Effect of age on the prognosis of intrahepatic cholangiocarcinoma
Article information
Abstract
Background/Aims
Intrahepatic cholangiocarcinoma (iCCA) is a subgroup of cholangiocarcinoma and is the second- most-common primary hepatic tumor. Several predictive and prognostic factors have been analyzed; however, in this study we focused on the influence of age. Our aim was to use real-world results to determine the influence of age in iCCA patients.
Methods
A retrospective analysis of patients treated between 2005 and 2016 at Konkuk University Medical Center. In total, 133 patients with iCCA were identified. The mass-forming, periductal-infiltrating, and intraductal-growth types were included; patients with extrahepatic or hilar-type cholangiocarcinoma were excluded. We defined two groups: a younger group, age < 65 years, and an older group, age ≥ 65 years. Statistical analyses using univariate and multivariate Cox regression analyses, including the Kaplan-Meier method, were conducted.
Results
In total, 114 patients were enrolled. The two groups differed with regard to treatment options such as surgery with adjuvant chemotherapy or palliative chemotherapy (p = 0.012, p < 0.001). The younger group had significantly longer survival than the older group (p = 0.017). In the younger group, patients who received therapy had longer survival than those who did not (hazard ratio, 3.942; 95% confidence interval, 2.053 to 7.569; p < 0.001). Multivariate analysis indicated that younger age, lower bilirubin, low CA 19-9, and no lymph-node involvement were independent factors for improved survival.
Conclusions
Younger patients and those who underwent surgery with adjuvant chemotherapy had longer survival. The younger the patient, the more treatments received, including palliative chemotherapy.
INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant transformation of bile duct epithelium that is categorized according to the site of origin. Intrahepatic cholangiocarcinoma (iCCA) is the second-most-common primary hepatic tumor. The incidence of iCCA is increasing worldwide; however, research is hampered by several of the condition’s characteristics. Although the etiology of CCA is unclear, it is related to hepatolithiasis, liver-fluke infection, liver cirrhosis, chronic hepatitis B or C, and inflammation of the bile duct, such as by primary biliary cholangitis or primary sclerosing cholangitis [1-5].
Young-onset gastric cancer or colorectal cancer is aggressive and has a poor prognosis in adolescents and young adults diagnosed with malignancy at the age of 15 to 45 years [6-9]. CCA may have a poorer prognosis in younger than older patients. A recent study reported molecular differences between young adults and older (> 45 years) patients with CCA [10].
iCCA has few treatment options and tends to have a poor outcome. Surgical intervention improves the survival and prognosis of patients [11,12], but iCCA is typically diagnosed at a non-operable stage. As recurrence is common after surgical resection, patients should be treated with adjuvant therapy [13-15].
The combination of cisplatin plus gemcitabine is the preferred systemic chemotherapy regimen for patients with unresectable and metastatic disease. Advanced molecular-based research has identified several targetable genetic aberrations, leading to the development of alternative treatment options such as fibroblast growth factor receptor (FGFR) inhibitors and neurotrophic tyrosine receptor kinase (NTRK)-gene-fusion-inhibitors. Isocitrate dehydrogenase 1 (IDH1) and B-Raf proto-oncogene, serine/threonine Kinase (BRAF) mutations have been characterized as targetable oncogenic mutations. Pembrolizumab is used as immunotherapy for mismatch-repair deficiency [16-20].
Often, iCCA treatment must be withdrawn because of malnutrition, general weakness, or decreased liver function. Some patients with iCCA, especially older patients, abandon treatment because of their general condition. In younger patients, treatment efficacy is often limited, and the disease progresses faster. Therefore, young patients with iCCA may have low survival rates [10,21]; further investigation is needed.
This study aimed to evaluate the prognosis of iCCA by age. Thus, we retrospectively analyzed the data to examine the effect of age in patients with iCCA.
METHODS
Study population
A retrospective chart review of patients treated at Konkuk University Medical Center between 2005 and 2016 was conducted. In total, 133 patients with iCCA were identified. They had been diagnosed based on imaging modalities such as computed tomography, magnetic resonance imaging, endoscopic retrograde cholangiopancreatography, or magnetic resonance cholangiopancreatography. Mass-forming-, periductal-infiltrating-, and intraductal-growth-type CCAs were included; extrahepatic and hilar-type CCAs were excluded. We excluded 19 patients, 13 because of loss to follow-up, three for insufficient data, and four who died from other causes (traffic accident, infection, and old age).
Pathology, TNM staging, and definition
Pathology reviews were conducted retrospectively. Most of the patients had adenocarcinoma, but some (n = 3) had hepatocellular carcinoma plus CCA. The tumor-node- metastasis (TNM) stage of iCCA was categorized based on the American Joint Committee 8th edition staging system [22]. The younger group was aged < 65 years and the older group was aged ≥ 65 years.
Statistical analysis and ethics
Statistical analysis was performed using the Mann-Whitney test, chi-squared test, or Fisher’s test as appropriate. Kaplan-Meier analysis by log-rank test was used to compare survival rates. Univariate and multivariate Cox regression analyses were conducted using a proportional-hazards model with backward selection for factors associated with the survival of patients with iCCA. Statistical significance was defined as p < 0.05. Statistical analysis was performed using SPSS software version 25.0 (IBM Co., Armonk, NY, USA). Kaplan-Meier curves were plotted using GraphPad Prism v. 5 (GraphPad Software Inc., San Diego, CA, USA).
This retrospective study complied with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Konkuk University Medical Center(KUMC 2021-11-062). The requirement for informed consent was waived by the IRB.
RESULTS
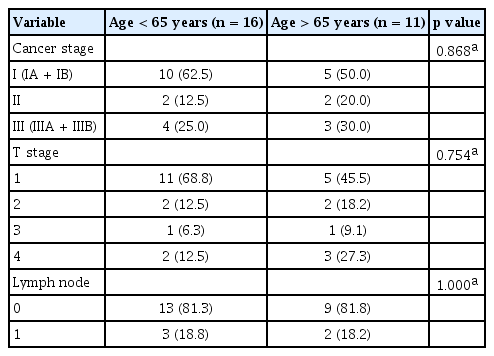
In total, 114 patients were enrolled and divided into the younger and older subgroups. Their baseline characteristics are shown in Table 1. The numbers of patients < 65 and ≥ 65 years of age were 51 (44.7%), and 63 (55.3%), respectively. The male:female ratios in the two groups were similar, as were their chemistry values, such as aspartate aminotransferase, alanine aminotransferase, total bilirubin, and albumin. The average level of serum carbohydrate antigen 19-9 (CA 19-9) was non-significantly higher in the older group. At the time of diagnosis, the T-stage distribution in the younger group was 19.6% T3 and 33.3% T4. The equivalent values in the older group were 15.9% and 54.0%, respectively. The numbers of patients with lymph node metastasis were 33 (64.7%) in the younger group and 40 (63.5%) in the older group (p = 0.893). Distant metastasis was absent in about two of thirds of the patients at diagnosis (p = 0.824).
The median survival time was 216 days (95% confidence interval [CI], 184.3 to 247.7). The mortality rates were 80.4% in the younger group and 84.1% in the older group (p = 0.602). The median survival time was 338 days in the younger group (95% CI, 191.4 to 484.6) compared to 194 days in the older group (95% CI, 111.7 to 276.3) (p = 0.017). The recurrence rate for the treatment group (surgery only or surgery with adjuvant chemotherapy) was 62.5% in the younger group and 72.1% in the older group.
Fig. 1 shows the results of Kaplan-Meier analysis of the iCCA patients. Patients < 65 years had longer median overall survival (p = 0.017). Younger patients who had received surgery or surgery with adjuvant chemotherapy had improved survival (p < 0.001). However, survival did not differ according to age in the curative treatment group (p = 0.08).
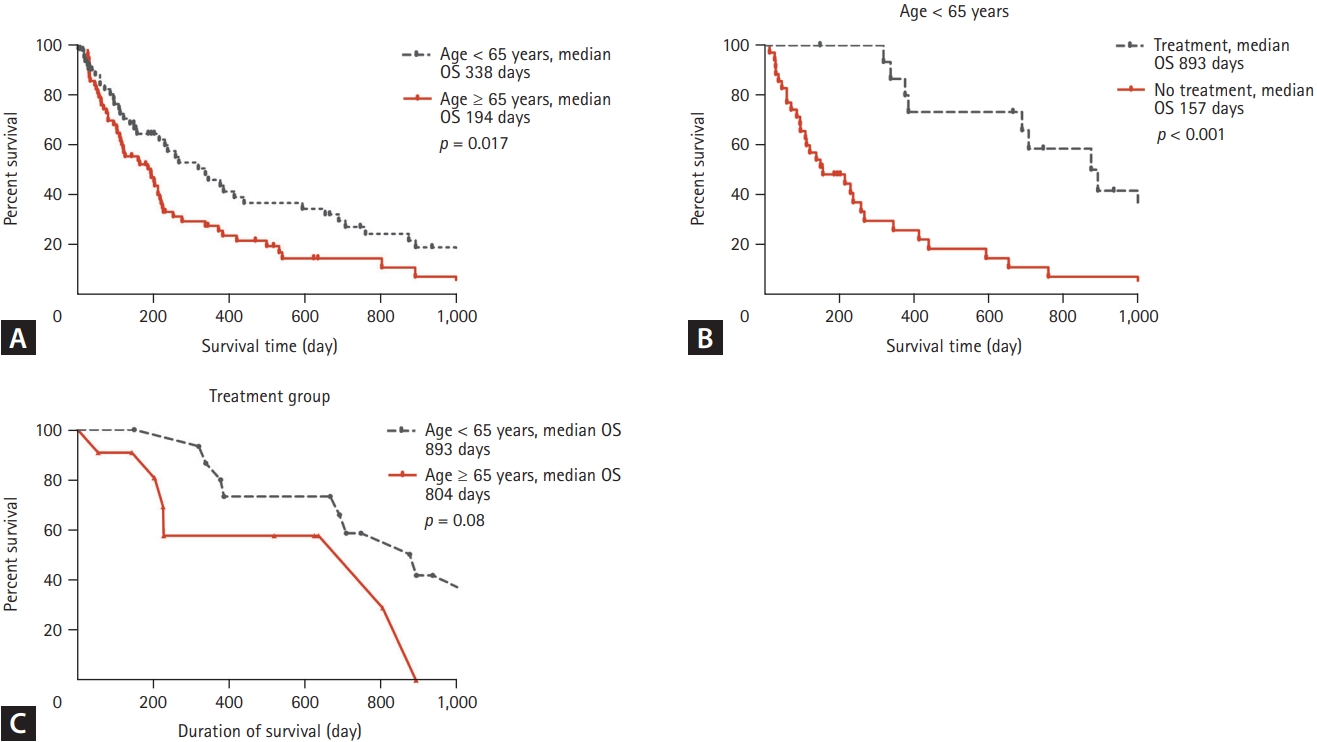
Kaplan-Meier analysis. Treatment group refers to patients who received surgery only or surgery with adjuvant chemotherapy. (A) The younger group (< 65 years) had longer survival (p = 0.017). (B) In the younger group, patients who received surgery only or surgery with adjuvant chemotherapy had longer survival (hazard ratio, 3.942; 95% confidence interval, 2.053 to 7.569; p < 0.001). (C) Survival according to age in the treatment group. The median survival for the younger group was 893 days, and that for the older group was 804 days. Although not statistically significant, the younger age group tended to have better survival (p = 0.08). OS, overall survival.
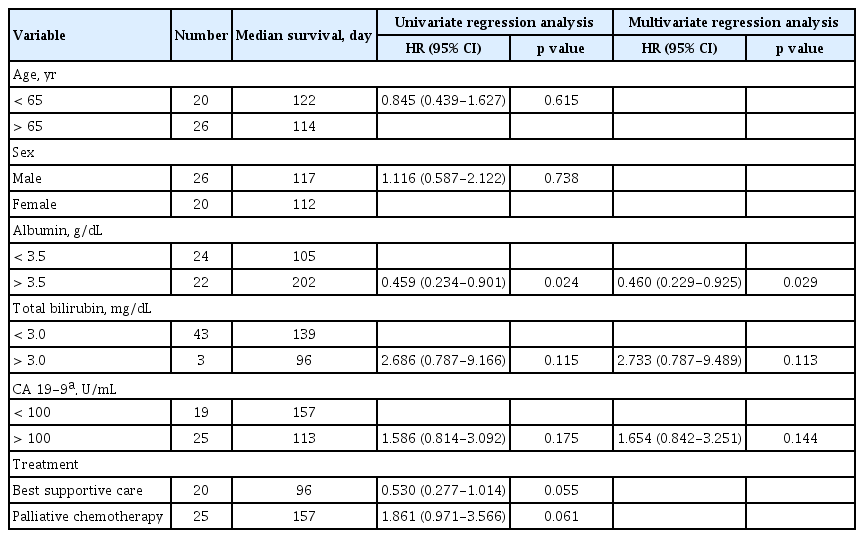
Table 2 shows the results of univariate and multivariate Cox regression analyses of factors associated with survival. In the simple Cox regression analysis, older age, low serum albumin level, high serum total bilirubin level, high serum CA 19-9 level, more advanced than stage T3, lymph-node involvement, and distant metastasis were associated with shorter survival (p < 0.05). By multiple Cox regression analysis with backward elimination, older age (p = 0.007), serum total bilirubin level (p = 0.012), serum CA 19-9 level (p < 0.001), and lymph-node involvement retained statistical significance(p < 0.001).
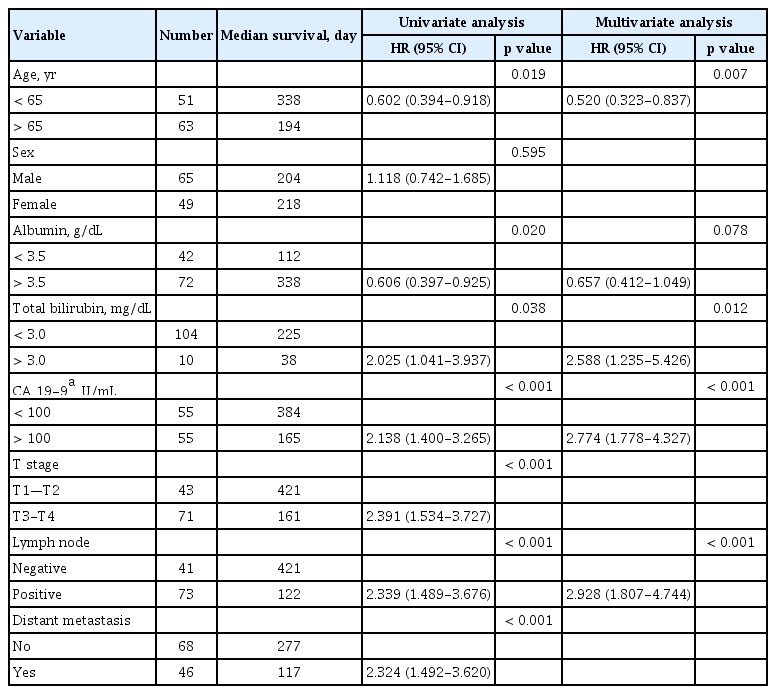
Univariate and multivariate Cox regression analyses of factors associated with the survival of patients with iCCA
Although the baseline characteristics of the two groups were similar, the ratio of treatment types was significantly different. Table 1 show that more patients in the younger group underwent surgery with adjuvant chemotherapy (p = 0.012). More patients in the older group received best supportive care, whereas more patients in the younger group underwent palliative chemotherapy (p < 0.001 and p = 0.001, respectively).
Table 3 shows another analysis comparing the cancer stage of patients who underwent surgery only or those who underwent surgery with adjuvant chemotherapy by age. Of the 27 patients, 16 were aged < 65 years. As there was only a small number of patients, we merged some cancer stages for the statistical analysis. No significant difference was observed in the distribution of cancer stages. Table 4 shows the results of univariate and multivariate Cox regression analyses for factors associated with survival in the metastasis group. The serum albumin level was significant in the multivariate Cox regression analysis (p = 0.029).
DISCUSSION
Young-onset malignancies tend to have a poor prognosis; they have unique biologic and genomic features, resulting in poor clinical behaviors, including resistance to therapy [6-9]. Few studies have evaluated young patients with iCCA. In this study, Fig. 1A shows that patients aged < 65 years had longer survival.
There might have been subjective differences between the two groups, such as fatigue, general weakness, and malnutrition. Older patients often have difficulty in maintaining chemotherapy or accepting invasive procedures, leading to a poor prognosis. Koch et al. [23] reported that multimodal treatments possibly improved the survival rate of patients with biliary tract cancer. Based on this report, it can be inferred that those who accept therapies may have the chance to live longer than those who do not.
Fig. 1B shows that survival was significantly improved by surgery with or without adjuvant chemotherapy in younger patients. Fig. 1C compares the effectiveness of therapy by patient age. The outcome of the therapy was better in younger patients than in older patients. Significantly more therapeutic interventions were conducted in younger patients. Therefore, it can be inferred that more active treatment can improve the survival of younger patients. There have been some reports that patients who received more treatment had better prognoses [14,15,24]. Likewise, in this study, therapy such as surgery with or without adjuvant chemotherapy improved survival. In this regard, a patient’s performance status is a key factor in treatment decision-making, including the need for surgery or adjuvant chemotherapy [3,25].
In this study, palliative chemotherapy did not improve survival in either group, for three reasons. First, in actual clinical situations, patients present with totally different medical conditions, including baseline illness and economic status. Second, the patients in this study received different first-line chemotherapy regimens (gemcitabine-based in 35 [63.6%] of the 55 patients who received palliative chemotherapy, and 5-fluorouracil [5-FU] based in 20 [36.4%]). Third, iCCA has a very poor prognosis, especially in a palliative setting. This could have been why chemotherapy did not improve survival.
This study had several limitations. First, it was of a retrospective design. Second, a small number of patients was enrolled, and although the incidence of iCCA is increasing, it is rarer than other cancers. We initially wanted to include three age groups, but the number of patients in each group would have precluded statistical analysis. Third, we could not analyze the quality of life of the patients. Although the younger patients who received multiple therapies may live longer, their quality of life might not be better because biliary tract malignancies result in many problems, such as infection and obstructive jaundice. Thus, a comprehensive approach is needed to treat patients with iCCA.
We hypothesized that younger patients with iCCA have a poorer prognosis compared to older patients with iCCA. However, in this study, younger patients received more treatments than older patients, which improved prognosis and survival. In addition, among the older group, patients who received intensive therapy lived longer. Thus, in the clinical setting, it is important to maintain a good performance status to increase the patient’s chances of treatment.
In conclusion, relatively young patients and those who received intensive treatment had longer survival. The younger the patient, the more intensive the treatment is, including palliative chemotherapy.
KEY MESSAGE
1. Several gastrointestinal cancers have poor prognosis in young patients.
2. However, in our study, younger patients with intrahepatic cholangiocarcinoma (iCCA) have a better prognosis compared to the elder patients with iCCA.
3. It is because the younger the patient, they could receive more active treatment.
Notes
No potential conflict of interest relevant to this article was reported.