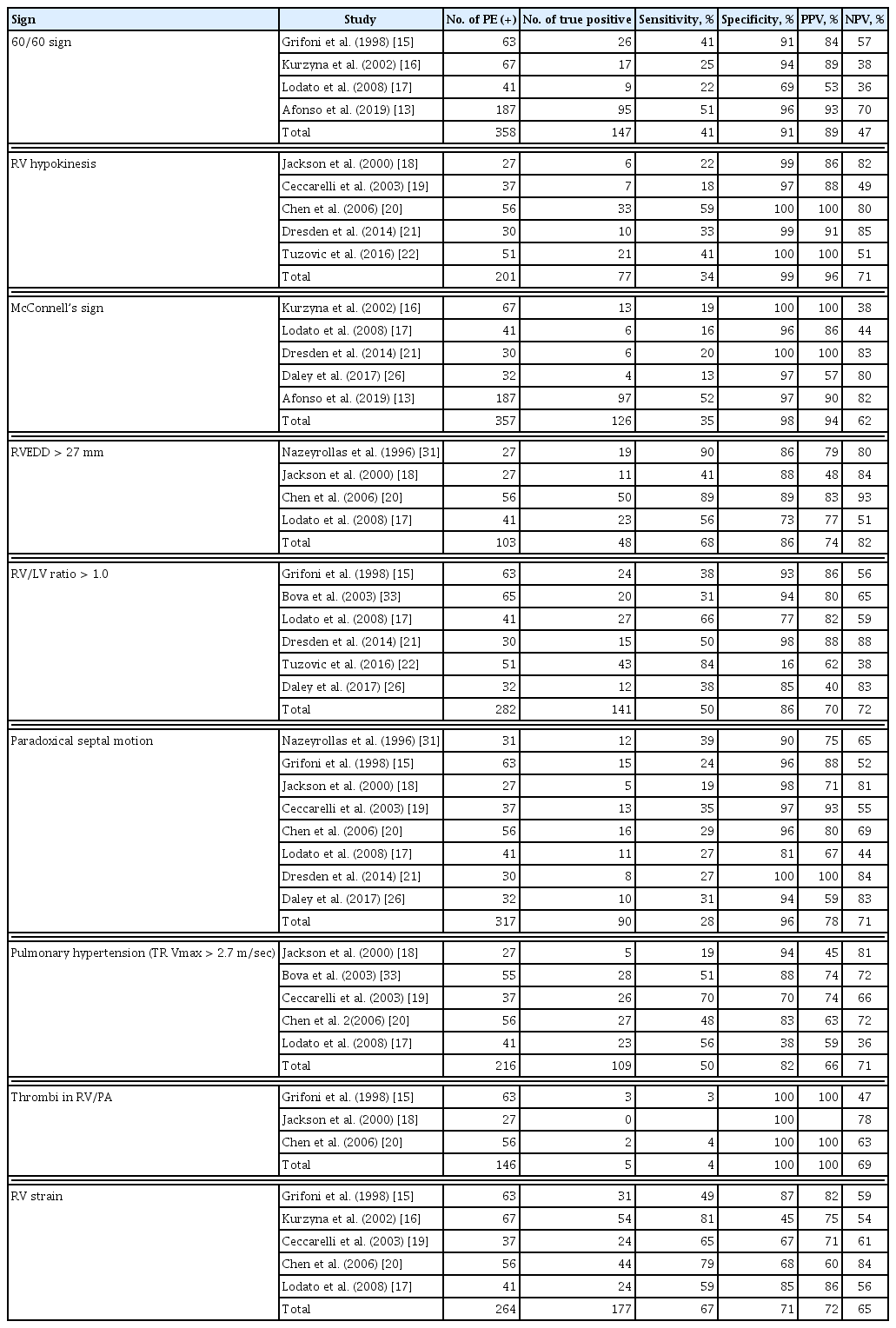
Herein, we illustrate a typical case of acute PE that a clinician may encounter in routine clinical practice. In this case, a 33-year-old man with a history of myotonic dystrophy presented with worsening dyspnea for 5 days. His initial blood pressure was 108/64 mmHg, heart rate was 120/min, respiratory rate was 25/min, and body temperature was 36.5°C. On physical examination, lung sounds were clear, and no cardiac murmur was found. The initial chest radiograph was normal (Fig. 1A). However, the electrocardiogram demonstrated sinus tachycardia and an S wave in lead I (S1), Q wave in lead III (Q3), T wave inversion in lead III (T3), and precordial leads V1–3 (Fig. 1B). We performed echocardiography to evaluate the cause of dyspnea. Echocardiography showed a D-shaped left ventricle (LV) (Fig. 1C), McConnell’s sign (Fig. 1D), linear thrombus in the main pulmonary artery (Fig. 1E), echocardiographic signs of the increased pulmonary arterial systolic pressure (Fig. 1F and 1G), and decreased right ventricular (RV) contractility assessed by tricuspid annular plane systolic excursion (TAPSE) (Fig. 1H). After the echocardiographic examination, we suspected the presence of acute PE and confirmed the diagnosis using contrast-enhanced computed tomography (CT) of the chest. After subcutaneous injection of low-molecular-weight heparin for 5 days, the patient was treated with oral warfarin therapy. Although echocardiographic examination was not used to confirm the diagnosis in this case, it provided diagnostic clues regarding the patient’s clinical presentation.
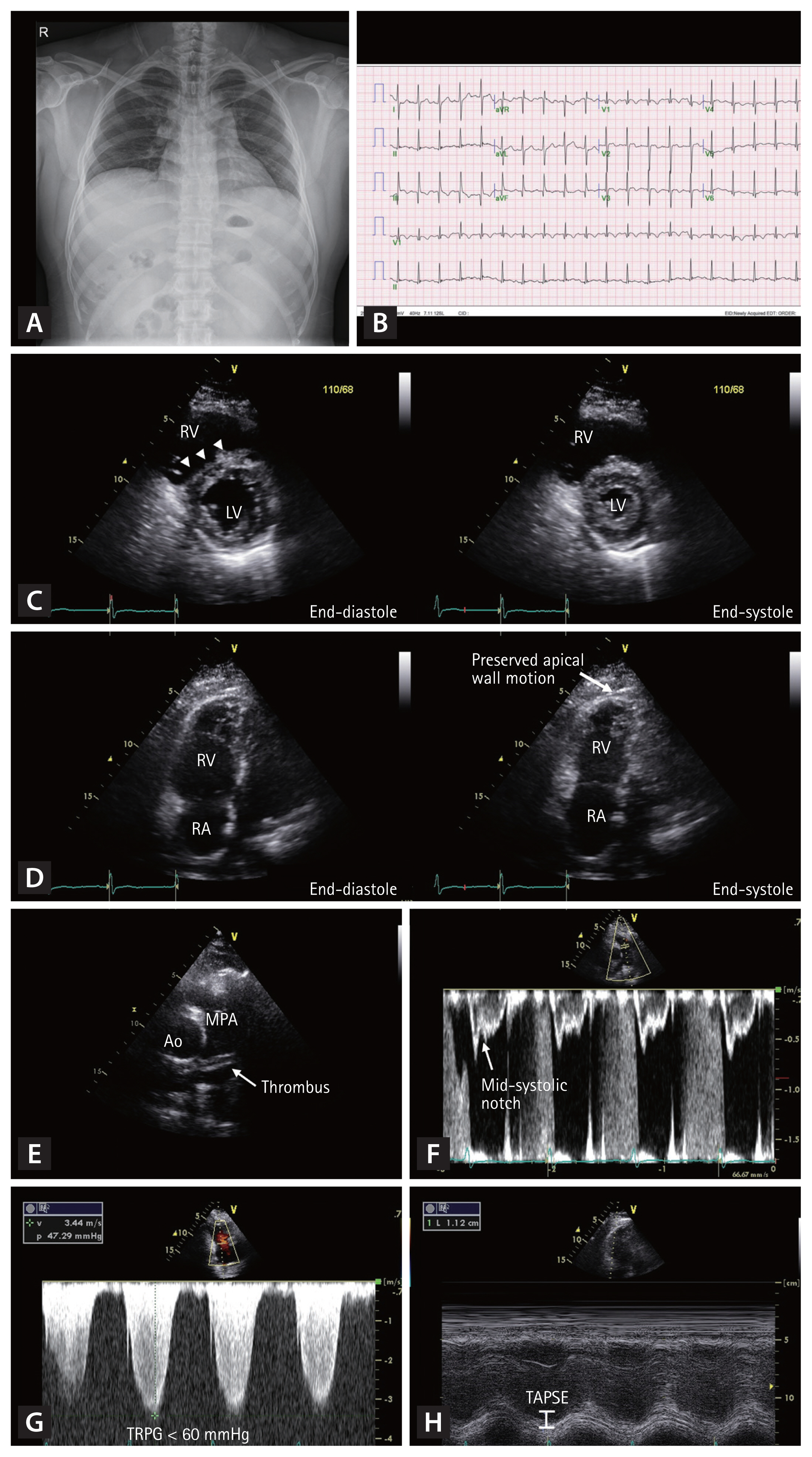
Typical case of an acute pulmonary embolism (PE) in a 33-year-old man. The initial chest radiography is normal (A). The electrocardiogram demonstrates sinus tachycardia, S wave in lead I (S1), Q wave in the lead III (Q3), T wave inversion in lead III (T3), and precordial leads V1–3 (B). Echocardiography shows D-shaped left ventricle (C, arrowheads), McConnell’s sign (D, arrow), thrombus in the main pulmonary artery (E), mid-systolic notching in the pulsed-wave Doppler tracing of right ventricular outflow tract (F), increased maximal velocity of tricuspid regurgitation (G), and decreased right ventricular contractility assessed by tricuspid annular plane systolic excursion (H). RV, right ventricle; LV, left ventricle; RA, right atrium; Ao, aorta; MPA, main pulmonary artery; TRPG, tricuspid valve peak systolic gradient; TAPSE, tricuspid annular plane systolic excursion.
Echocardiography is the most used imaging modality for evaluating and managing acute PE. Besides to give clues about other etiologies for chest pain or dyspnea, echocardiography can be used in the early risk stratification and impact management strategies in patients with acute PE. In addition, it can be used to predict prognosis in these patients. Throughout this review, we discuss the role of echocardiography in diagnosing and predicting outcomes in patients with signs or symptoms of PE. Further, we describe the impact of echocardiography on risk stratification and management strategies of PE.
RECOMMENDATION OF ECHOCARDIOGRAPHY IN RECENT TREATMENT GUIDELINES
Bedside echocardiography is indicated for diagnosing suspected acute PE in high-risk patients in the recent guidelines for the diagnosis and management of acute PE by the European Society of Cardiology (ESC, class I, level of evidence C) [10]. In addition, echocardiographic parameters are valuable markers for early risk stratification of patients with acute PE [10]. The presence of RV dysfunction assessed by echocardiography can be an indication for thrombolysis, particularly in patients with hemodynamic instability [10,11]. The American Heart Association guidelines indicate the presence of RV dysfunction in patients with RV dilatation (RV to LV end-diastolic diameter ratio [RV/LV ratio] > 0.9) or RV systolic dysfunction on echocardiographic studies [11]. However, in the ESC and American Society of Hematology guidelines, thrombolytic therapy is not indicated in patients with acute PE who are hemodynamically stable and do not have RV dysfunction [10,12].
ECHOCARDIOGRAPHIC FINDINGS IN PATIENTS WITH PE
Although computed tomography pulmonary angiography (CTPA) with contrast enhancement and lung ventilation/ perfusion scan are the gold standard for diagnosing acute PE [10], echocardiography plays a key role in its diagnosis. As the most available, and noninvasive diagnostic modality that does not require contrast agents or radiation, echocardiography is a feasible imaging modality for diagnosing acute PE.
Acute PE causes RV pressure overload, potentially leading to RV systolic dysfunction. Echocardiography can provide several clues to rule in or rule out acute PE, including regional wall motion abnormality in patients with acute myocardial infarction and dissecting aortic flaps in patients with acute aortic dissection in an emergency setting. We searched all published articles in English focused on the calculation of diagnostic accuracy of transthoracic echocardiography in the PubMed (National Library of Medicine, www.pubmed.gov) database. We selected 13 studies reporting quantitative data and reanalyzed diagnostic power. Table 1 shows the diagnostic power of several echocardiographic signs for acute PE. An illustration showing parameters found in patients with PE is presented in Fig. 2.
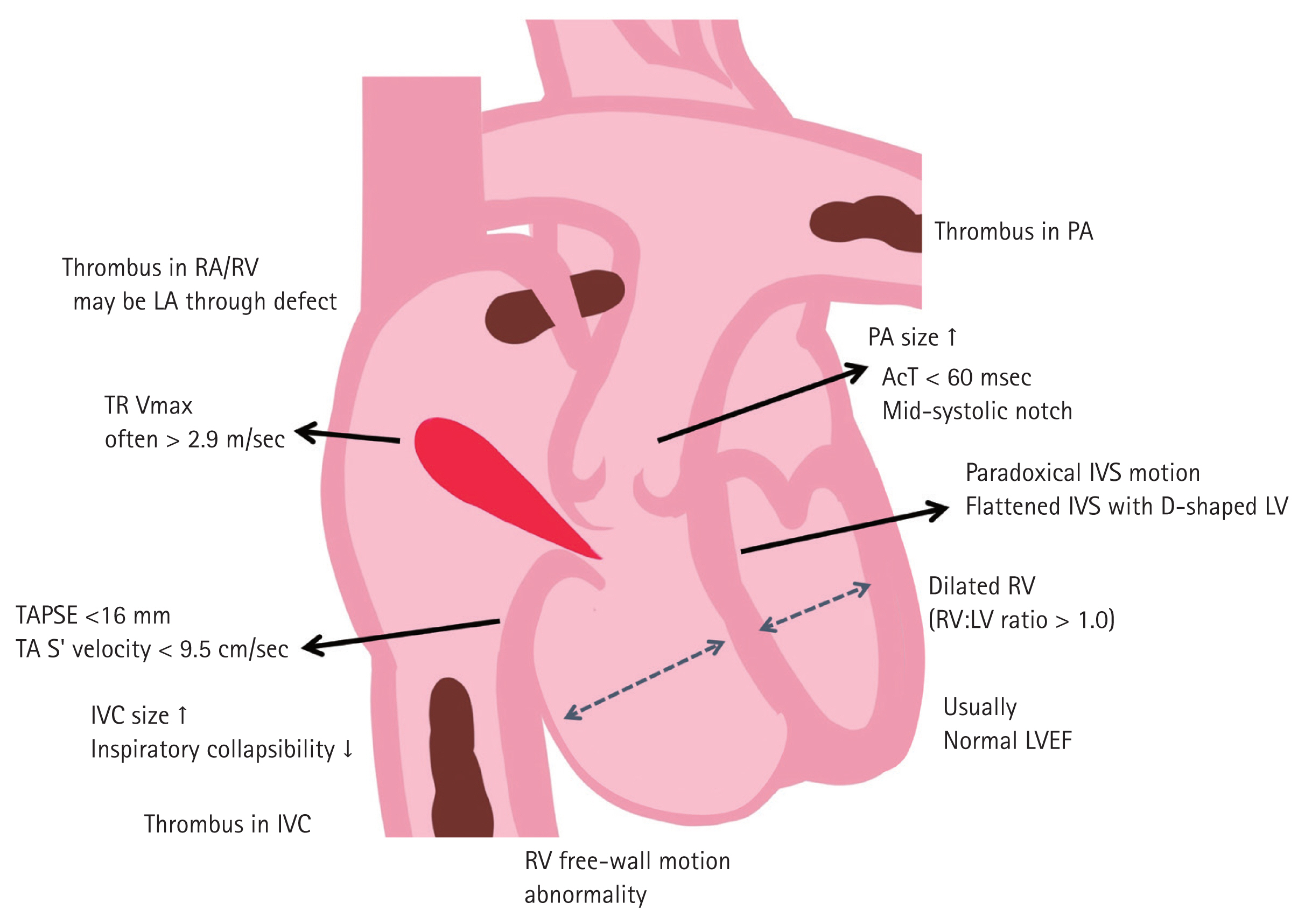
Schematic illustration of echocardiographic findings can be found in acute pulmonary embolism. RA, right atrium; RV, right ventricle; LA, left atrium; TR Vmax, maximal velocity of tricuspid regurgitation; TAPSE, tricuspid annular plane systolic excursion; TA, tricuspid annulus; IVC, inferior vena cava; PA, pulmonary artery; AcT, acceleration time of right ventricular outflow tract; IVS, interventricular septum; LV, left ventricle; LVEF, left ventricular ejection fraction.
The “60/60” sign
The “60/60 sign” is defined as a pulmonary flow acceleration time < 60 ms in the presence of a tricuspid regurgitation (TR) pressure gradient of < 60 mmHg or a TR velocity < 3.9 m/sec, a Doppler echocardiographic PE sign based on a distorted RV ejection pattern. The pulsed wave Doppler-derived systolic flow velocity curve pattern in the RV outflow tract reflects the pulmonary arterial pressure level. The presence of short acceleration time and mid-systolic deceleration with a notched pattern is considered diagnostic of severe pulmonary hypertension; however, these patterns have also been observed in acute PE. Early systolic notching on the pulmonary Doppler flow tracing was found in 92% of patients with massive or submassive PE, and it was superior to McConnell’s sign in PE diagnosis [13].
However, with a relatively mild increase in systolic pulmonary arterial pressure, the most disturbed systolic flow velocity curve was found in patients with acute PE who manifested the shortest acceleration time and early mid-systolic deceleration. Whenever short acceleration time (< 60 ms) is found in patients with no more than a moderate increase in the TR pressure gradient (< 60 mmHg) as assessed by Doppler echocardiography, acute PE should be strongly suspected (specificity 98%, sensitivity 48%) [14]. In our re-analysis, the presence of the “60/60” sign had a sensitivity of 41% and a specificity of 91% in detecting acute PE in 358 patients (Table 1) [13,15–17].
Decreased RV free wall motion and systolic dysfunction
In patients with acute PE, RV hypokinesis can be observed. However, it can also be observed in patients with RV infarction. Its sensitivity and specificity were 34% and 99%, respectively, in our analysis of 201 patients with acute PE (Table 1) [18–22]. The presence of pulmonary hypertension in patients with acute PE helps to distinguish this finding from that in RV infarction [23].
Patients with acute PE show decreased RV free wall motion with preserved RV apical motion (McConnell’s sign), which is suggestive of acute PE [24]. Initially, McConnell et al. [24] reported that McConnell’s sign could diagnose acute PE with a sensitivity of 77% and a specificity of 94%. However, several studies reported that this finding was present in approximately 35% of 357 patients with acute PE (Table 1) [13,16,17,21,25,26]. The combination of the “60/60” and McConnell signs increased the sensitivity without compromising the specificity of echocardiographic acute PE diagnosis in a cohort of patients with a high prevalence of previous cardiorespiratory disorders [16].
There are several echocardiographic markers of RV systolic dysfunction. Decreased TAPSE may be present in patients with acute PE [27]. A TAPSE < 1.6 cm was associated with higher pulmonary arterial pressure, higher incidence of RV dilatation and free wall hypokinesis. In addition, a tricuspid annular systolic velocity < 10 cm/sec, derived from Doppler tissue imaging of the lateral tricuspid annulus, is another RV systolic dysfunction marker [28]. Midventricular peak systolic strains of RV using speckle-tracking echocardiography provide objective findings of decreased RV free wall motion [29]. These strain values improved with the resolution of regional wall motion abnormality. However, these echocardiographic parameters have low sensitivity for detecting acute PE as stand-alone findings. Moreover, these indices may be normal in hemodynamically stable patients with PE [30].
RV dilatation
RV dilatation could occur due to increased RV afterload; it can be diagnosed when the RV basal diameter is > 42 mm and the RV diameter at the midlevel is > 35 mm [28]. Right ventricular end-diastolic dimension (RVEDD) > 27 mm was found in 68% of 103 patients with acute PE (Table 1) [17,18,20,31]. However, its positive predictive value was only 74%.
Furthermore, an RV/LV ratio > 1.0 indicates RV dilatation [32]. It can be found in about 27.4% of patients with acute PE [25]. In one prospective study with 146 patients, RV dilatation on bedside echocardiography had high specificity (98%) and poor sensitivity (50%) in detecting PE [21]. PE diagnosis was more sensitive in younger patients or patients without evidence of lung diseases, including chronic pulmonary obstructive disease. The presence of an RV/LV ratio > 1.0 showed a sensitivity of 50% and a specificity of 86% in 282 patients with acute PE (Table 1) [15,17,21,22,26,33].
Morphology and motion of the interventricular septum
Patients with RV pressure overload can have a flattened or bowed interventricular septum (IVS) toward the LV at end-diastole and end-systole. In the parasternal short axis view, the LV becomes a D-shaped cavity as the IVS flattens and loses its convexity due to increased RV pressure during diastole [34]. Paradoxical septal motion on echocardiography, along with RV dilatation, RV hypokinesis, McConnell’s sign (RV free wall hypokinesis with apical sparing), and increased TR velocities, are evidences of RV dysfunction [15]. In a prospective observational study of 146 patients with moderate- to high-risk of PE or confirmed PE, advanced RV dysfunction signs, such as paradoxical septal motion, RV hypokinesis, and McConnell’s sign on bedside echocardiography had a high specificity for PE diagnosis. However, these had low sensitivity similar to RV dilatation and increased the pretest probability of a diagnosis before a definitive imaging test [21]. In addition, this study suggests that signs of advanced RV dysfunction were associated with a higher thrombus burden. These findings were more significant in patients with more proximal emboli, such as the saddle, lobar, and mainstem emboli in the pulmonary arterial bed. In our analysis, a paradoxical septal motion was observed in 90 of 317 patients with acute PE, with a sensitivity of 28% and a specificity of 96% (Table 1) [15,17–21,26,31].
Pulmonary hypertension
Pulmonary hypertension is high blood pressure affecting the pulmonary arteries. In patients with acute PE, increased total pulmonary resistance originates from mechanical obstruction of the pulmonary arteries and humoral factors secreted due to hypoxia [35]. The method of choice for noninvasive estimation of pulmonary arterial pressure is continuous wave Doppler measurement of the peak velocity of the regurgitant jet across the tricuspid valve (maximal velocity of tricuspid regurgitation [TR Vmax]). Based on the simplified Bernoulli equation and straightforward pathophysiological concepts, this method proved highly reliable in numerous cardiovascular diseases [36]. Grifoni et al. [15] reported that documenting a RV-to-atrial pressure gradient > 30 mmHg (equivalent to a TR Vmax > 2.7 m/sec) on echocardiography was poorly sensitive but highly specific in patients undergoing evaluation for possible PE. In a previous study evaluating the diagnostic utility of various noninvasive investigations for PE compared with CTPA, pulmonary hypertension on echocardiography showed a high sensitivity (83%) and positive predictive value (86%) in patients with clinically suspected PE [37]. Other studies reported similar findings in various clinical situations. In a prospective cohort study of emergency department patients with suspected PE, elevated pulmonary artery or RV systolic pressure (> 44 mmHg) had a sensitivity of 8% and a specificity of 96%, with a moderate possibility of a positive ratio (4.0) [18]. Nazeyrollas et al. [31] reported a possibility of a positive ratio ranging from 3.9 to 5.1 in an intensive care setting. In contrast, a normal TR Vmax < 2.5 m/sec is usually found in normal controls than in patients with acute PE, helping in ruling out and the decision-making on further invasive testing [31,38]. In our analysis involving 216 patients with acute PE, its sensitivity and specificity were 50% and 82%, respectively (Table 1) [17–20,33].
Visualization of emboli in the right heart and pulmonary arteries
Emboli, usually originating from deep vein thrombi, can be found anywhere in the inferior and superior venae cavae, right atrium (RA), RV, and pulmonary arteries. Since thrombi form in deep veins, emboli are usually elongated in shape. Sometimes, emboli from tumors or myxomas can be round in shape [18,20,39]. In one study with 130 patients with acute PE, RA thrombi were found in 23 (18%) [40]. In another study with 1,113 patients with acute PE, the incidence of right heart thrombi was 3.8% (42 patients) on baseline echocardiography [41]. The presence of mobile right heart thrombi was associated with acute PE in about 97% of cases, showing a high mortality rate of approximately 44% [41,42]. Right heart thrombi were found in five (4%) of 146 patients with acute PE (Table 1) [15,18,20]. However, its specificity and positive predictive value were 100%, suggesting that the presence of mobile thrombi can confirm PE. Furthermore, the presence of mobile RA thrombi or mobile RA thrombi prolapsed into the RV, particularly in patients with echocardiographic signs of RV pressure overload, can be a confirmative sign of acute PE.
Echocardiographic signs of RV strain
Echocardiographic RV strain parameters include RV dilatation (RVEDD > 30 mm at the apical four-chamber view), Mc-Connell’s sign, paradoxical interventricular septal motion, and visible thrombi in the right heart or pulmonary arteries [20]. In our analysis, RV strain was found in 173 of 264 patients with acute PE (67%) [15–17,19,20].
Diagnostic power of echocardiographic findings
In a previous systematic review and meta-analysis, 22 studies showed consistently high specificity and low sensitivity for echocardiography in diagnosing PE [43]. Moreover, we reanalyzed the sensitivity and specificity of several studies; these results are presented in Table 1.
Among 12 unique signs suggestive of acute PE, right heart strain was the most common sign (sensitivity 73%, specificity 75%) [16–18,20,33,44]. Table 1 shows the high specificity for all the signs, with none of them having a specificity < 80%; however, sensitivity was much lower, with only an increased RVEDD having a sensitivity > 70% for acute PE. These meta-analysis findings revealed that various echocardiographic parameters suggesting acute PE can be used to diagnose PE. However, echocardiographic signs showed a low negative predictive value; therefore, none of them can rule out PE except the presence of clots in the right heart chambers and pulmonary arteries. In clinically insignificant PE with no significant RV hemodynamic effects, echocardiography is usually normal. Therefore, it is important to note that signs can be interpreted differently depending on the situation. In addition, the positive predictive value of echocardiographic signs was insufficient as a stand-alone finding. Signs of RV overload or dysfunction may be observed even in the absence of acute PE with concomitant cardiac or respiratory disease condition. Thus, echocardiography is limited as a potential gold standard for ruling in or out a PE diagnosis, and clinicians should consider further advanced imaging tests in case of a discordance between clinical judgement and the echocardiographic parameters. However, multiple echocardiographic findings can be useful in diagnosing PE and deciding on the use of thrombolysis for patients who cannot receive other confirmatory studies, especially including the most critically ill patients.
ROLE OF ECHOCARDIOGRAPHY IN PE DIAGNOSIS IN SPECIAL SITUATIONS
Echocardiography can be useful in PE diagnosis, particularly in patients for whom performing CT is impossible or difficult [45]. Although echocardiography is not recommended in the diagnostic work-up for hemodynamically stable patients with PE, it can be included in the evaluation of suspected high-risk PE [45].
Considering this, echocardiography in pregnant women may be useful for PE diagnosis because it has no known adverse effect on the fetus. PE is a leading cause of pregnancy-related mortality in developed countries, accounting for 20% of maternal deaths in high-income countries [46]. VTE risk is higher in pregnant women than in non-pregnant women of similar age; it increases during pregnancy and reaches a peak during the post-partum period [47]. The most common PE symptoms include dyspnea (73%), pleuritic chest pain (66%), cough (37%), and hemoptysis (13%). The abrupt onset of pleuritic chest pain may be the first unique symptom. However, accurate clinical suspicion or VTE diagnosis during pregnancy is challenging because of the overlap of signs and symptoms between physiologic changes during pregnancy and the development of PE. The pregnant patient must undergo specific diagnostic tests to establish or exclude PE diagnosis because routine laboratory findings are not specific for confirming the diagnosis. Because radiation exposure during pregnancy can be associated with fetal teratogenic and oncogenic effects, conventional pulmonary angiography or standard-dose CT should be avoided, particularly in the first trimester [45]. Patients with chronic kidney disease (CKD) also have a high-risk of VTE due to activation of procoagulants, decreased anticoagulants, or decreased fibrinolytic activity [48]. A clinical diagnosis of acute PE in patients with CKD is frequently considered; however, the diagnostic approach is challenging. Current guidelines recommend CTPA as the gold standard for an imaging test. However, CTPA is prohibited for use in patients with severe renal impairment due to the increased risk of contrast-induced complications [10]. Thus, for these special populations, a D-dimer test and duplex ultrasound of the lower extremities play essential roles in PE diagnosis. Lung ventilation/perfusion scan, a lower-radiation and contrast medium-sparing procedure, may preferentially be applied if an imaging test is necessary, but it may not be used in an emergency setting [49]. Echocardiography can provide signs that may help in the differential diagnosis of shock; thus, the absence of echocardiographic findings of RV pressure overload can exclude PE as the cause of hemodynamic instability. Moreover, echocardiographic findings suggesting causes other than acute PE can help exclude the need for CT. However, a low-dose CT or ventilation/perfusion scan should be used to distinguish PE in patients with unequivocal echocardiographic signs of RV pressure overload.
ROLE OF ECHOCARDIOGRAPHY IN RISK STRATIFICATION
Acute PE mortality risk levels are conventionally divided into high, intermediate (high/low), and low levels. This stratification can be achieved using four diagnostic tools: the patient’s hemodynamic state, Pulmonary Embolism Severity Index (PESI) score, echocardiographic RV systolic dysfunction parameters, and cardiac markers (troponin). The ESC guidelines classify patients with acute PE into the following categories: (1) high-risk, defined as the presence of shock or persistent hypotension; (2) intermediate-risk, defined as not at high-risk, and a simplified PESI (sPESI) score ≥ 1; and (3) low-risk, defined as the absence of hypotension and an sPESI score of 0 [50]. Risk stratification is essential for allocating each patient to the appropriate treatment and considering more aggressive initial treatments, such as thrombolytic therapy or surgical embolectomy [51]. The presence of RV systolic dysfunction is a poor prognostic factor and represents a more advanced pathophysiologic PE stage. The assessment of RV systolic function is one of the crucial factors for the risk stratification and further management [52]. Although many methods exist for evaluating RV systolic function, no single measurement is accepted as the gold standard. The ESC risk stratification approaches use alternate imaging modalities, such as echocardiography and CT angiography, to assess RV systolic function. However, in hemodynamically stable patients with acute PE, further risk assessment is required based on clinical imaging, cardiac biomarkers (mostly related to RV function and myocardial injury), and the presence of comorbidities. Thus, current risk stratification has focused on identifying low-risk PE in hemodynamically stable patients. Of the 17 prognostic models of acute PE, PESI, and sPESI are the most widely validated and are now included in the risk classification of the ESC guidelines [53,54]. A recently updated prognostic model showed improvement in the prognosis of acute PE by adding one or more clinical-, biological-, and imaging-based markers of RV systolic dysfunction and myocardial injury to the existing models [55].
ROLE OF ECHOCARDIOGRAPHY IN THERAPEUTIC STRATEGIES
The standard treatment for most patients with PE in the acute phase is anticoagulation and thrombolytic therapy [10]. Recently, more effective therapies and interventions, such as greater use of anticoagulants, thrombolysis, and surgical embolectomy in practice, have contributed to the reduction in mortality and PE recurrence.
Because thrombolytic therapy dissolves thrombi in the pulmonary arterial system, it lowers pulmonary arterial pressure and improves RV systolic function more rapidly than anticoagulation only in selected patients with acute PE [56,57]. In patients with high-risk PE, thrombolysis was associated with a reduction in mortality (odds ratio [OR], 0.66; 95% confidence interval [CI], 0.45 to 0.97) [58]. All treatment guidelines recommend thrombolysis as the initial treatment of choice for patients with acute PE who are hemodynamically unstable. Thus, early identification of RV systolic dysfunction in high-risk patients with acute PE is essential in promptly deciding whether to administer thrombolytic therapy [59]. Many critically ill patients cannot undergo more advanced imaging; therefore, bedside echocardiography should be performed at an emergency department or intensive care unit to identify RV systolic dysfunction [60,61].
The role of thrombolysis is unclear in patients with intermediate-risk acute PE. Several large randomized controlled trials have investigated the impact of thrombolytic treatment. The Pulmonary Embolism Thrombolysis (PEITHO) trial compared systemic thrombolysis plus anticoagulation with anticoagulation alone in the largest trial involving 1,005 adult patients with intermediate-risk PE. The incidence of death or hemodynamic decompensation was 2.6% in the group receiving tenecteplase group; this value was much lower than the 5.6% in the group receiving heparin (OR, 0.44; 95% CI, 0.23 to 0.87; p = 0.02) [62]. Although thrombolytic therapy was associated with a significant reduction in the risk of hemodynamic decompensation or collapse, there was an increased risk of bleeding, including major extracranial bleeding (6.3% vs. 1.2% in heparin alone) and hemorrhagic stroke (2.4% vs. 0.2% in heparin alone). Furthermore, thrombolytic therapy showed no reduction in overall mortality, persistent exertional dyspnea or functional limitation, and chronic thromboembolic pulmonary hypertension on long-term follow-up (median duration, 38 months) in the PEITHO trial (709 of 1,006 initially randomized patients) [63]. Therefore, current guidelines do not recommend routine primary reperfusion treatment in patients with intermediate-risk PE [64,65]. “Rescue” thrombolysis should be reserved for these patients if they develop signs of hemodynamic instability despite anticoagulant treatment. Although echocardiographic evidence of RV dysfunction is not a compelling indication for systemic thrombolysis even in intermediate-high-risk patients, there has been an overall uptrend in the use of cardiac imaging in hemodynamically stable patients with PE over the past decade [66]. Because of its easy availability, low procedural cost and risk, and potential for guiding appropriate early management, echocardiography has been used increasingly in non-massive PE at the time of admission [67].
PROGNOSTIC VALUE OF ECHOCARDIOGRAPHIC PARAMETERS IN ACUTE PE
Presence of RV systolic dysfunction
The echocardiographic criteria for RV systolic dysfunction include RV dilatation and an increased end-diastolic RV/ LV ratio, RV free wall hypokinesis, increased TR Vmax, or combinations of these conditions [45]. Although the overall positive predictive power was low (< 10%), systematic reviews and meta-analyses have shown that RV dysfunction detected using echocardiography was associated with an increased risk of short-term mortality, even in hemodynamically stable patients with acute PE [68]. This might be related to the difficulty in standardizing the definition of RV systolic dysfunction in echocardiographic parameters between studies. Nevertheless, the echocardiographic assessment of the RV systolic function is widely recognized as a valuable tool for the prognostic assessment of hemodynamically stable patients with acute PE in current clinical practice (Table 2).
Decreased TAPSE
TAPSE is a well-known, easy-to-measure, and reproducible echocardiographic parameter. It represents the RV longitudinal function measuring the longitudinal displacement of the systolic excursion of the tricuspid annular segment. A TAPSE < 16 mm is regarded as decreased RV systolic function and is the most frequently reported finding associated with unfavorable prognoses in acute PE. Patients with decreased TAPSE (< 16 mm) during diagnosis have a two-fold higher mortality rate (hazard ratio, 2.3; 95% CI, 1.2 to 4.7; p = 0.02) [27]. In some prospective observational studies, TAPSE ≤ 15–16 mm was an independent predictor of increased risk of PE-related mortality or rescue thrombolysis, even after adjusting for other echocardiographic findings of RV dysfunction [45]. Furthermore, TAPSE is a better predictor of acute PE-related outcomes than the RV/LV diameter ratio in normotensive patients [69]. Patients with TAPSE > 20 mm can be considered very low-risk patients who may be candidates for a short hospital stay or even outpatient treatment.
Presence of thrombi in the right heart
According to several studies, right heart thrombi can be detected using echocardiography or CT angiography in approximately 4% of unselected patients with acute symptomatic PE [70]. Prevalence of right heart thrombi may reach 18% among patients with PE in the intensive care setting. However, it is rarely found in normotensive patients without echocardiographic evidence of RV dysfunction (1.0%) [71]. The presence of right heart thrombi was associated with high early mortality in acute PE [72]. Systematic reviews and meta-analyses reported that right heart thrombi increased the risk of short-term all-cause mortality (OR, 3.0; 95% CI, 2.2 to 4.1) and PE-related mortality (OR, 4.8; 95% CI, 2.0 to 11.3) [73]. Associations between right heart thrombi and mortality were consistent after adjusting for demographics, cardiovascular comorbidities, and sPESI score [71]. In low-risk patients without hemodynamic instability or RV systolic dysfunction, the presence of right heart thrombi had no impact on mortality. In particular, the prognosis was more related to the hemodynamic status than to thrombi characteristics [71].
Decreased RV longitudinal strain
Recently, several researchers have shown considerable interest in evaluating RV function based on RV strain in patients with acute PE. Visual estimation of RV systolic function, TAPSE, RV fractional area change, Tei index, and tissue Doppler tricuspid valve annulus systolic wave has been used to estimate RV strain. However, these parameters have significant limitations [74]. Conversely, RV longitudinal strain measured by two-dimensional speckle-tracking echocardiography can assess RV myocardial systolic function without the confounding effect of angle dependency and tethering. RV strain value is closely related to prognosis in patients with hemodynamic stable acute PE. A small-scale prospective study reported that three-dimensional echocardiography-based RV ejection fraction and mid-portion of RV free wall longitudinal strain were independently associated with 6-month adverse outcomes in acute submassive PE [75]. These results remained even in the multivariate analysis after adjusting for cardiac biomarkers and other measured echocardiographic parameters. Lee et al. [76] demonstrated that RV free and global wall strains are independent prognostic markers for in-hospital events, such as in-hospital PE-related death or escalation of therapies.
CONCLUSIONS
Acute PE is the third leading cause of cardiovascular death, and its prevalence is increasing. Echocardiography has been validated for early risk stratification of patients presenting with acute PE and impacts management strategies. Despite decades of research, the role of echocardiography in diagnosing and predicting outcomes of acute PE remains under-estimated. The use of CTPA has facilitated early diagnosis in patients with suspected PE. However, echocardiography is the most available, noninvasive, and feasible imaging option (before performing CTPA) for patients with suspected PE.
Echocardiography has high specificity in diagnosing suspected PE in patients with adequate risk. Thus, it is helpful as a rule in test in the initial diagnosis of acute PE. Echocardiography may be particularly useful at the bedside in the emergency department or intensive care unit for patients who cannot undergo other confirmatory studies, especially those who are in the most critical condition. In addition, it can be a useful option in pregnant patients suspected of having acute PE. More effective therapies and interventions have contributed to reducing mortality and recurrence of acute PE in the last decade. Thrombolytic therapy is the initial treatment of choice in hemodynamically unstable patients with acute PE. Thus, echocardiography is widely used to identify RV dysfunction in high-risk patients with massive PE. For low-risk hemodynamically stable patients, echocardiography rarely yields additional prognostic information. Current guidelines do not recommend echocardiography as part of the diagnostic work-up in non-high-risk patients. However, the presence of RV systolic dysfunction is a poor prognostic factor and represents a more advanced pathophysiologic PE stage. RV dysfunction detected using echocardiography was associated with an increased risk of short-term mortality, even in hemodynamically stable patients with acute PE. Although echocardiography is not recommended for diagnostic work-up in hemodynamically stable patients with PE, echocardiographic assessment of RV systolic function is now widely recognized as a valuable tool for prognostic assessment of hemodynamically stable patients in current clinical practice. Furthermore, a recently updated prognostic model showed that specific echocardiographic RV dysfunction markers have the potential to improve prognosis beyond existing risk models. Furthermore, there has been an overall uptrend in cardiac imaging in the last decade, particularly echocardiography, even in patients with hemodynamically stable PE. Extensive validation and impact studies are needed to guide appropriate patient selection for echocardiography use in hemodynamically stable PE.
Acknowledgments
We would like to specially thank Sung-Won Park for drawing an excellent illustration.
Notes
No potential conflict of interest relevant to this article was reported.