Capecitabine and temozolomide for metastatic intermediate to high-grade pancreatic neuroendocrine neoplasm: a single center experience
Article information
Abstract
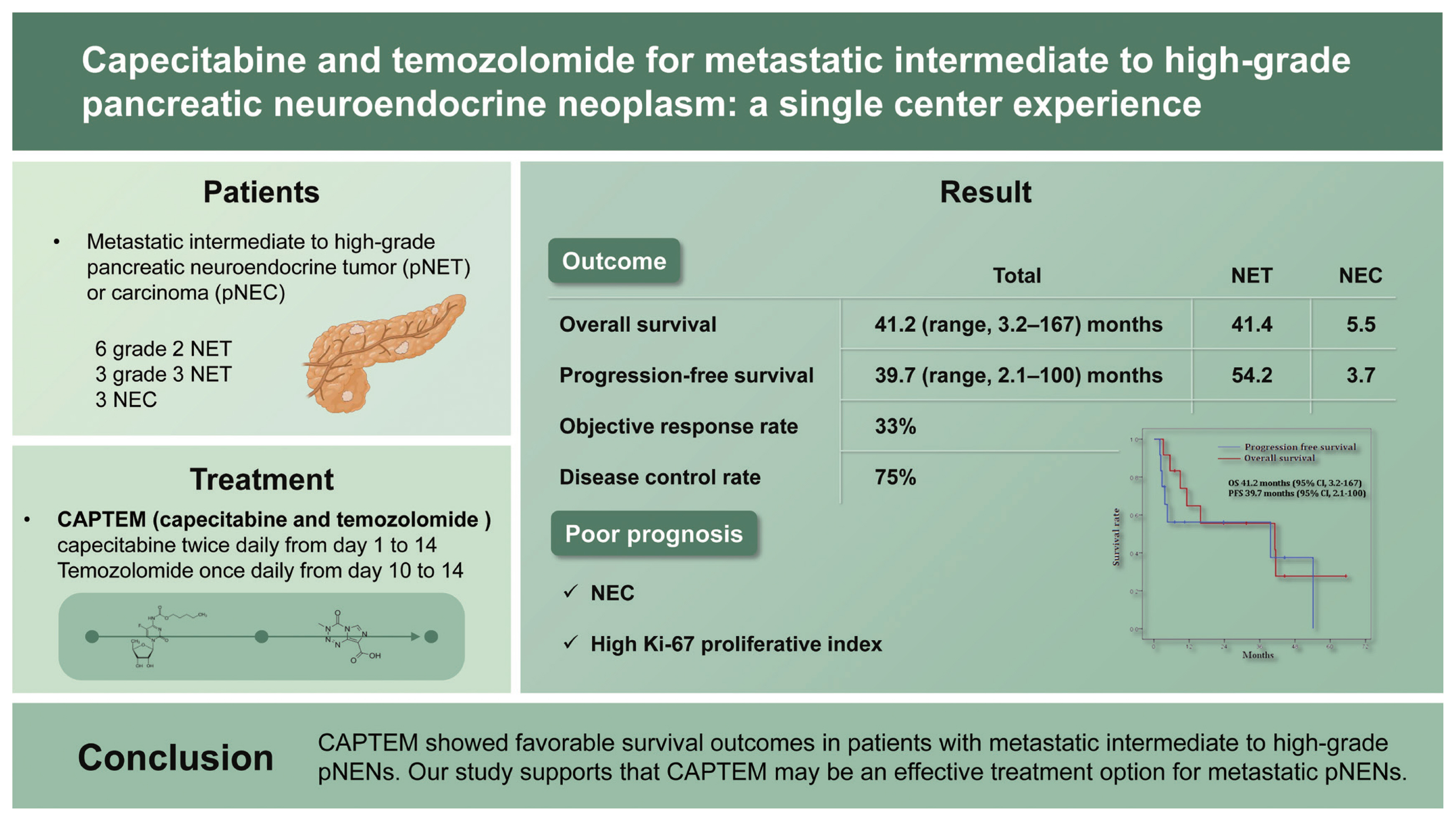
Background/Aims
The combination of capecitabine and temozolomide (CAPTEM) is one of the treatment options for metastatic pancreatic neuroendocrine neoplasms (pNENs). This study aims to evaluate the efficacy of CAPTEM in patients with metastatic intermediate to high-grade pancreatic neuroendocrine tumor (pNET) or carcinoma (pNEC).
Methods
This study was conducted retrospectively in a single center. Patients were treated for intermediate to high-grade tumor with 750 mg/m2 of capecitabine twice daily from day 1 to 14 and 200 mg/m2 of temozolomide once daily from day 10 to 14, repeating twice in a cycle of 28 days. The primary outcomes were durations of overall survival (OS) and progression-free survival (PFS). The secondary outcomes consisted of objective response rate and disease control rate.
Results
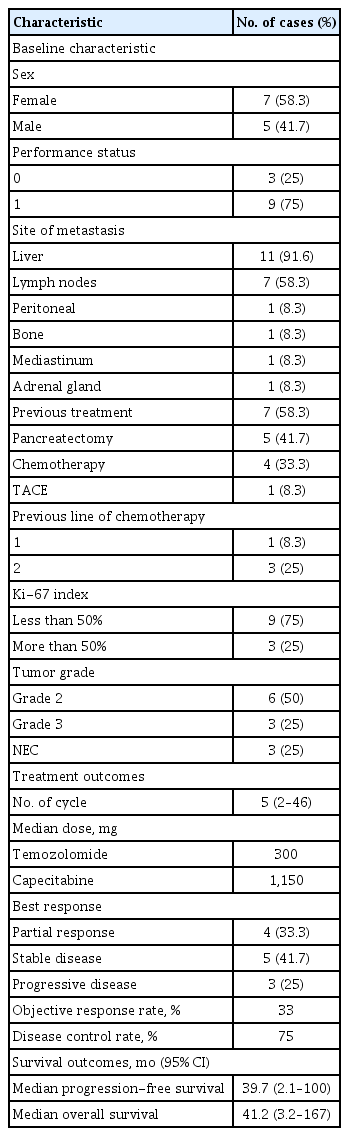
A total of 12 patients (grade 2 NET in six, grade 3 NET in three, NEC in three patients) who received CAPTEM were included in this study. Patients received a median of five cycles (range, 2 to 46) of CAPTEM. The median dose combined 1,150 mg of capecitabine and 300 mg of temozolomide. The median OS and PFS were 41.2 months (range, 3.2 to 167) and 39.7 months (range, 2.1 to 100), respectively. Patients with NET had longer OS and PFS compared to those of patients with NEC (p = 0.002 and p = 0.028). High Ki-67 proliferative index (> 50%) was significantly associated with poor survival outcomes.
Conclusions
CAPTEM showed favorable survival outcomes in patients with metastatic intermediate to high-grade pNENs. Our study supports that CAPTEM may be an effective treatment option for metastatic pNENs.
INTRODUCTION
Pancreatic neuroendocrine neoplasms (pNENs) are rare neoplasms, which account for approximately 1% to 2% of all pancreatic tumors. The incidence of pNEN has been increasing in recent decades. While cytotoxic chemotherapy is rarely used for lower grade (grade 1) NETs, it is an essential treatment option for metastatic intermediate (grade 2) to high-grade (grade 3) NETs or neuroendocrine carcinomas (NECs) [1].
Although there is no consensus on the ideal cytotoxic chemotherapy for pNEN, streptozocin/5-fluorouracil, temozolomide, and capecitabine and temozolomide (CAPTEM) have been used for metastatic pNEN in general. In patients treated with streptozocin/5-fluorouracil, progression-free survival (PFS) and overall survival (OS) were reported as 23 and 52 months, respectively [2]. Patients using temozolomide alone or CAPTEM showed similar PFS of 11 to 20 months, with a slightly higher response rate in those using CAPTEM [3,4].
Previous studies have demonstrated high and durable response rates of CAPTEM in metastatic low and intermediate-grade pancreatic neuroendocrine tumor (pNET) [5]. Although a previous single-center report showed that CAPTEM exhibited improved survival rates also in metastatic intermediate to high-grade pancreatic and extra-pancreatic NETs, its utility in Asian population with metastatic intermediate to high-grade pNET or pancreatic NEC has not yet been established well [6]. Therefore, this study aims to evaluate the efficacy of CAPTEM in metastatic intermediate to high-grade pNENs in the Republic of Korea.
METHODS
Study design and patients
This study was conducted retrospectively in a single center. Patients who received at least one cycle of CAPTEM for metastatic pNEN from September 2014 to July 2020 at the pancreatobiliary and liver cancer center of National Cancer Center were included in this study. Patients with the Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 were considered for inclusion. Patients who rejected to give informed consent or receive treatment were excluded. Patients were further divided into subgroups by tumor differentiation (NET or NEC), tumor grade (grade 2 or grade 3) and Ki-67 index (< 50% or ≥ 50%) for subgroup analysis. Tumor tissues were obtained from primary or metastatic sites using endoscopic ultrasound-guided biopsy or ultrasound-guided percutaneous core needle biopsy for histopathologic diagnosis. The grading of pNEN was assessed and classified based on the 2017 World Health Organization classification [7]. Patient information such as demographics (age and sex), previous treatment, histopathological and radiologic data (computed tomography [CT], magnetic resonance imaging [MRI], positron emission tomography scans) was collected from the patients’ electronic medical records. The cut-off date for data analysis was August 16, 2021. This study was conducted by following the Declaration of Helsinki, and the protocol was approved by the Institutional Review Boards at the National Cancer Center in Korea (IRB No. NCC2022-0069). Written informed consent by the patients was waived due to a retrospective nature of our study.
Treatment and assessments
Patients were treated with 750 mg/m2 of capecitabine twice daily from day 1 to 14 and 200 mg/m2 of temozolomide once daily from day 10 to 14, repeating such procedure twice in a cycle of 28 days [8]. Dose reduction or schedule adjustment was performed in consideration of individual patient’s general condition and toxicity at the discretion of the clinician. Patients were evaluated with a blood test after every cycle of chemotherapy to monitor the level of toxicity. Response to treatment was assessed every 8 weeks through CT and/or MRI images according to the guidelines of Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Treatment-related adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v.5.0).
The primary outcomes were OS, defined as the duration from the start of CAPTEM treatment to the patient’s death of any cause or the last follow-up, and PFS, defined as the duration from the start of the CAPTEM regimen to disease progression or the last follow-up. The secondary outcome consisted of treatment response. Objective response rate (ORR) was defined as the percentage of patients with complete or partial response. Disease control rate (DCR) was defined as the percentage of patients showing complete response, partial response, or stable disease.
Statistical analysis
All statistical analyses were performed using SAS systems version 9.2 (SAS Institute Inc., Cary, NC, USA). Continuous variables were compared by independent Students’ t analysis. Non-continuous variables were compared by chi-square or Fisher’s exact test. Survival curves for OS and PFS were estimated using the Kaplan-Meier method and compared using the log-rank test. Median PFS and median OS were expressed with 95% confidence intervals (CIs). Analyses with subgroups were performed to evaluate the PFS, OS, ORR and DCR, stratified by Ki-67 proliferative rate and tumor grade. The cox proportional-hazards model was used to identify further individual factors associated with the prognosis. Unadjusted and adjusted hazard ratios (HRs) were presented with 95% CIs.
RESULTS
A total of 12 patients treated with CAPTEM were included in our study. The baseline characteristics of patients are listed in Table 1. The median age of patients was 62 years old (range, 38 to 74). Five (41.7%) patients were male. Seven (58%) patients previously received treatment for pNENs prior to this study. Five (41.7%) patients underwent primary curative-intent surgical resection. Out of four (33.3%) patients that received previous chemotherapy, three patients were previously treated with second-line chemotherapy before this study. Transcatheter arterial chemoembolization for multiple liver metastases was previously performed in one patient. The most common distant metastasis site was liver (67%), followed by lymph node (50%) and peritoneum (17%). Half of the patients had a histologic result of grade 2 NET. There were three (25%) patients with grade 3 NET and three (25%) patients with NEC.
The treatment details are presented in Fig. 1 and Supplementary Table 1. Patients received a median of 5 cycles of CAPTEM (range, 2 to 46). The median dose consisted of 1,150 mg of capecitabine and 300 mg of temozolomide. The best response was partial response for four patients, stable disease for five patients, and progressive disease for three patients. These results indicated that the ORR and DCR were 33.3% and 75%, respectively. Four patients have continued the CAPTEM regimen at the cut-off point (Fig. 1).
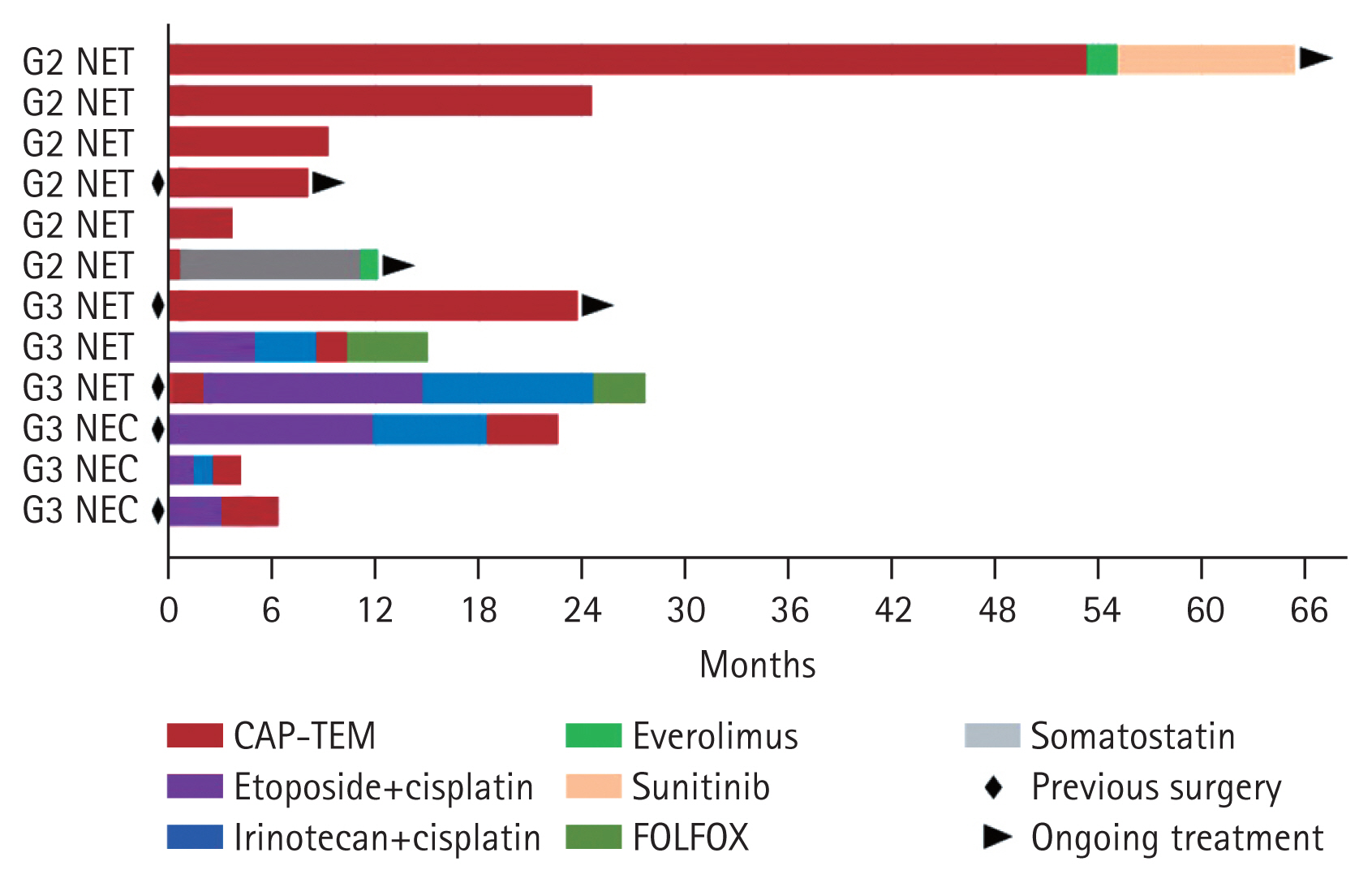
Treatment summary. NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma; CAPTEM, capecitabine and temozolomide; FOLFOX, fluorouracil, leucovorin, and oxaliplatin.
After the median follow-up duration of 44.5 months (range, 2.3 to 65.3), seven (58.3%) patients experienced disease progression or death. The median OS and PFS were 41.2 months (95% CI, 3.2 to 167) and 39.7 months (95% CI, 2.1 to 100), respectively (Fig. 2). We analyzed the survival outcomes according to the subgroups. Patients with NET had longer OS (41.4 months vs. 5.5 months, p = 0.002) and PFS (54.2 months vs. 3.7 months, p = 0.028) compared to patients with NEC. In regard to the tumor grade, patients with grade 2 showed longer survival outcomes than patients with grade 3. However, statistical significance was not achieved in OS (41.4 months vs. 11.2 months, p = 0.133). We additionally compared survival outcomes according to the cut-off level of Ki-67 (50%), resulting in a statistically significant difference between OS (41.4 months vs. 11.2 months, p = 0.031) and PFS (54.2 months vs. 2.8 months, p = 0.014).
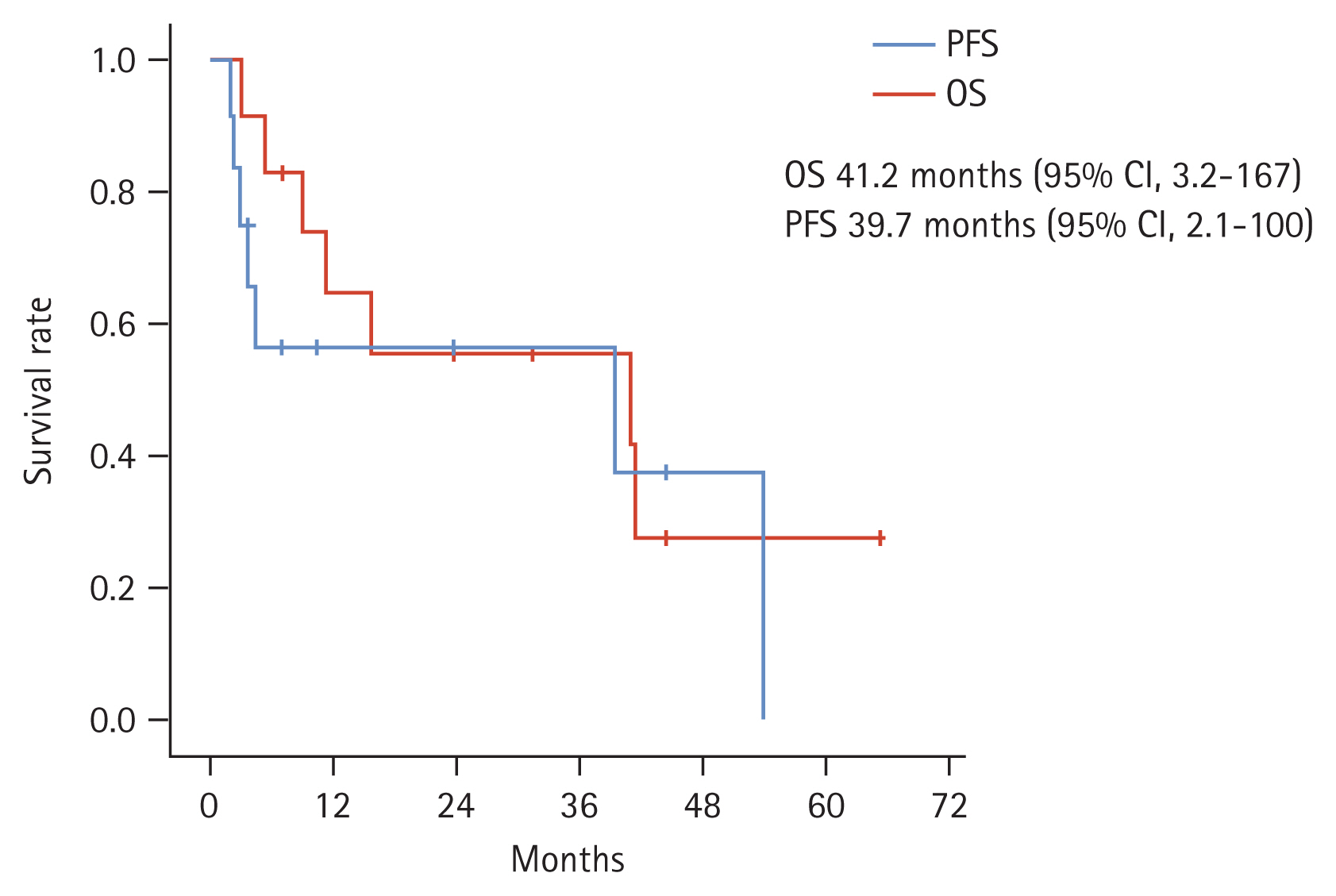
Survival plots for overall survival (OS) and progression-free survival (PFS). CI, confidence interval.
We explored the associated factors of survival outcomes (Table 2). Factors such as metastasis to two or more organ sites (HR, 8.95; 95% CI, 1.06 to 75.5; p = 0.044) and poorly differentiated NEC (HR, 16.3; 95% CI, 1.65 to 161; p = 0.017) were significantly associated with poor OS. A high Ki-67 proliferative index of over 50% was also associated with poor OS, but a statistical significance showed marginal (HR, 5.82; 95% CI, 0.96 to 35.4; p = 0.056). High Ki-67 (> 50%) was the only factor significantly associated with poor PFS (HR, 7.09; 95% CI, 1.16 to 43; p = 0.034).
A total of eight patients exhibited treatment-related adverse events of all severity: headache in two, anorexia in three, nausea/vomiting in two, pruritus in one and arthralgia in one patient. Among four patients that experienced adverse events of grade 3 or higher, two patients experienced thrombocytopenia, one experienced anemia, and one experienced obstructive jaundice.
DISCUSSION
This retrospective study revealed that CAPTEM showed favorable oncologic outcomes for patients with metastatic pNETs. The ORR and DCR were 33.3% and 75%, respectively. The median OS and PFS were 41.2 and 39.7 months, respectively. Factors such as poorly differentiated NEC and Ki-67 > 50% were significantly associated with poor OS outcomes. We also presented a patient exhibiting dramatic response and a prolonged survival after receiving the CAPTEM treatment. Most toxicities were manageable and rarely associated with cessation of treatment.
CAPTEM is one of the available options of systemic cytotoxic therapy for advanced pNEN. Our oncologic outcomes for CAPTEM were comparable to those in previous studies. According to a recent study of 116 patients with advanced NEN, consisting of 47 (41%) patients with pNEN and 18 (16%) with poorly differentiated tumors, the median OS, ORR, and DCR were reported as 35 months, 38%, and 77% in pNENs [9], respectively, similar to our data. Another recent retrospective study evaluating CAPTEM in 100 patients with advanced NET reported the median OS, ORR, and DCR as 75.2 months, 51%, and 87%, respectively, which showed better survival outcome compared to our study [4]. Such difference in survival outcomes may be due to the discrepancy in patient severity between the two studies; while the majority of patients (74%) in this study had grade 2 NET, half of our patients had more severe diagnoses of grade 3 NET or NEC.
There is limited data on the efficacy of CAPTEM for grade 3 pNENs. A retrospective study of 32 patients with grade 3 NENs treated with CAPTEM reported longer median OS and PFS in patients with grade 3 NET than in patients with grade 3 NEC (OS, 22 months vs. 4.6 months; PFS, 15.3 months vs. 3.3 months, respectively) [10]. In our study, we also demonstrated that survival outcomes were poor in patients with NEC and grade 3 NEN. A high Ki-67 index (> 50%) was a consistent prognostic factor for both OS and PFS according to our subgroup and univariate analysis. The proliferative index can predict the response to CAPTEM and the resulting survival in patients with grade 3 NENs. Another study that analyzed the improvement of PFS in patients with low grade 3 (Ki-67 < 55%) and high grade 3 (Ki-67 ≥ 55%) receiving CAPTEM demonstrated the survival benefits of CAPTEM in patients with low grade 3 tumor [6]. Therefore, CAPTEM may be beneficial for pNEN with Ki-67 < 55% [9].
Our study also reported a notable case, in which a patient exhibited dramatic response to the CAPTEM regimen. A 42 years old male who presented to our clinic with abdominal pain showed extensive liver masses in abdominal CT (Fig. 3A). After percutaneous liver biopsy, he was pathologically diagnosed with grade 2 (Ki-67 index of 3%) pNET. The patient received systemic chemotherapy with 1,000 mg of capecitabine twice daily from day 1 to 14 and 300 mg of temozolomide daily from day 10 to 14. After three cycles of CAPTEM, a follow-up CT scan showed dramatic reduction in size and decrease in number of liver metastases, which became visibly indiscernible during subsequent treatments (Fig. 3B). He experienced disease aggravation after a total of 46 cycles of CAPTEM and was still alive with a 65-month survival after receiving third-line systemic treatment (sunitinib followed by everolimus).

(A) Initial abdominal computed tomography (CT) scan before capecitabine and temozolomide (CAPTEM) treatment and (B) abdominal CT scan after 46 cycles of CAPTEM.
Our study has several limitations. First, this analysis was performed with a small sample size at a single center, with the lack of multivariate analysis. Thus, caution is necessary in interpretation and generalization of our results. The rarity of pNEN hinders the recruitment of patients and hence, further research with a larger sample size in multicenter studies is required. Although we conducted this study retrospectively, patients who received CAPTEM were enrolled in a consecutive order, thus, making effort to minimize selection and recall bias.
Genomic sequencing may serve as a breakthrough that overcomes difficulties of researching rare diseases such as pNEN [11]. Somatic mutations of MEN1 tumor suppressor gene, as well as mutations in alpha-thalassemia/mental retardation X-linked (ATRX) and death domain-associated protein (DAXX) genes, occur in almost half of sporadic pNENs. Approximately 15% of pNENs have somatic mutations in genes related with the mammalian target of rapamycin (mTOR) pathway [12]. Although this study does not include any analysis on genomic sequencing, further collecting and analyzing genome and/or transcriptome data of pNENs may identify subtypes of pNENs and find potential response predictors of the CAPTEM treatment.
In conclusion, through our analysis the CAPTEM regimen proved durable survival outcomes in patients with metastatic pNENs. Most toxicities of CAPTEM were manageable. Our study supports that CAPTEM may be an effective treatment option for patients with intermediate to high-grade (grade 2 or grade 3) pNENs. Further research is warranted to compare the efficacy of CAPTEM to that of other systemic therapies.
KEY MESSAGE
1. Capecitabine and temozolomide (CAPTEM) showed favorable survival outcomes in patients with metastatic intermediate to high-grade pancreatic neuroendocrine neoplasms.
2. A high Ki-67 index (> 50%) can predict poor survival outcomes for CAPTEM.
Acknowledgments
The present study was supported by grants from the National Cancer Center, Korea (Grant numbers 1910190, 2212470-1). We thank the patients, their families, investigators, site staff and researchers that have participated in this study.
Notes
No potential conflict of interest relevant to this article was reported.