The efficacy of denosumab in Korean male patients with osteoporosis
Article information
Abstract
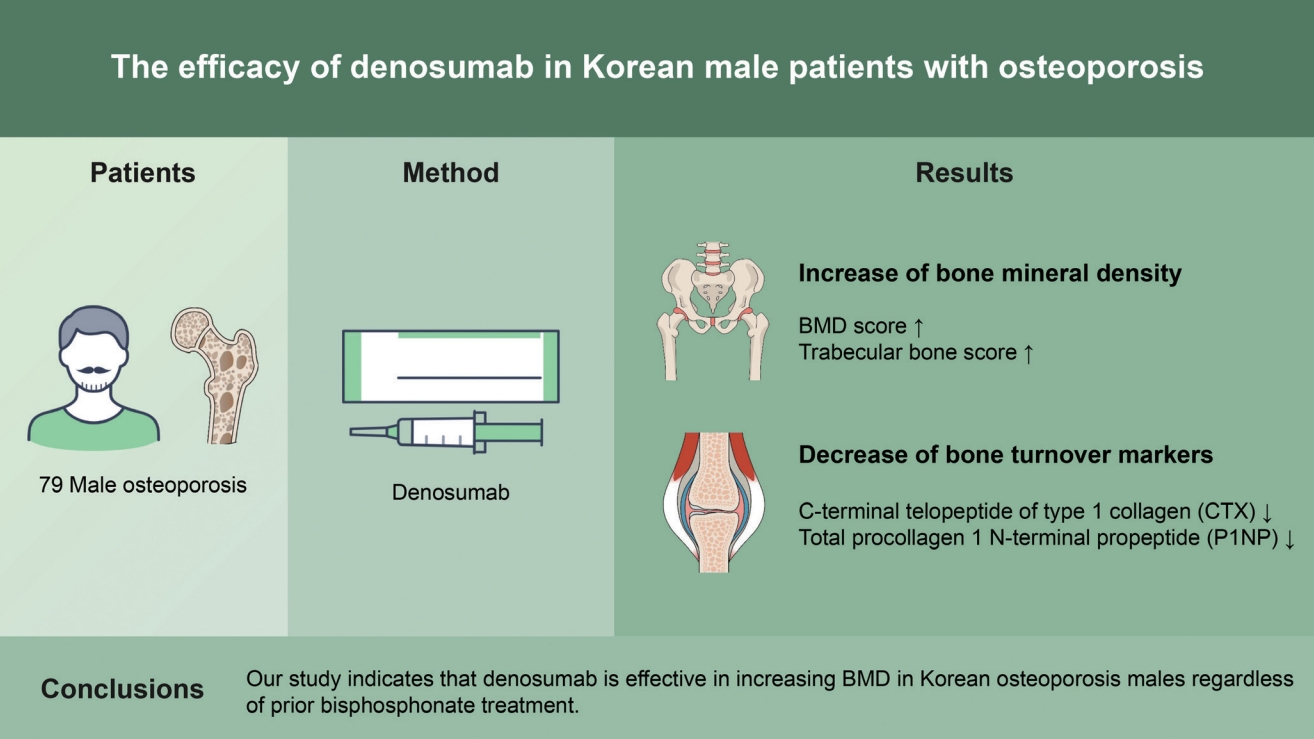
Background/Aims
Despite the prominence of denosumab as the number one prescribed anti-osteoporosis drug in Korea, the effects of denosumab in male osteoporosis patients were not researched sufficiently. Moreover, concerns on rebound vertebral fractures associated with poor denosumab adherence exist.
Methods
We retrospectively evaluated 147 Korean male osteoporosis patients treated with denosumab. After 12 months of treatment, 60 patients were lost during follow-up, and eight were excluded due to missing data. Out of the initial 147 patients, 79 were considered eligible for the analysis of the efficacy of denosumab. 54 patients were initially drug-naïve, and 25 had previously received bisphosphonate therapy.
Results
In 54 drug-naïve patients, significant increases in bone mineral density (BMD) were observed in all measurement sites: 5.2% ± 3.7% in the lumbar spine, 2.3% ± 2.8% in the femoral neck, and 1.9% ± 2.8% in the total hip (p < 0.01, respectively). Trabecular bone score showed an increase of 0.5% ± 5.8% in drug-naïve patients. Likewise, in 25 patients with previous bisphosphonate treatment, increase in BMD were observed as well: 4.8% ± 3.5% in the lumbar spine, 1.4% ± 3.6% in the femoral neck, and 0.8% ± 2.1% in the total hip (p < 0.01, p = 0.06, p = 0.06, respectively). Significant declines of −55.1% ± 31.8% in C-terminal telopeptide of type 1 collagen (CTX), and −62.9% ± 21.3% in total procollagen 1 N-terminal propeptide (P1NP), in drug-naïve patients; and −37.7% ± 41.5%, in CTX and −55.4% ± 30.1%, in P1NP in patients with previous bisphosphonate treatment were exhibited after 12 months of treatment. The adherence rates of the second and third dosing schedules were 79.9% and 56.8%, respectively.
Conclusions
Our study indicates that denosumab is effective in increasing BMD in Korean osteoporosis males regardless of prior bisphosphonate treatment.
INTRODUCTION
Osteoporosis is a major disease that affects more than 200 million people worldwide [1]. Osteoporosis leads to a decrease in bone strength, thus increasing the risk of fracture and leading to notable morbidity and deterioration in the quality of life [2,3]. Whereas the risk of osteoporosis-related fractures has been extensively studied in women, there are less available data on men, leading to the inevitable underdiagnosis of male osteoporosis.
Eight out of 10 male subjects with osteoporosis in Korea did not take anti-osteoporosis medication [4]. Although men do not experience the rapid bone loss present at menopause in women, they go through substantial amounts of bone loss with aging. This results in low bone mass and microstructural worsening with a subsequent susceptibility to fracture. In Korea, the number of osteoporotic fractures in males has gradually increased since 2008 [4]. Globally, an estimate of 39% of all osteoporotic fractures occurs in men over the age of 50 [5]. By 2050, the incidence of hip fracture in men is expected to rise by 310% worldwide, a significantly higher increase compared to that in women (240%) [6]. The importance of osteoporosis treatment in men should be socially evoked, since mortality after hip fracture is higher in men than in women at any point of lifetime [7,8].
Currently, there are several approved options for the treatment of osteoporosis in men. Bisphosphonates (BPs), teriparatide, and denosumab are approved for osteoporosis in men in Europe, the United States and South Korea. However, the data from clinical trials are scarce, and many currently known effects of antiosteoporotic drugs in men have been derived from studies in women.
Denosumab, the first-approved biologic agent for the treatment of osteoporosis, is a potent antiresorptive drug that led to a significant reduction in the risk of hip, vertebral, and non-vertebral fractures [9]. In September 2012, denosumab was approved by the Food and Drug Administration (FDA) to increase bone mass in male osteoporosis of high fracture risk—defined as a history of osteoporotic fracture or multiple risk factors for fracture—or in treatment failure or intolerance to other available osteoporosis therapies [10]. Likewise in Korea, denosumab gained insurance coverage as a primary treatment for osteoporosis in April 2019. According to the most recent update from the Health Insurance Review and Assessment Service in Korea, denosumab is the number one prescribed anti-osteoporosis drug, accounting for 30% of the whole market share in osteoporosis treatment. Regarding male patients only, the use of denosumab has nearly doubled by 2020, occupying 12.2% of the market share.
The effect of denosumab on bone mineral density (BMD) has currently not been verified in not only Korean but also Asian males. In addition, no study has reported the efficacy of denosumab in male osteoporosis patients on bone microarchitectural texture. Furthermore, while BPs had been the most widely used anti-osteoporosis drug in the past, numerous patients previously on BPs have switched to denosumab. However, no current study has evaluated the effect of denosumab in male patients previously on BPs.
Moreover, adherence to denosumab is crucial for the benefit of therapy. Concerns about loss of gained BMD and rebound vertebral fractures associated with non-adherence to denosumab injection exist [11]. However, there has been a lack of reports on the rate of adherence to denosumab in Korean males.
Thus, this study aims to determine the effectiveness of denosumab on BMD and bone microarchitecture in male osteoporosis patients, arranged into treatment-naïve and prior BPs receiving groups. Adherence to denosumab therapy was evaluated together.
METHODS
Data sources
We retrospectively evaluated 147 male patients with osteoporosis receiving denosumab treatment in Yeouido St. Mary’s Hospital and Seoul St. Mary’s Hospital. Patients treated with denosumab for the first time from April 2019 to December 2020 were enrolled in this research. The inclusion criteria were males aged 50 years or older diagnosed with osteoporosis. Participants with cardiac, liver or renal disease; endocrine or metabolic abnormalities; or inflammatory disease were excluded. 60 patients were lost during follow-ups and eight were excluded due to missing data. Out of the initial 147 patients, 79 had their follow-up BMD values after 12 months of treatment, and were considered eligible for the analysis of the efficacy of denosumab (Fig. 1).
Adherence was defined as receiving the second and third denosumab injections, i.e., at 6 and 12-month (with an allowable delay of another 8 weeks) after the date of the index administration. Out of the initial 147 patients, 139 without missing data were evaluated for the rate of adherence.
Measurement
The BMD of the lumbar spine, femoral neck, and total hip were measured in grams per square centimeter using dual-energy X-ray absorptiometry (DEXA) (Hologic Horizon, Hologic Inc., Bedford, MA, USA). The hip and femoral neck references were created by adjusting the National Health and Nutritional Examination Survey white male reference data downward by 10.01%. The anteroposterior spine reference was based on measurements of the L2–L4 lumbar region from a population based study of 5,627 native Japanese subjects developed in coordination with the Japan Society for Bone and Mineral Research. The coefficient of variation according to precision was determined to be 1.2% at the lumbar spine and 1.9% at the femoral neck in Yeouido St. Mary’s Hospital. The variation was 1.0% for the lumbar spine, 1.5% for the femoral neck, and 0.9% for the total hip in Seoul St. Mary’s Hospital. Trabecular bone score (TBS) was used to assess bone microarchitectural texture [12–14]. TBS was assessed only in drug-naïve patients. TBS has been recommended by international guidelines as an additional tool to identify and improve patients at risk for fracture and to monitor therapeutic interventions [15]. All TBS measurements were performed using TBS iNsight software version 3.0.2.0 (Medimaps Group, Geneva, Switzerland). We used a conservative estimation of the least significant change of 5.8% for TBS, based on the largest published value [16]. BMD and TBS were assessed at the baseline and 12 months after the initial administration of denosumab. Blood samples were collected after overnight fasting, and biochemical tests such as serum cross-linked C-terminal telopeptide of type 1 collagen (CTX), total procollagen 1 N-terminal propeptide (P1NP), serum 25(OH) vitamin D, albumin, calcium and phosphate were checked before denosumab therapy and every 6 months.
Treatment schemes
Patients underwent DEXA every 12-month interval after the first administration of denosumab. Denosumab (60 mg, Amgen Inc., Thousand Oaks, CA, USA) was administered subcutaneously in the upper arm every 6 months. All participants were under supplementation of an average of 1,000 IU vitamin D and 750 mg of oral calcium carbonate. This study was approved by the Institutional Review Board of the Catholic University of Korea (KC21RISI0868). Written informed consent by the patients was waived due to a retrospective nature of our study.
Statistical analysis
All data were statistically analyzed using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). All descriptive data are presented as mean ± standard deviation for continuous measures and percentages for categorical measures. A p ≤ 0.05 was considered to be statistically significant. The comparison of the means and proportions was performed with independent sample t test or the Mann-Whitney test. The comparison of categorical variables was performed with Fisher’s exact test or chi-square analysis. Percentage changes in BMD and TBS were calculated as the absolute change from the baseline to follow-up divided by the baseline value. The Wilcoxon signed-rank test or paired T test was employed to evaluate the differences in percentage change from the baseline.
RESULTS
General baseline parameters
The baseline characteristics of patients who had 12 months of denosumab treatment are summarized in Table 1. All 79 participants were male patients aged 50 to 91, and the mean age was 68.9 ± 9.9. Nineteen (24.0%) patients had a history of fracture in at least one skeletal site. Fifty-four participants were anti-osteoporotic drug-naïve patients, and their baseline bone turnover markers (BTMs) were 0.479 ± 0.269 ng/mL for CTX and 68.9 ± 58.7 ng/mL for P1NP. Twenty-five patients had undergone prior BPs treatment before denosumab. Their mean duration of BPs treatment was 30.9 ± 21.2 months. The mean duration from the end of BPs treatment to the start of denosumab was 6.5 ± 10.2 months. More than half of patients who received prior BPs treatment switched to denosumab due to the low efficacy of BPs (28%) or due to the fear of long-term use induced complications (28%). The BTMs in prior BPs treatment group were 0.302 ± 0.364 ng/mL for CTX and 70.0 ± 97.4 ng/mL for P1NP. The baseline TBS was 1.312 ± 0.091 in drug-naïve patients. No statistically significant difference could be found between the study groups’ baseline characteristics except for the level of 25-hydroxyvitamin D.
Change in BMD and BTM
After 12 months of denosumab treatment, significant increases in BMD were observed in all measurement sites compared to the baseline in drug-naïve patients, as demonstrated in figures of 5.2% ± 3.7% in the lumbar spine, 2.3% ± 2.8% in the femoral neck, and 1.9% ± 2.8% in the total hip (p < 0.01, respectively). TBS showed an increase of 0.5% ± 5.8% in drug-naïve patients. In patients with previous BPs treatment, each BMD resulted as 4.8% ± 3.5% in the lumbar spine, 1.4% ± 3.6% in the femoral neck and 0.8% ± 2.1% in the total hip (p < 0.01, p = 0.06, p = 0.06, respectively). The increases in BMD were greater in patients in drug-naïve group than in patients with previous BPs treatment group without statistical significance (Fig. 2). CTX and P1NP significantly declined after 12 months of treatment in both groups: −55.1% ± 31.8% in CTX and −62.9% ± 21.3% in P1NP (p < 0.01, respectively) in drug-naïve patients; and −37.7% ± 41.5% in CTX and −55.4% ± 30.1% in P1NP (p < 0.01, respectively) in patients with previous BP treatment. CTX and P1NP showed a sharp decrease for the first 6 months and then remained declined up to 12 months (Fig. 3).
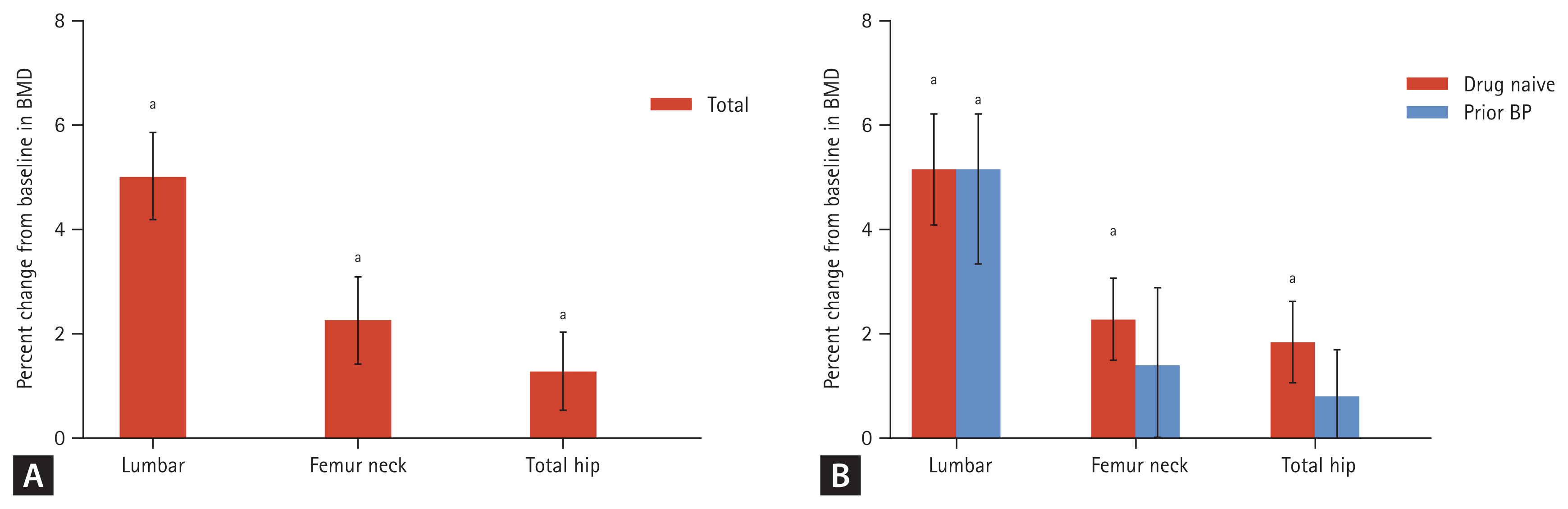
Bone mineral density (BMD) percentage change at 12 months after treatment with denosumab (A) in total patients, (B) in drug-naïve/prior bisphosphonate (BP) treatment groups. Error bars represent the 95% confidence interval of the mean. ap < 0.05.
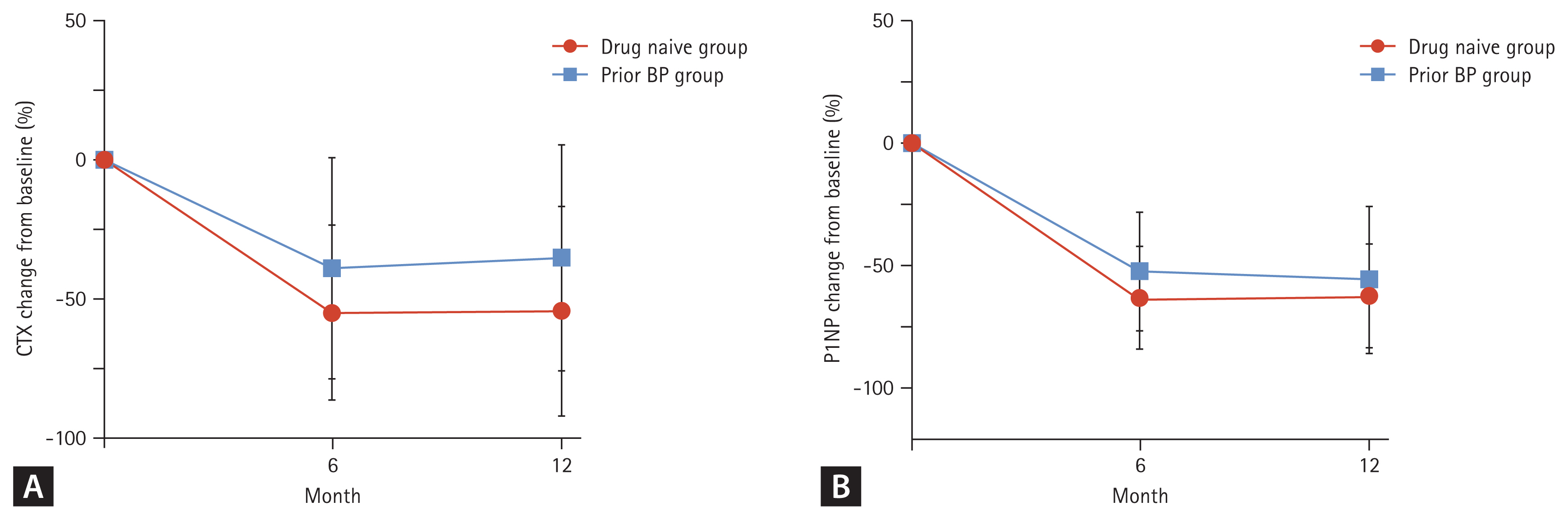
(A) Change of cross-linked C-terminal telopeptide of type 1 collagen (CTX) after 12 months of denosumab in drug-naïve (n = 27) and prior bisphosphonate (BP) treatment (n = 12) groups. (B) Change of total procollagen 1 N-terminal propeptide (P1NP) after 12 months of denosumab in drug-naïve (n = 20) and prior BP treatment (n = 6) groups.
Adverse events and changes in T score
No significant change in the corrected calcium level was observed during the treatment. Serious adverse drug reactions that required drug discontinuation were not reported. There was no report of severe hypocalcemia involving admission, osteonecrosis of the jaw, fracture healing complication or atypical femoral fracture. The lowest T score of under −2.5 is a value provided by the World Health Organization as the diagnosis of osteoporosis. After 12 months of treatment with denosumab, the lowest T scores exceeded −2.5 in 12.2% of drug-naïve patients and 13.6% of prior BPs patients.
Adherence to the dosing schedule of denosumab therapy
139 patients (94 drug-naïve and 45 with prior BPs treatment) started treatment with denosumab (eight patients with missing data excluded). In total, 79.9% of patients were found to be adherent to the second dosing schedule. To the third dosing schedule, 56.8% of patients were adherent. Subgroup analysis revealed that adherence rates were 75.5% and 88.9% at the second dosing schedule and 58.5% and 53.3% at the third dosing schedule in drug-naïve and prior BPs treatment patients, respectively. Thus, adherence rates were not significantly different between the two patient groups (p = 0.69) (Fig. 4).
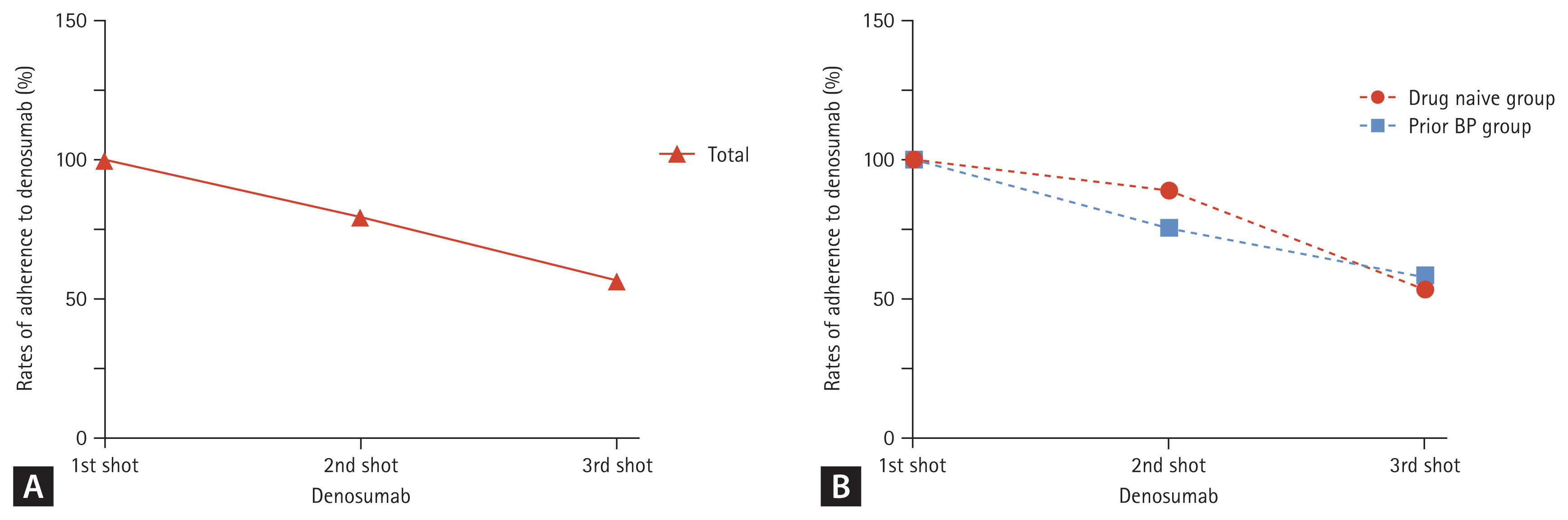
Rate of adherence to denosumab at 2nd and at 3rd dosing schedules. Adherence was defined as receiving the subsequent injection within 6 months + 8 weeks of the previous injection. (A) Adherence of total candidate at baseline (n = 139). (B) Adherence in drug-naïve (n = 94) and prior bisphosphonates (BPs) treatment group (n = 45).
DISCUSSION
In this retrospective cohort study, denosumab effectively increased BMD in male osteoporosis patients after 12 months of treatment. Currently, only few studies evaluated the efficacy and safety of denosumab in a population of men with low BMD. Our results showing a significant increase of BMD after 12 months of treatment (5.2% in the lumbar spine, 2.3% in the femoral neck, and 1.9% in the total hip) correspond well with the results of the A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Compare the Efficacy and Safety of Denosumab Vs Placebo in Males with Osteoporosis (ADAMO) study [17], which included 117 men with low BMD. Our increase in the lumbar spine was at a similar percentage to that expected from the ADAMO study (around 5.7%) while the annual femur neck BMD increase was somewhat higher than that from the ADAMO study (around 2.1%) [17]. Plus, our data were quite similar to the increases in BMD observed in Smith et al. [18], which involved prostate cancer patients with low bone mass or a history of fragility fracture, receiving androgen deprivation therapy (HALT trial) and those in Makras et al. [19], which evaluated the effect of denosumab in male HIV-infected patients (5.8% in the lumbar spine and 3.0% in the femoral neck). The same results were shown regarding BTM. Similar to our research, in which serum CTX level decreased by −55.1% after 6 months and −54.6% after 12 months in denosumab-naïve group, serum CTX levels declined by −65% after 6 months and −60% after 12 months in ADA-MO study [17].
Bone loss in most men is mainly the result of a negative remodeling balance due to reduced bone formation, often resulting in trabecular thinning, whereas bone loss in women is due to increased bone resorption, resulting in trabecular separation [20,21]. Interestingly, although bone metabolism differs between men and women, our study demonstrated that the increase of BMD with denosumab treatment in males were comparable to that in postmenopausal women with osteoporosis (3.0% to 6.7% at the lumbar spine and of 1.9% to 3.6% at the total hip) according to previous studies [9,22], suggesting that denosumab is effective regardless of sex.
To the best of our knowledge, there has not been any research on sequential BPs and denosumab therapy in male osteoporosis patients. Thus, many currently known effects of denosumab in males after prior BPs has been derived from studies in women. Effects of denosumab in postmenopausal women with prior BPs treatment demonstrated blunted increases in BMD compared to those who were drug-naïve. Kendler et al. [23] showed a 3.03% increase in the lumbar spine and a 1.90% increase in the total hip in postmenopausal women who were treated with alendronate for 34.5 months before the denosumab injection. Corresponding results were reported in a recent study [24] in 321 post-menopausal women with 6.2 months of prior BPs therapy. Similarly, in our study with male osteoporosis patients, prior BPs patients showed blunted increases in BMD compared to those of drug-naïve patients (4.8% increase in the lumbar spine, 1.4% in the femoral neck, and 0.8% in the total hip). Such discrepancy in the outcomes may be attributed to the difference of the study populations. Thirty-six percent of our study subjects did not switch to denosumab treatment immediately after the end of BPs use; those with prior BPs treatment had a mean cessation of 6.5 months before starting denosumab. Although blunted, denosumab showed an increase of BMD in males that had switched from prior BPs treatment.
BMD accounts for only 60% to 70% of bone strength [25]. DEXA only measures the mineral component of the bone and does not offer insight into bone microarchitecture, which is an important element for fragility fractures, leading to a newly emerged interest in bone microarchitecture. TBS has been recommended by international guidelines as an additional tool to identify and improve patients at risk for fracture and to monitor therapeutic interventions [16]. In previous studies, denosumab exhibited a 0.6% to 1.9% increase of TBS from the baseline after 12 months of therapy in postmenopausal women [26,27]. Similarly, our study exhibited 0.5% ± 5.8% increase of TBS in men with osteoporosis. Antiresorptive agents such as denosumab increase BMD through mineralization of the bone matrix without increasing the matrix volume itself responsible for microarchitecture [28]. Our result, lower than the least significant change in TBS, is in accord with Tsai et al. [29] which reported insignificant changes in trabecular microarchitecture after 24 months of denosumab treatment.
Adherence in our study at the third dosing schedule of denosumab was as less as 56.8%, indicating that four to 10 male patients on average are lost to follow-up after a year of their first injection. No currently available study has shown the adherence of denosumab in males alone so far. In previous studies targeting women, the adherence to denosumab in 12 months was estimated to range from 64% to 82% [30–33]. The poorer adherence of the present study might have resulted from the difference in sex. Previous researches that have explored adherence to osteoporosis therapies have reported conflicting findings on men; while some found men less likely to be adherent [34], others suggested otherwise [35]. Therefore, further analysis on such issue is necessary. Furthermore, our research was mainly investigated in the era of COVID-19, which may have served as a factor for patients’ hesitancy to visit hospitals and clinics for ongoing management of osteoporosis [36], resulting in a lower adherence rate.
As previously mentioned, the termination of denosumab without further bone treatment tends to increase the risk of rebound fracture. Clinicians need to take the poor adherence in male osteoporosis patients into account in the use of denosumab.
This is the first study identifying the effect of denosumab in Asian osteoporosis male patients on BMD in drug-naïve and in prior BPs treatment patients. There are several limitations in this study. First, the current analysis did not include placebo patients. Secondly, the sample size was insufficient to demonstrate significant statistical difference in all BMD areas in both patient groups. Longer duration of assessment would have made the increase of BMD more apparent, especially in cortical bone rich region. Thirdly, long-term effects of denosumab on the prevention of fractures in male patients were not reported. However, since the reduction of fracture risk was associated with the increase of BMD according to the Fracture Reduction Evaluation of Denosumab in Osteoporosis study [9] and HALT trial [18], additionally noting the similarity of the mean increase in BMD between our current and aforementioned studies, it is plausible to suggest that denosumab reduces fracture risks in men with osteoporosis.
In conclusion, our study indicates that denosumab is effective and safe in Korean males with osteoporosis regardless of receiving prior BPs treatment. Twelve months of denosumab therapy in men with osteoporosis increased BMD at all skeletal sites and reduced BTMs. Denosumab therapy was well-tolerated, and the effect of denosumab was meaningful for the treatment of disease in men. However, the adherence to denosumab was poorer than estimated. Physicians should be careful to increase compliance when administering denosumab.
KEY MESSAGE
1. In men with osteoporosis, denosumab treatment demonstrated a significant increase in bone mineral density and decrease in bone turnover markers.
2. In men with osteoporosis, denosumab treatment was effective in not only anti-osteoporosis drug naive patients but also in patients previously treated with bisphosphonate.
Notes
No potential conflict of interest relevant to this article was reported.