Perioperative and anesthetic management of patients with rheumatoid arthritis
Article information
Abstract
Our understanding and management of rheumatoid arthritis (RA) have greatly improved, but perioperative and anesthetic management remain challenging. RA is not limited to joints; systemic evaluation is thus required when planning perioperative management. Especially, careful airway evaluation is needed; management of airway-related arthritis is challenging. A multidisciplinary approach is essential to prevent complications without exacerbating RA disease activity. Guidelines published in 2017 are available for perioperative management of anti-rheumatic medication in patients with rheumatic diseases undergoing elective total hip or total knee arthroplasty. However, the guidelines focus only on anti-rheumatic medications, and do not consider all aspects of perioperative management (including anesthesia). Here, we discuss the perioperative and anesthetic management of patients with RA.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disorder that mainly affects the synovial joint [1,2]. However, the precise etiology remains unknown, although the inflammation is autoimmune in nature. RA is characterized by symmetrical erosive polyarthropathy, most commonly of the joints of the hands and feet [3]. However, RA can affect internal organs, i.e., can be systemic. Patients with RA commonly require orthopedic surgery under anesthesia for RA management or surgery unrelated to RA [4]. This study reviews perioperative and anesthetic issues in RA patients.
CLINICAL FEATURES, DIAGNOSIS, AND MANAGEMENT OF RA
RA typically presents as persistent and painful joint swelling with morning stiffness. Usually, the small joints of the wrist and hand are involved, but the atlanto-axial joints and large joints (such as those of the hip and knee) may also be affected. Joint inflammation and destruction with muscle atrophy and weakness cause pain. Progressive joint synovial inflammation triggers cartilage degradation and bone erosion, and ultimately joint destruction with laxity/disintegration of the tendon and ligament [3]. For example, the hand joints exhibit swan neck and boutonniere deformities, which may require surgical correction [5]. The diagnosis of RA is partly clinical (joint involvement and long symptom duration) and partly based on laboratory findings. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for RA include the presence of autoantibodies (rheumatoid factor and anti-cyclic citrullinated peptide antibody) and an acute phase reactant (C-reactive protein), along with a high erythrocyte sedimentation rate [6,7]. The goal of management is clinical remission or maintenance of low-level disease activity via early aggressive use of conventional and targeted synthetic agents, and biological disease-modifying anti-rheumatic drugs (DMARDs) [8]. These improve symptoms, inhibit disease progression, maintain joint function and facilitate the performance of activities of daily living [9].
PRE- AND INTRA-OPERATIVE CONSIDERATIONS
Detailed RA history-taking, including RA disease severity and duration, systemic involvement, and medication status, is essential to avoid missing a subtle symptom or sign [10]. Careful physical examination, especially of the airway, is also essential when planning anesthesia. A low body mass index (“rheumatoid cachexia”) may indicate sustained, poorly controlled disease. As RA increases morbidity and mortality, mechanical ventilator support should be considered postoperatively [4]. Apart from joint disabilities, systemic complications and current medications must be reviewed prior to anesthesia. The goals of anesthetic management are establishment of a safe airway with preservation of cervical spine integrity (via careful positioning and manipulation); successful management of any systemic condition, and prevention of RA progression and disease related-complications.
RA-related joint problems
The joint deformities caused by bone and cartilage destruction impact anesthetic management as follows. First, joint deformities affect patient positioning and hinder vascular assessment and evaluation of regional anesthesia. The need for appropriate positioning during surgery requires additional surgical support and time, which is associated with postoperative adverse effects. Finally, axial joint deformities render the cervical spine unstable, which makes intubation difficult. However, RA patients with axial joint disorders are often asymptomatic, so careful assessment thereof is essential prior to anesthesia.
Airway-related joint disorder
1) Atlanto-axial subluxation
The range of motion (flexion and extension) of the cervical spine should be assessed preoperatively. Attenuation of the transverse ligament and erosion of the odontoid process can cause atlanto-axial subluxation (AAS) and, in turn, atlanto-axial instability and spinal cord compression, with or without compression of the vertebral arteries [4]. During airway management, neurological complications (including quadriplegia and sudden death) can occur, but are fortunately rare. The anesthesiologist must be aware that the standard sniffing position for endotracheal intubation using a laryngoscope involves head hyperextension and neck flexion, and may thus exacerbate AAS and cause neurological injury [11]. The clinical symptoms of an AAS neurological disorder are difficult to distinguish from those of simple joint or muscle disorders and peripheral neuropathy. Moreover, the proportion of patients with such symptoms is low. Based on plain radiographs, the prevalence of AAS was estimated as 3% to 44% [12,13]. Preoperative plain radiography of the cervical spine has been recommended for all RA patients, as it can reveal cervical spine instability [14]. However, no evidence-based guidelines or consensus have emerged. If a plain radiograph reveals narrowing of the atlanto-axial joint space, airway management may be difficult [15]. Anesthetic management should be based on radiographic data; it is essential to prevent or minimize neurological and vascular complications. Magnetic resonance imaging (MRI) is better for assessing the cervical spine than radiography, although the data correlate weakly with clinical symptoms. If a neurological sign is present, cervical spine MRI is essential. The risk of AAS increases over time and is higher in seropositive patients and those with more severe RA. Involvement of the sub-axial cervical spine is less common than AAS, and limitation of cervical spine motion makes central venous access difficult.
2) Temporomandibular joint disorders
Temporomandibular joint disorders can be associated with difficulties in mouth-opening and mastication, but may also be asymptomatic. However, careful airway examination, including using the Mallampati test with the mouth open and mandible protruding, is essential to prevent airway accidents. If an RA patient exhibits cervical spine instability, or a limitation of neck motion or mouth-opening, airway management may be difficult; awake fiberoptic bronchoscopy-guided endotracheal intubation is recommended in such cases [16].
3) Cricoarytenitis
Cricoarytenitis is associated with hoarseness, stridor, and a foreign body sensation in the larynx; a laryngeal mass is also occasionally observed. When one of these symptoms or signs is observed, the larynx should be directly examined. Difficulty in airway management should be anticipated such that appropriate equipment is available. Endotracheal intubation should be avoided if possible; if not, the endotracheal tube should have the smallest possible diameter. Use of a face mask or supra-glottic airway device should be preferred. Awake fiberoptic bronchoscopy-guided endotracheal intubation is sometimes recommended for such patients, although surgical tracheostomy under local anesthesia is an alternative [17]. Close observation of airway obstruction caused by edema is required during recovery from extubation.
4) Other joint disorders with skin lesions
A joint deformity, or thin and fragile skin makes intravenous assessment difficult. A wrist joint deformity may render the radial artery inaccessible. Preoperatively, the range of motion of affected joints should be assessed to optimize patient positioning during and after surgery. Careful positioning during surgery is essential. All pressure points should be secured to avoid pressure sores. Chronic steroid use triggers osteoporosis; such patients must be manipulated gently. Eye care should also be considered; 15% of RA patients suffer from keratoconjunctivitis and are thus at risk of corneal ulceration, especially when prone [18].
Regional anesthesia obviates the need for airway management. However, joint deformities make it difficult to palpate anatomical landmarks. Central neuraxial block using non-steroidal anti-inflammatory drugs (NSAIDs) does not increase the risk of hematoma. All procedures must be aseptic; immunosuppressive agents increase the risk of infection.
RA-related systemic complications
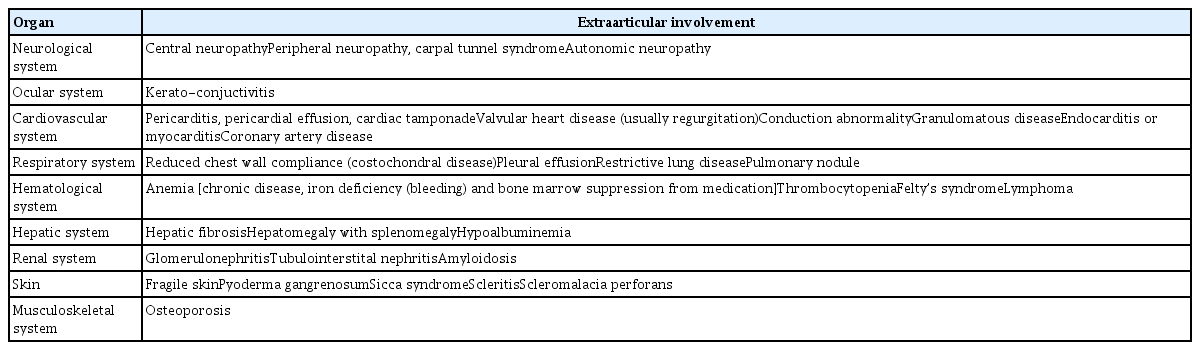
RA-related systemic complications were summarized in Table 1. Although no preoperative evaluation guideline is available for RA patients, RA does not appear to increase the risk of perioperative mortality [19]. Careful preoperative evaluation of extraarticular complications is required when planning anesthesia. Arterial blood gas analysis, a pulmonary function test, and echocardiography may be required, depending on the systemic complications. As RA patients do not exhibit the classical symptoms and signs of cardiovascular disease, it is easy to miss the disease. RA patients are at increased risk of atherosclerosis (stroke and myocardial infarction) [20,21]. The presentation is usually insidious and triggers sudden cardiac death [22]. Heart failure is a major cause of death in the absence of a history of ischemic heart disease, in contrast to non-RA patients [23,24]. A higher body mass index is associated with a higher cardiovascular mortality rate in the general population. However, RA patients with a lower body mass index have a higher likelihood of cardiovascular mortality due to rheumatoid cachexia and sustained and poorly controlled chronic inflammation [25]. Cardiac arrhythmia is an important cause of sudden death in RA patients; it is associated with rheumatoid nodules, amyloidosis, and ischemic heart disease [22]. The chronic inflammation seen in RA is likely to affect the vessels, although this remains to be confirmed. Anemia is common in RA patients, and is attributable to chronic disease or medications such as glucocorticoids and NSAIDs. RA patients are more likely to require perioperative blood transfusions. The effects of RA on the pharmacokinetics and pharmacodynamics of the drugs used to induce anesthesia remain poorly understood. RA-associated systemic disorders (especially hepatic and renal disorders) and RA medications change drug pharmacokinetics and pharmacodynamics. Therefore, the drugs should be carefully titrated.
Medications for RA
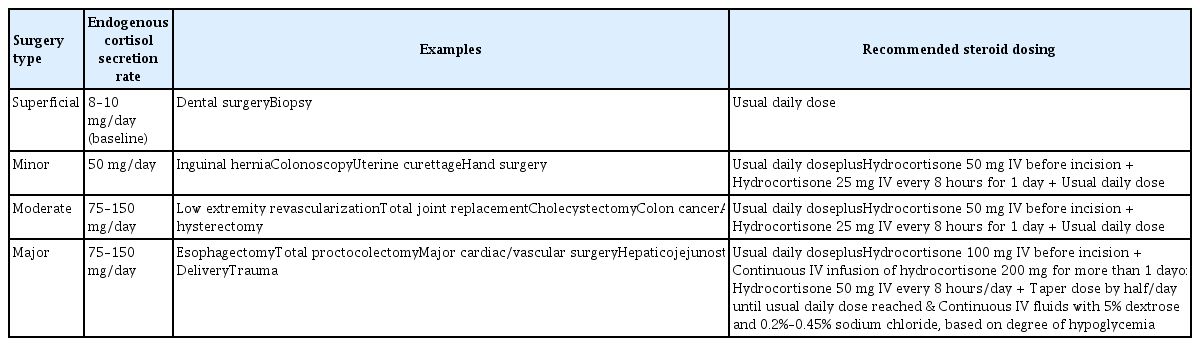
To control or manage RA, various medications were used (Table 2). NSAIDs and glucocorticoids are commonly used to relieve pain and decrease joint inflammation. Janssens and Hartstein [16] stated that the adverse effects of opioids outweighed the benefits; opioids are nonetheless used for pain relief. Conventional NSAIDs and glucocorticoids increase the risk of gastrointestinal bleeding; prophylaxis for gastrointestinal complications is required. Long-term glucocorticoid use is problematic because they increase the infection rate and wound-healing complications in a dose-dependent manner. Normally, the adrenal gland secretes about 8 to 10 mg of cortisol daily. Perioperative glucocorticoid coverage is required by patients on a prednisolone dose > 10 mg/day to control the surgical stress response [26]. Although the transient increase in cortisol secretion in response to stress varies among individuals, it is typically up to 50 mg/day for minor procedures and 75 to 150 mg/day for more complex ones; it rarely exceeds 200 mg/day (Table 3) [27]. The blood glucose level and blood pressure should be closely monitored. The use of nitrous oxide to induce anesthesia should be avoided in patients on methotrexate to treat folate depletion. Patients on glucocorticoids or DMARDs are susceptible to infection; strict application of an aseptic technique and appropriate antibiotic prophylaxis are required [28].
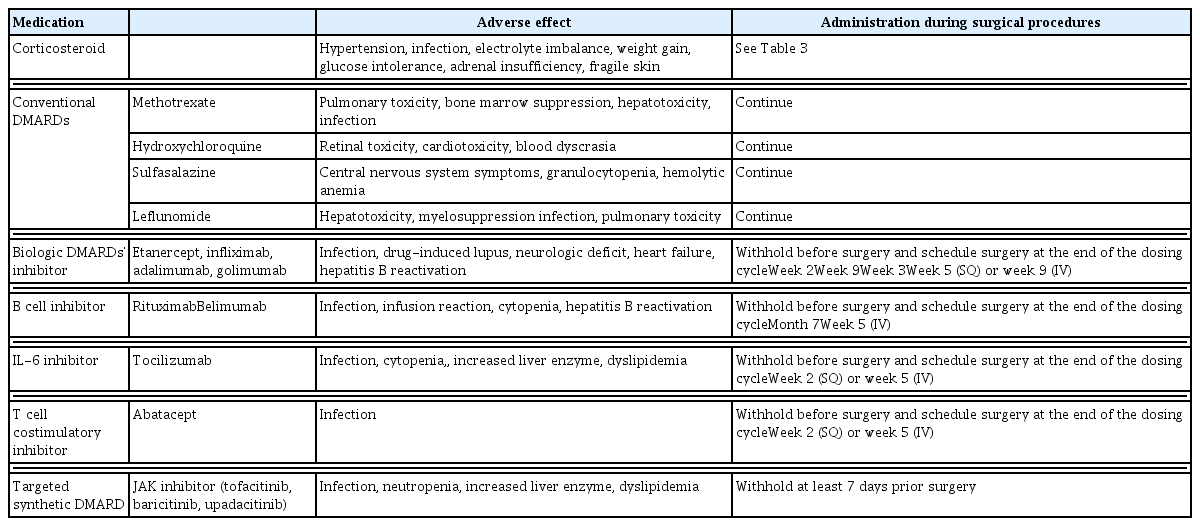
Common adverse effects associated with medications for rheumatoid arthritis and recommendations on how to deal with them during elective surgical procedures
Conventional and biological DMARDs have been used to slow disease progression and prevent joint destruction and deformity. The 2017 guidelines of the American College of Rheumatology/American Association of hip and knee surgeons suggest that conventional synthetic DMARDs (methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine) can be continued in patients undergoing elective joint surgery; however, this remains controversial in clinical practice [26,29,30]. All current biological and targeted synthetic DMARDs should be stopped prior to surgery (at the end of a dosing cycle for biological DMARDs). Tofacitinib must be withheld for at least 7 days. Medications may be restarted when the wound is healing well without any sign of infection [26,28,29].
POSTOPERATIVE MANAGEMENT
Airway and breathing status should be closely monitored after successful anesthesia. The possibility of tracheal edema, and thus a need for re-intubation, should be borne in mind. Standard breathing exercises should be initiated as soon as possible to prevent the development of respiratory problems, and postoperative pain should be appropriately controlled to allow for early recovery and mobilization. However, the use of opioids for postoperative pain management requires caution, as they can affect airway management. Appropriate perioperative thromboprophylaxis is required [4]. RA patients tend to exhibit delayed ambulation, associated with hypercoagulability. Any sign of infection should be carefully monitored, and any immunosuppressive agent temporarily stopped.
CONCLUSIONS
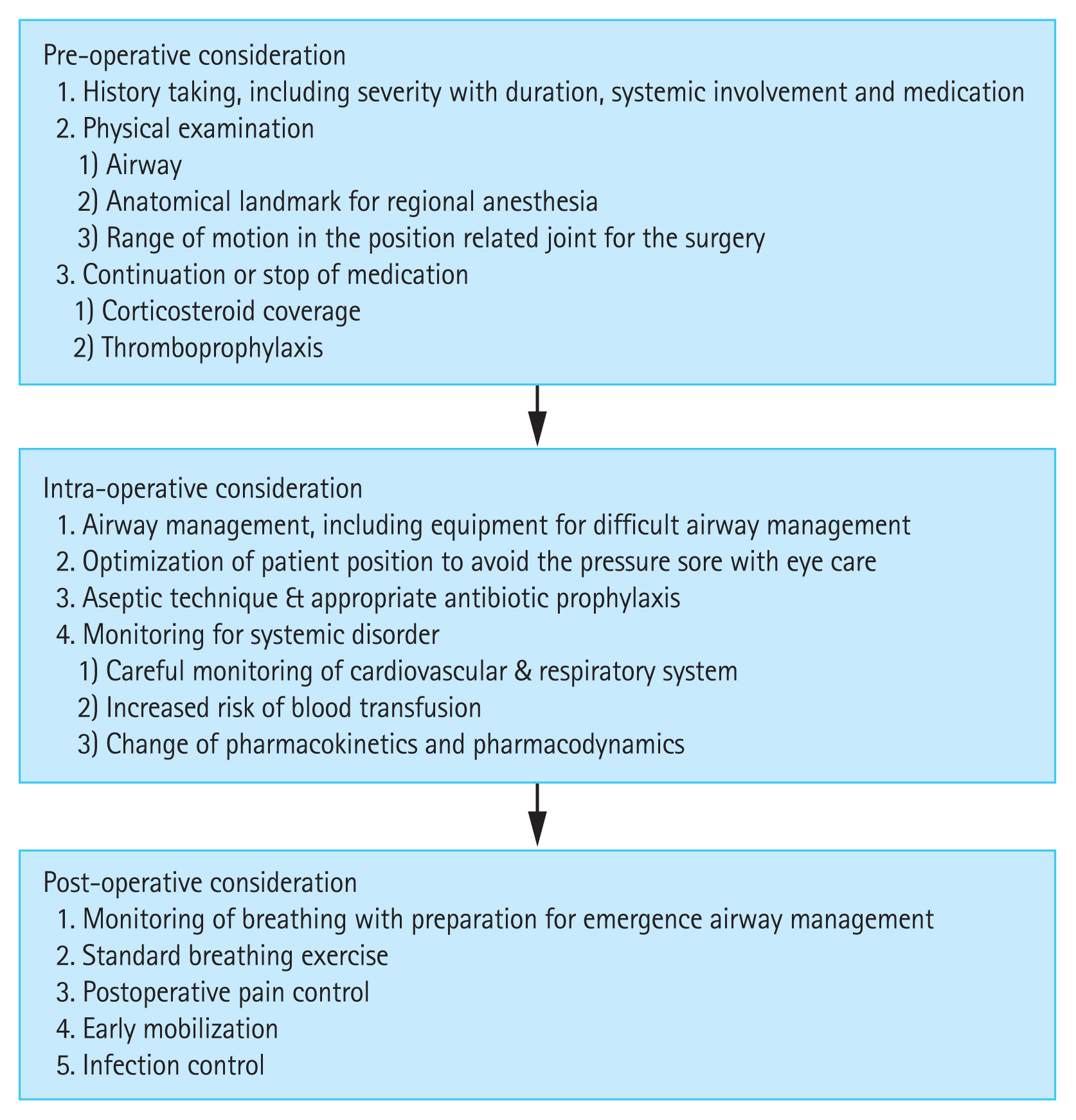
The perioperative management of RA patients is summarized in Fig. 1. Prior to anesthesia, careful preoperative evaluation is required to prevent complications and minimize injury. Anesthetic management strategies must consider RA-related systemic problems and postoperative management should be individualized.
Notes
No potential conflict of interest relevant to this article was reported.