Helicobacter pylori eradication reduces risk for recurrence of gastric hyperplastic polyp after endoscopic resection
Article information
Abstract
Background/Aims
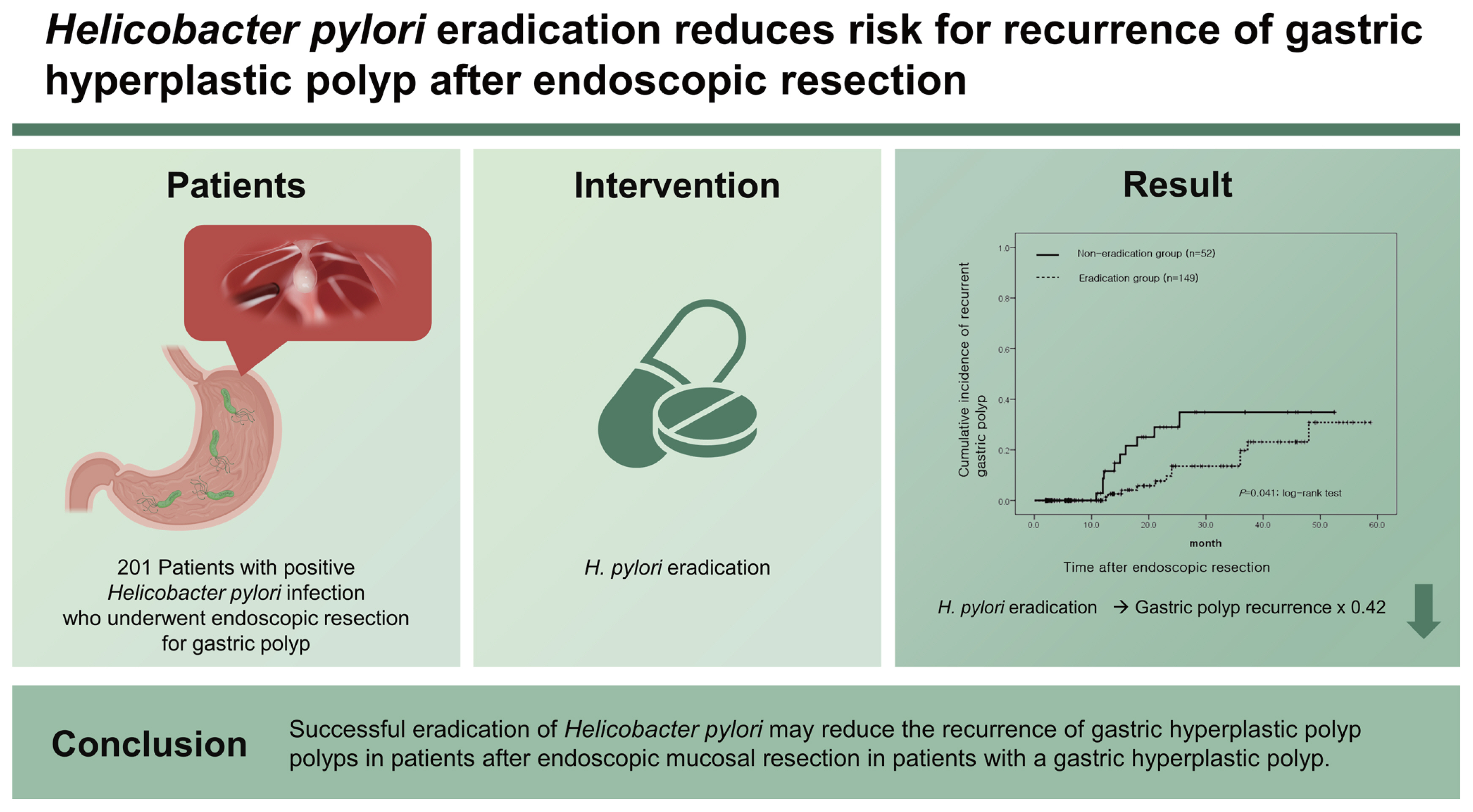
Little is known about the effect of Helicobacter pylori eradication on the recurrence of gastric hyperplastic polyps after endoscopic resection. Thus, we evaluated the recurrence rate of gastric hyperplastic polyps based on H. pylori eradication following endoscopic resection.
Methods
We retrospectively reviewed the medical records of 201 patients with H. pylori infection who underwent endoscopic resection for gastric hyperplastic polyps at six medical centers. H. pylori status was assessed by histological analysis and a rapid urease test. A total of 149 patients underwent successful H. pylori eradication (eradication group), whereas 52 patients had persistent H. pylori infections (non-eradication group). The recurrence rate of gastric hyperplastic polyps and the risk factors according to H. pylori status were analyzed.
Results
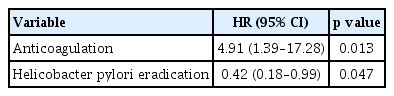
During the mean follow-up period of 18.3 months, recurrent gastric polyps developed after endoscopic resection in 10 patients (19.2% [10/52]) in the non-eradication group and 12 patients (8.1% [12/149]) in the eradication group. The cumulative incidence of recurrent gastric hyperplastic polyps was significantly higher in the non-eradication group than in the eradication group (p = 0.041, log-rank test). In the adjusted analysis, H. pylori eradication reduced the recurrence of gastric hyperplastic polyps (hazard ratio [HR], 0.42; 95% confidence interval [CI], 0.18 to 0.99), whereas anticoagulation therapy increased the risk of recurrence of gastric hyperplastic polyps (HR, 4.91; 95% CI, 1.39 to 17.28).
Conclusions
Successful eradication of H. pylori may reduce the recurrence of gastric hyperplastic polyps in patients after endoscopic mucosal resection.
INTRODUCTION
Recently, as endoscopy has become more accessible, the detection rate of gastric polyps has improved. Gastric polyps can be broadly described as lesions protruding into the stomach, but more precisely, as neoplastic or hyperplastic lesions arising from the mucosa of the stomach [1]. The gastric polyps most commonly identified by endoscopy are hyperplastic, fundic gland, and adenomatous. Gastric hyperplastic polyps often exhibit proliferation and expansion of the foveolar epithelium and glands. A well-known cause of these particular histological characteristics is a Helicobacter pylori infection [2]. In regions with high H. pylori prevalence, gastric hyperplastic polyps account for up to 75% of the gastric polyps detected [3].
Most gastric hyperplastic polyps are asymptomatic and are incidentally detected on endoscopy. Observation through regular follow-up is a possible course of action if the polyp is small. However, the risk of focal adenocarcinoma rises with an increase in the size of the polyp; a previous study reported this risk to be as high as 2.1% [4]. Therefore, endoscopic resection is recommended for symptomatic or large-sized gastric hyperplastic polyps. In general, for gastric hyperplastic polyps > 10 mm in size, endoscopic resection is recommended to exclude neoplastic foci and prevent neoplastic transformation [5]. The guidelines of the American Society of Gastrointestinal Endoscopy recommend endoscopic resection for gastric polyps > 5 mm [6].
As gastric hyperplastic polyps are associated with H. pylori infection, removal of the underlying conditions that cause mucosal damage can be effective. Hence, H. pylori eradication may be considered as a treatment option. Case-control studies and randomized controlled trials have reported that H. pylori eradication in patients with gastric hyperplastic polyps reduces the size of the polyp and improves inflammation in the gastric mucosa [7–9]. Guidelines on the management of H. pylori infection in Japan recommend H. pylori eradication in patients with multiple gastric hyperplastic polyps (evidence level II) [10]. Similarly, the British Society of Gastroenterology recommends testing and treatment for H. pylori when the presence of gastric hyperplastic polyps is noted (high evidence, definite recommendation) [11]. However, it is not well known whether H. pylori infection affects the recurrence of hyperplastic polyps after endoscopic resection. For this reason, we evaluated the recurrence rate of gastric hyperplastic polyps based on H. pylori eradication after endoscopic resection of gastric hyperplastic polyps.
METHODS
Patients
This study included patients endoscopically diagnosed with gastric hyperplastic polyps (≥ 0.5 cm) who underwent endoscopic resection at six tertiary-care referral centers in Korea from January 2011 to October 2018. An evaluation of electronic database records showed that endoscopic and pathological reviews were performed to confirm H. pylori infection, and cases in which H. pylori infection was either negative or could not be tested were excluded. Likewise, patients who did not undergo follow-up endoscopy after endoscopic resection were excluded from the study. Baseline characteristics, including age, sex, underlying disease, drug history, smoking status, and alcohol consumption were obtained from the patients’ medical records.
This study was approved by the Institutional Review Board of Kyungpook National University Hospital (KNUCH2019-07-011) and Soonchunhyang University Cheonan Hospital (SCHCA 2019-04-031). The requirement for patient consent was waived owing to the retrospective nature of the study.
H. pylori infection and eradication
H. pylori infection was confirmed using a rapid urease test (campylobacter-like organism [CLO] test) or cresyl violet staining of biopsy tissue obtained from the gastric antrum and body through endoscopy. If either test result was positive, the patient was considered positive for H. pylori infection. Currently, in Korea, H. pylori eradication therapy for gastric hyperplastic polyps does not fall under insurance coverage; therefore, it is administered according to the patient’s preference. The standard triple therapy (amoxicillin 1.0 g twice a day [bid], clarithromycin 0.5 g twice a day, proton pump inhibitor twice a day) was administered as first-line therapy according to the guidelines for the diagnosis and treatment of H. pylori infection in Korea [12]. H. pylori eradication was confirmed by the C13 urea breath test, 4 to 8 weeks after the eradication treatment. Bismuth-based quadruple therapy (proton pump inhibitor twice a day, tetracycline 500 mg four times a day, metronidazole 500 mg thrice a day, and bismuth subcitrate 120 mg four times a day) was administered as second-line therapy in patients who did not benefit from standard triple therapy. Patients who did not benefit from eradication therapy and those who did not undergo eradication therapy were defined as the non-eradication group, while those in which H. pylori eradication was successful after therapy were defined as the eradication group.
Endoscopic evaluation and follow-up
The polyps identified in this study were removed via polypectomy or endoscopic mucosal resection. The location of the lesion was classified as either in the body or antrum, and multiple polyps were defined as three or more polyps. Any polyp that was not removed by endoscopic resection was defined as a remnant gastric polyp. The size of the polyps was measured using endoscopically resected tissue. A pathologist examined resected tissue specimens. Gastric hyperplastic polyps were defined as hyperplastic polyps or foveolar hyperplasia based on the pathologic results. Recurrence was defined as the new occurrence of gastric hyperplastic polyps at the resection site or other sites on follow-up endoscopy. Additionally, the occurrence of neoplastic lesions such as dysplasia or adenocarcinoma was assessed. In remnant polyps, gastric polyp regression was defined as either the disappearance of polyps (defined as the complete disappearance of gastric hyperplastic polyps) or a reduction in polyps (defined as a greater than 50% decrease in the number of gastric hyperplastic polyps or a greater than 50% decrease in the size of the polyps). No change/new lesion/increase were defined as any response other than disappearance or reduction [13]. Endoscopic findings were analyzed by endoscopists with more than 5 years of endoscopic experience at each institution, and two endoscopists (S.Y.N. and Y.S.C.) were consulted when a decision was difficult.
Statistical analyses
The significance of the differences was determined using the chi-square test or t test, as appropriate. Kaplan-Meier analysis was performed to estimate the cumulative incidence of recurrent gastric hyperplastic polyps after endoscopic resection according to H. pylori eradication. Cox regression analysis with hazard ratios (HRs) and 95% confidence intervals (CIs) was performed to evaluate the statistical significance of the recurrence of gastric hyperplastic polyps. Statistical significance was set at p < 0.05. All data were analyzed using the SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Baseline characteristics of the enrolled patients
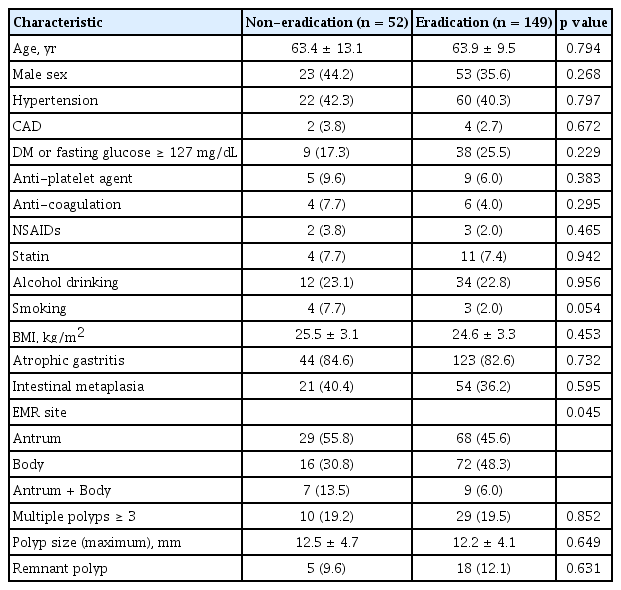
Between 2011 and 2018, the records of 380 patients who underwent endoscopic resection for gastric hyperplastic polyps were analyzed. Among the 380 patients, 143 had negative H. pylori test results, 28 did not undergo follow-up endoscopy, and 8 were not followed up after eradication treatment. These patients were subsequently excluded. Of the 201 patients included in the final analysis, 158 received H. pylori eradication therapy and 149 had successful eradications. Of the nine unsuccessful H. pylori eradications, four underwent bismuth-based quadruple therapy. Hence, 149 and 52 patients were classified into the eradication and non-eradication groups, respectively (Fig. 1). The mean age of the two groups was 63.4 ± 13.1 years in the non-eradication group and 63.9 ± 9.5 years in the eradication group (p = 0.79). There were no statistical differences between the two groups in terms of sex, diabetes, antiplatelet and anticoagulant use, smoking, or alcohol consumption (Table 1).
The endoscopic characteristics of the two groups are summarized in Table 1. The antrum was a more prevalent site for endoscopic resection in the non-eradication group than in the eradication group (55.8% vs. 45.6%, p = 0.045). The median maximum diameter of the lesion was 12.5 ± 4.7 mm in the non-eradication group and 12.2 ± 4.1 mm in the eradication group, with no significant differences between the two groups. There was no significant difference between the two groups regarding the presence of atrophic gastritis, intestinal metaplasia, multiple polyps, or remnant polyps.
Effects of H. pylori eradication on the recurrence of gastric hyperplastic polyps after endoscopic resection
During the follow-up period, the recurrence of gastric hyperplastic polyps was observed in 10 out of 52 patients (19.2%) in the non-eradication group and in 12 out of 149 patients (8.1%) in the eradication group (Table 2). In the Kaplan-Meier analysis, the cumulative incidence rate of recurrence of gastric hyperplastic polyps in the non-eradication group was higher than that in the eradication group (p = 0.041, log-rank test) (Fig. 2). When comparing the incidence of neoplastic lesions between the two groups, neoplastic lesions occurred in three patients (5.8%) in the non-eradication group and in one patient (0.7%) in the eradication group (p = 0.054). The recurrence of hyperplastic polyps or neoplastic lesions was significantly higher in the non-eradication group (12 [23.1%] in the non-eradication group and 13 [8.7%] in the eradication group).
Risk factors related to recurrence of gastric hyperplastic polyp
The demographic and endoscopic factors affecting the recurrence of gastric polyps were analyzed (Table 3). Multivariate analysis was performed using Cox regression analysis on the results of the univariate analysis and variables that may have had clinical significance (including age, sex, past medication history, and H. pylori eradication). As a result, multivariate analysis confirmed that both H. pylori eradication (HR, 0.42; 95% CI, 0.18 to 0.99) and anticoagulation (HR, 4.91; 95% CI, 1.39 to 17.28) affect the recurrence rate of hyperplastic gastric polyps (Table 4).

Comparison of clinical and endoscopic characteristics according to the recurrence of gastric hyperplastic polyps
DISCUSSION
In this retrospective multicenter study, the cumulative incidence of gastric hyperplastic polyp recurrence after endoscopic resection was higher in the non-eradication group compared to that in the eradication group. In the non-eradication group, gastric hyperplastic polyps predominantly recurred 1 to 2 years after endoscopic resection, whereas gastric polyps recurred gradually with time in the eradication group. H. pylori eradication therapy significantly reduces the recurrence of gastric hyperplastic polyps after endoscopic resection, whereas anticoagulation therapy increases the risk of recurrence.
This was a retrospective study that investigated whether H. pylori eradication therapy could prevent the recurrence of hyperplastic polyps after endoscopic resection. Our investigation showed that during a mean follow-up period of 18.3 months, gastric hyperplastic polyps recurred in 22 patients. The cumulative incidence of gastric hyperplastic polyps was higher in the non-eradication group (19.2%) than in the eradication group (8.1%). A reduction in the recurrence of gastric hyperplastic polyps, after H. pylori eradication, is associated with a reduction or loss of inflammation induced by H. pylori. Gastric hyperplastic polyps often occur as a result of a prominent, reparative, or regenerative phenomenon, and H. pylori infection is well known as a causative factor [14]. H. pylori infection causes damage to the gastric mucosa and increases the expression of inflammatory mediators such as cyclooxygenase-2, interleukin-1β, and hepatocyte growth factor [7,15,16]. It has been reported that these inflammatory mediators cause gastric epithelium proliferation and foveolar hyperplasia, resulting in gastric hyperplastic polyps [17,18].
In previous observational studies, approximately 90% of patients who underwent H. pylori eradication showed partial loss or complete regression of the lesion [19]. In a recent randomized controlled trial involving 27 patients with gastric hyperplastic polyps, all patients demonstrated polyp regression in the eradication group, whereas no regression was observed in the non-eradication group [13]. However, it is rarely reported whether the presence of H. pylori affects recurrence after endoscopic resection of gastric hyperplastic polyps. In a study conducted in Korea by Kang et al. [20], 79 patients were followed-up after endoscopic resection of gastric hyperplastic polyps. In this study, the recurrence rate of gastric hyperplastic polyps was higher in the H. pylori-persistent group (42.9%) than in the H. pylori-eradicated group (21.7%), but this was not statistically significant. Additionally, the previous study had limitations in that it was a single-center study with a small sample size. Therefore, our study provides sufficient evidence for the relationship between H. pylori infection and the recurrence of gastric hyperplastic polyps after endoscopic resection.
In this study, neoplastic lesions occurred in four patients during the follow-up period. The non-eradication group had a higher incidence rate (5.8%) of gastric dysplasia than the eradication group (0.7%), but the difference was not statistically significant. This was probably due to the small number of patients. However, previous studies have reported that H. pylori eradication reduces the incidence of gastric dysplasia. A retrospective study conducted on 282 patients diagnosed with gastric dysplasia reported that H. pylori eradication therapy was able to reduce the incidence of metachronous gastric neoplasms after endoscopic resection [21]. In contrast, a study on 129 patients diagnosed with gastric dysplasia reported that H. pylori eradication did not reduce the recurrence of gastric adenoma after endoscopic resection [22]. Gastric hyperplastic polyps are thought to be markers for mucosa that is predisposed to developing carcinomas. The incidence of adenocarcinoma developing in the background gastric mucosa, associated with a hyperplastic polyp (but not within the polyp), is 6% [23,24]. Therefore, it can be stated that H. pylori eradication in gastric hyperplastic polyps provides a secondary benefit of preventing gastric neoplasms as well as reducing the recurrence of gastric polyps. However, evidence for this is currently lacking, and further research is required.
Gastric hyperplastic polyps that are 0.5 to 1 cm or more in size are associated with an increased risk of neoplastic lesions; therefore, endoscopic resection and a thorough pathologic examination of the resected tissue are required [25]. According to the American Society for Gastrointestinal Endoscopy Guideline, gastric polyps ≥ 5 mm in size are known to increase the risk of neoplastic lesions [6]. In the case of small polyps, follow-up endoscopic examinations are performed according to the results of the histological examination. However, in a cohort study on gastric hyperplastic polyps less than 1 cm diagnosed through Korea’s national cancer screening program, 84% of the polyps disappeared in the group that received treatment for H. pylori and 34% regressed in the untreated group. Therefore, successful H. pylori eradication increases the possibility of the disappearance of hyperplastic polyps (adjusted odds ratio, 5.56; 95% CI, 2.63 to 11.11) [26]. A total of 23 patients had remnant polyps in this study.
This study demonstrated that anticoagulation therapy increased the risk of recurrence of gastric hyperplastic polyps, whereas age, sex, chronic disease, smoking, drinking, nonsteroidal anti-inflammatory drugs, statins, and polyp site were not associated with recurrence of gastric hyperplastic polyps after endoscopic resection. The association between anticoagulants and gastric hyperplastic polyps has never been reported. Therefore, it is difficult to elucidate a clear mechanism behind this association. Anticoagulants are used to treat underlying diseases, such as atrial fibrillation, and have been reported to be associated with atrial fibrillation and inflammatory gastrointestinal conditions. Additionally, it is possible to hypothesize that anticoagulants may influence inflammation and immune responses against H. pylori infection by inhibiting the coagulation cascade. Fibrin, an end product of the coagulation cascade, is known to play a protective role during infection by limiting bacterial dissemination and activating the antimicrobial properties of monocytes and macrophages [27,28]. A previous study showed an increased level of fibrin in the gastric mucosa of H. pylori-positive patients [29]. Therefore, the use of anticoagulants has the potential to induce an excessive inflammatory response to H. pylori infection, along with inhibition of the coagulation pathway. Further research on the relationship between the host immune response to H. pylori infection and the coagulation pathway is required.
As this was a retrospective study, selection bias was inevitable. However, this study may be of clinical significance in providing evidence for the usefulness of H. pylori eradication therapy after endoscopic resection of gastric hyperplastic polyps. Randomized controlled trials and large-scale studies on the prevention of gastric hyperplastic polyps after endoscopic resection are needed in the future. To our knowledge, this is the first report to show that anticoagulation therapy increases the risk of recurrence of gastric hyperplastic polyps. In the future, the association between anticoagulation therapy and gastric hyperplastic polyps should be investigated.
In conclusion, H. pylori eradication reduces the recurrence of gastric hyperplastic polyps after endoscopic resection. This study provides evidence of the usefulness of H. pylori eradication after endoscopic resection in patients with gastric hyperplastic polyps. Moreover, further studies are required to elucidate the association between anticoagulation therapy and hyperplastic gastric polyps.
KEY MESSAGE
1. Persistent Helicobacter pylori infection and use of anticoagulants were associated with the recurrence of hyperplastic gastric polyps.
2. H. pylori eradication reduced the recurrence of gastric hyperplastic polyps after endoscopic resection.
Notes
No potential conflict of interest relevant to this article was reported.