A retrospective observational study of the appropriate starting dose of febuxostat in patients with gout
Article information
Abstract
Background/Aims
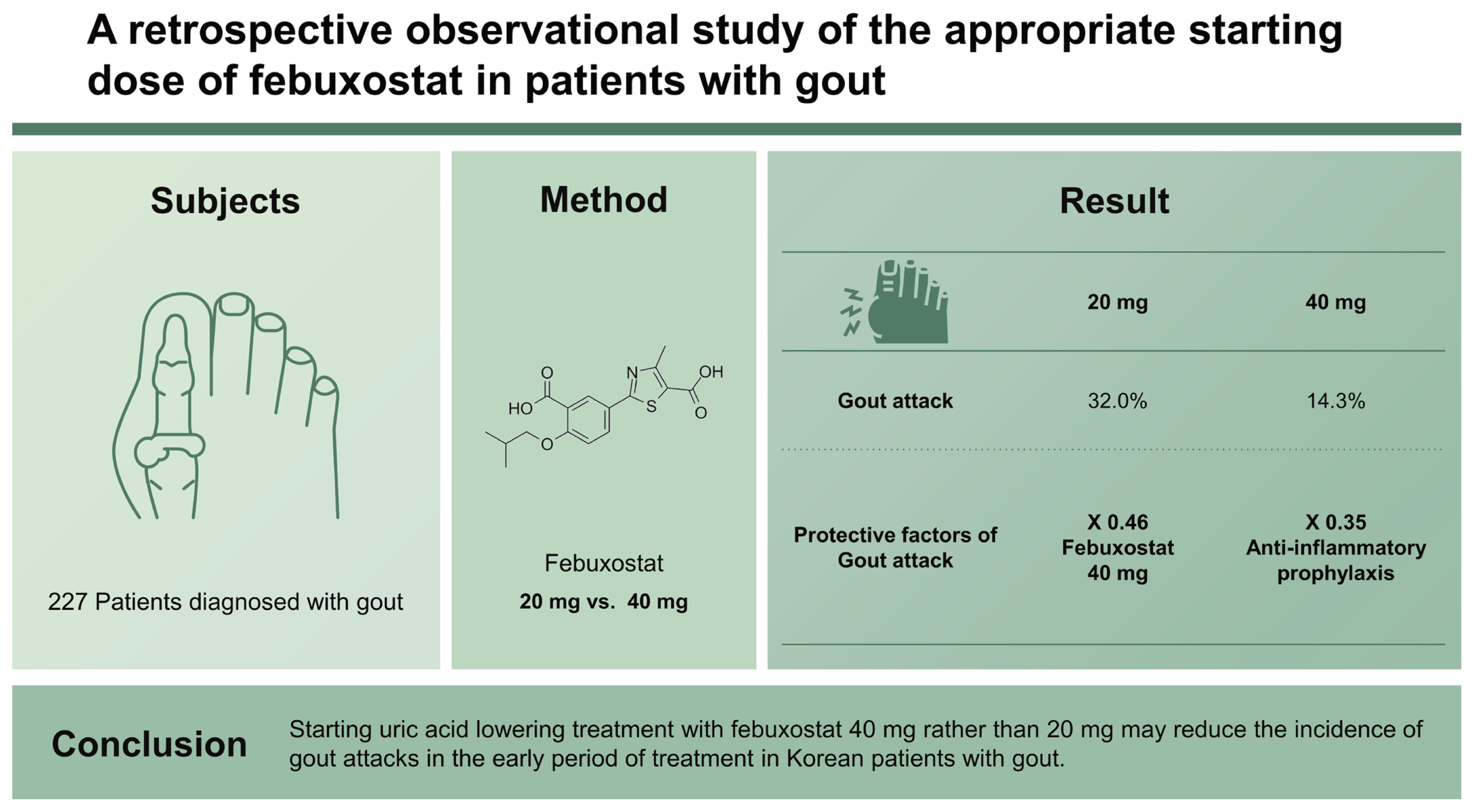
The occurrence of gout attacks at the start of uric acid lowering treatment worsens compliance. We aimed to determine the appropriate dose of febuxostat to reduce the occurrence of gout attacks during the initial treatment period.
Methods
We retrospectively analyzed the data of patients diagnosed with gout who underwent treatment at Jeju National University Hospital between May 2018 and May 2020.
Results
Two-hundred and twenty-seven patients were included, with a mean age of 53.2 ± 16.4 years, and 219 (96.5%) were male. The patients were divided into two groups according to the starting dose of febuxostat (20 mg vs. 40 mg). There were no significant differences in mean age, disease duration, colchicine, estimated glomerular filtration rate (eGFR), initial uric acid levels, and presence of subcutaneous tophi between the two groups. Gout attacks occurred more frequently in the 20 mg group than in the 40 mg group during the first 3 months of treatment (32.0% vs. 14.3%, p = 0.002), particularly during the first month (21.3% vs. 7.5%, p = 0.005). Multivariate logistic regression analysis was conducted adjusting for the effects of disease duration, the presence of subcutaneous tophi, eGFR, and initial uric acid levels. A febuxostat starting dose of 40 mg (odds ratio, 0.464; 95% confidence interval [CI], 0.246 to 0.862; p = 0.015) and anti-inflammatory prophylaxis (odds ratio, 0.359; 95% CI, 0.158 to 0.813; p = 0.014) were found to be independent factors associated with a gout attack.
Conclusions
Starting uric acid lowering treatment with febuxostat 40 mg rather than 20 mg may reduce the incidence of gout attacks in the early period of treatment in Korean patients with gout.
INTRODUCTION
Gout is the most common form of inflammatory arthritis and its incidence is increasing due to dietary changes [1]. The goal of gout treatment is to reduce the frequency of gout attacks and prevent complications, such as joint deformity due to tophi, kidney stones, renal failure, metabolic syndrome, and coronary artery disease [2–6]. Treatment guidelines and clinical specialists recommend starting treatment with low-dose uric acid lowering agents [7,8]. Gout attacks generally occur when there is an abrupt change in serum uric acid levels [9], for example when starting uric acid-lowering therapy [10]. Gout attacks occurring at the beginning of therapy negatively affect patient adherence to treatment. Furthermore, in the early stages of gout, there is a long interval between gout attacks so many patients voluntarily stop treatment [11]. However, discontinuation of treatment is associated with an increasing frequency of gout attacks and complications. Therefore, it is important to prevent the occurrence of gout attacks in the early phase of treatment so that patients adhere to the treatment regimen. This study aimed to determine the optimal febuxostat dose to reduce the incidence of gout attacks during the early phase of uric acid-lowering treatment in Korean patients with gout.
METHODS
This hospital-based observational study was approved by the Ethics Committee of the Institutional Review Board of Jeju University Hospital (approval number 2021-11-005). Written informed consent by the patients was waived due to a retrospective nature of our study.
Study design and patient selection
We retrospectively reviewed the data of patients attending Jeju National University Hospital between May 2018 and May 2020 with a diagnosis of gout and who continued treatment for more than 3 months. The diagnosis of gout was based on the 2015 American College of Rheumatology (ACR) gout classification criteria [12]. Patients who started uric acid lowering treatment with febuxostat were selected for analysis. According to the treatment starting dose, patients were divided into two groups (20 mg vs. 40 mg). We retrospectively analyzed patient medical records, medical history, physical examination, and test results.
Definition and drug
In this study, a gout attack was defined as a gout flare in a broad sense including a mobilization flare. Obesity was defined as a body mass index (BMI) of 25 kg/m2 or more according to the criteria of the Korean Society for the Study of Obesity [13]. Prophylaxis was defined as pharmacological anti-inflammatory prophylaxis for the prevention of gout attacks, and colchicine, nonsteroidal anti-inflammatory drugs, and prednisolone were used for anti-inflammatory prophylaxis. The dose of febuxostat was increased if uric acid levels of 6 mg/dL or less were not achieved at 1 and 3 months after the start of treatment.
Data collection
Blood test results including the uric acid levels were obtained by reviewing the medical records. At the time of diagnosis, comorbidity and disease duration were ascertained from the patient medical history and waist circumference, height, weight, and the presence of subcutaneous tophi on physical examination were recorded. Estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. BMI was calculated using height and weight measured at the time of diagnosis. Gout attacks occurring after the start of treatment were confirmed by patients at the outpatient visit.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and nominal variables were expressed as frequencies. The t test and the chi-squared test were used to determine the significance of the differences between the groups. Logistic regression analysis was performed to confirm the influence of predictors affecting the frequency of gout attacks. The independent variables included in the multivariate logistic regression analysis are variables that are known to affect gout attacks, those that were found to significantly affect gout attack occurrence in univariate logistic regression analysis (p < 0.05), or those which we anticipated would affect the frequency of gout attacks. A p < 0.05 was regarded as significant. All statistical analyzes were performed using IBM SPSS Statistics version 20.0 for Windows (IBM Co., Armonk, NY, USA).
RESULTS
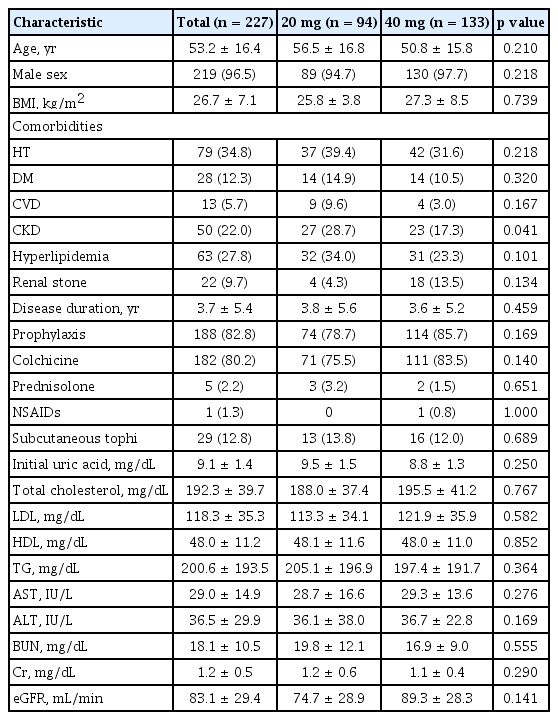
Two-hundred and twenty-seven patients were enrolled in this study. Patients mean age was 53.2 ± 16.4 years, and 219 patients (96.5%) were male. The mean duration of disease was 3.7 ± 5.4 years, and the initial uric acid concentration was 9.1 ± 1.4 mg/dL. Patients mean BMI was 26.7 ± 7.1 kg/m2, mean eGFR was 83.1 ± 29.4 mL/min and 60.1% of the patients were obese.
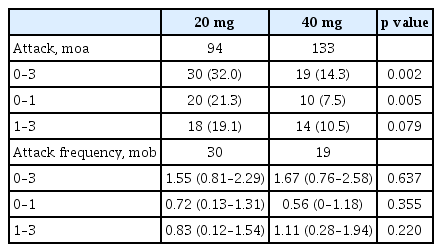
Forty-nine patients (21.6%) experienced gout attacks within 3 months of starting uric acid lowering treatment. These patients were categorized into two groups according to the starting dose of febuxostat (20 mg vs. 40 mg). There were no significant differences in the mean age (56.5 ± 16.8 years vs. 50.8 ± 15.8 years, p = 0.210), mean duration of disease (3.8 ± 5.6 years vs. 3.6 ± 5.2 years, p = 0.459), the number of patients treated with colchicine (69 [73.4%] vs. 111 [83.5%], p = 0.066), the number of patients with subcutaneous tophi (13 [13.8%] vs. 16 [12%], p = 0.689), eGFR (74.7 ± 28.9 mL/min vs. 89.3 ± 28.3 mL/min, p = 0.141), and initial uric acid levels (9.5 ± 1.5 mg/dL vs. 8.8 ± 1.3 mg/ dL, p = 0.250) (Table 1). There were no significant differences in comorbid conditions (hypertension, diabetes mellitus, cardiovascular disease, dyslipidemia, and renal stones), lipid profile, and liver function test results. The number of patients with gout attacks (30 [32.0%] vs. 19 [14.3%], p = 0.002) was significantly lower in the febuxostat 40 mg group during the first 3 months of treatment (Table 2).
When analyzing the incidence of gout attacks after the start of therapy, the number of patients with gout attacks between 0 and 1 month (20 [21.3%] vs. 10 [7.5%], p = 0.005) was significantly lower in the 40 mg febuxostat group; however, the number of patients with gout attacks between 1 and 3 months (18 [19.1%] vs. 14 [10.5%], p = 0.079) was not significantly different between the two groups. There was no significant difference between the two groups when comparing the frequency of gout attacks in patients with gout attacks (Table 2).
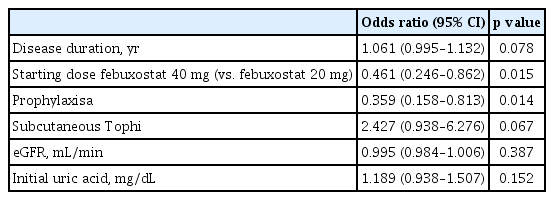
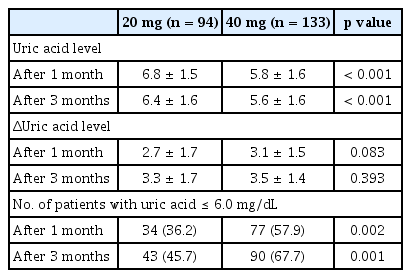
Regression analysis was performed to determine the factors influencing the occurrence of gout attacks. The results of univariate regression analysis showed that disease duration (odds ratio [OR], 1.064; 95% confidence interval [CI], 1.009 to 1.120; p = 0.021), subcutaneous tophi (OR, 2.350; 95% CI, 1.069 to 5.167; p = 0.034), and initial uric acid levels (OR, 1.254; 95% CI, 1.027 to 1.531; p = 0.026) were predictors of gout attacks. In addition, febuxostat 40 mg use (OR, 0.407; 95% CI, 0.232 to 0.715; p = 0.002) and anti-inflammatory prophylaxis (OR, 0.413; 95% CI, 0.205 to 0.833; p =0.013) were predictors of a lower occurrence of gout attacks. Multivariate regression analysis using these variables revealed that febuxostat 40 mg (OR, 0.461; 95% CI, 0.246 to 0.862; p = 0.015) and anti-inflammatory prophylaxis (OR, 0.359; 95% CI, 0.158 to 0.813; p = 0.014) predicted a lower occurrence of gout attacks even after adjusting for the effects of other variables (Table 3). Uric acid levels after the 1st month of treatment (6.8 ± 1.5 mg/dL vs. 5.8 ± 1.6 mg/dL, p < 0.001) and at 3 months following the initiation of treatment (6.4 ± 1.6 mg/dL vs. 5.4 ± 1.6 mg/dL, p < 0.001) were significantly lower in the febuxostat 40 mg group (Table 4). Although the change in uric acid levels was greater in the febuxostat 40 mg group this difference was not significant (Table 4).
Furthermore, the proportion of patients with uric acid levels below 6 mg/dL was significantly higher in the febuxostat 40 mg group in the first month of treatment (36.2% vs. 57.9%, p = 0.002) and at 3 months (45.7% vs. 67.7%, p = 0.001) (Table 4).
DISCUSSION
In countries with good access to medical care, such as Korea, it is important to provide education about gout and about reducing the frequency of gout attacks to improve treatment compliance [14]. The prevention of gout attacks in the early stage of treatment is crucial to ensure that patients continue treatment.
The ACR 2020 guidelines recommend starting febuxostat treatment with a low dose of 40 mg or less [8], and clinical experts also recommend starting febuxostat at a dose of 20 or 40 mg [7]. The results of our study show that in patients with gout, starting treatment with febuxostat 40 mg reduces the incidence of gout attacks after the commencement of therapy. Patients starting treatment with febuxostat 40 mg had significantly fewer gout attacks than with a febuxostat starting dose of 200 mg (20 [21.3%] vs. 10 [7.5%], p = 0.005) during the 1st month of treatment with no significant differences observed between the two groups thereafter. If patient uric acid levels were not within the target range at the 1st month follow-up, the dose of febuxostat was adjusted. Therefore, it is possible that there was no difference in the occurrence of gout attacks between 1 and 3 months.
Although there was an anti-inflammatory effect of prophylaxis in preventing gout attacks, there was no significant difference in the rate of prophylaxis between the two groups. In addition, even after adjusting for the effects of anti-inflammatory prophylaxis, starting treatment with a febuxostat dose of 40 mg lowered the risk of gout attacks more than starting with a 20 mg dose. We postulated that gout attacks would occur more frequently in patients starting treatment with febuxostat 40 mg than in those starting treatment with febuxostat 20 mg because of the large changes in uric acid levels. However, in this study, although a larger change in uric acid levels was observed in the febuxostat 40 mg group, the difference between the two groups was not significant. The proportion of patients who achieved the target uric acid level < 6 mg/dL was higher in the febuxostat 40 mg group. Therefore, starting uric acid-lowering treatment with febuxostat 40 mg does not cause a sudden change in uric acid levels resulting in gout attacks but may prevent gout attacks by improving uric acid level control.
Multivariate regression analysis revealed a febuxostat starting dose of 40 mg and anti-inflammatory prophylaxis were factors that could lower the risk of gout attack during the first 3 months of treatment. Unlike univariate regression analysis, multivariate analysis showed that disease duration, the presence of subcutaneous tophi, and eGFR were not independent factors influencing the occurrence of gout attacks. A previous study found a significant increase in the occurrence of gout attacks in the early period of treatment in patients with gout duration longer than 13.4 years [15]; however, in our study gout duration was shorter (3.7 years) and had no effect on the occurrence of gout attacks in the early period of treatment. Our results are consistent with the findings reported by Abhishek et al. [15] that the presence of subcutaneous tophi does not affect the risk of gout attacks following the start of treatment. Rothenbacher at al. [16] showed that chronic renal failure (chronic kidney disease [CKD] stage 3 or higher) increases the risk of gout attacks after treatment; However, most of the patients in our study were classified as CKD stage 1 and 2 (78%) so eGFR did not appear to affect the occurrence of gout attacks after the start of treatment.
This study has several limitations. First, as this was a retrospective study, BMI and renal function were evaluated using tests performed at the time of initial diagnosis. Additionally, the effects of alcohol consumption and smoking status could not be evaluated. However, because BMI and renal function do not generally change rapidly within 3 months, it is unlikely that changes in these variables affected the frequency of gout attacks. Second, there is a possibility that recall bias occurred because the frequency of gout attacks was investigated through questionnaires administered when patients attended outpatient clinics. However, since the intervals between outpatient visits were not long, and gout attacks were characterized by severe pain, it is unlikely that the patient misremembered the frequency of gout attacks. Third, a febuxostat starting dose of 20 mg may have been prescribed to patients with frequent gout attacks or very high uric acid levels resulting in selection bias. Fourth, it is not known whether a febuxostat starting dose of 40 mg is the most effective option because this has not been compared with starting doses of 60 or 80 mg. However, clinical guidelines also recommend starting treatment at a low dose. When treatment is initiated with a dose higher than 40 mg, target uric acid levels can be achieved more quickly, although sudden fluctuations in uric acid levels can induce gout attacks. Furthermore, only 15.9% of patients ultimately required more than 60 mg of febuxostat. Therefore, we considered that it would be more effective to start treatment with febuxostat 40 mg and increase the dose if necessary.
Our study evaluated the association between the starting dose of febuxostat (20 mg vs. 40 mg) and gout attacks during the first 3 months after the initiation of uric acid lowering treatment. It is important to note that we identified an appropriate therapeutic dose for the Korean population characterized by a smaller physique than that of Westerners.
In conclusion, initiation of uric acid lowering treatment with febuxostat 40 mg, compared with febuxostat 20 mg, in patients with gout can reduce the occurrence of gout attacks in the early period of treatment.
KEY MESSAGE
1. In patients with gout, starting uric acid-lowering treatment with febuxostat 40 mg reduced the occurrence of gout attacks in the early period of treatment more than starting treatment with febuxostat 20 mg.
2.Febuxostat 40 mg achieved target uric acid levels more effectively than febuxostat 20 mg.
3. The use of febuxostat 40 mg rather than 20 mg combined with anti-inflammatory prophylaxis could lower the risk of gout attacks in the early period of treatment.
Acknowledgments
We thank Sehee Kim for support in reviewing and compiling research data.
Notes
CRedit authorship contributions
Joondon Lee: data curation, formal analysis, writing - original draft; Jinseok Kim: data curation, writing - review & editing; Byeongzu Ghang: data curation; Wooseong Jeong: conceptualization, data curation, formal analysis, funding acquisition, methodology, writing - original draft, writing - review & editing
Conflicts of interest
The authors disclose no conflicts.
Funding
This work was supported by a research grant from Jeju National University Hospital in 2019.