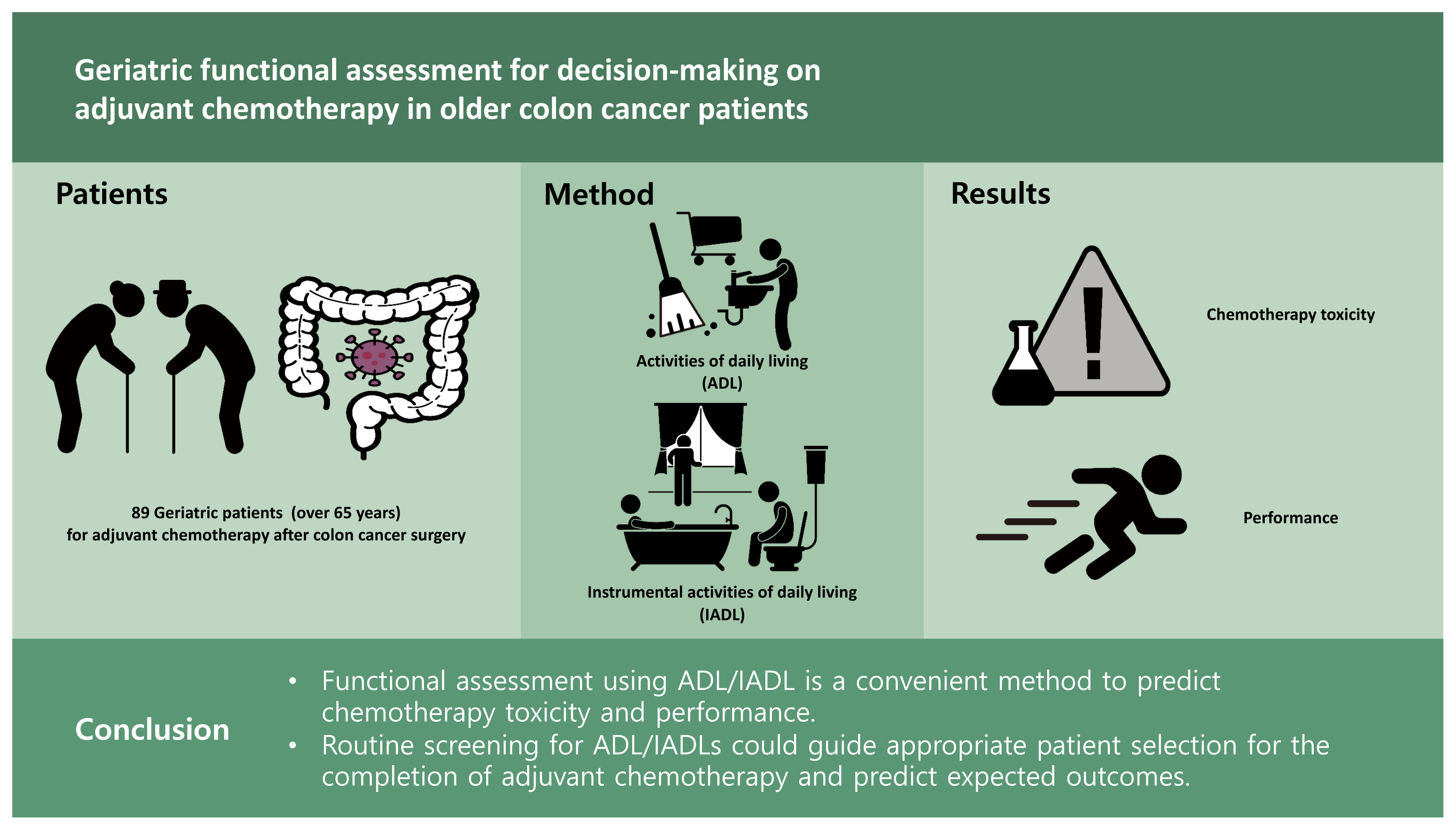
Geriatric functional assessment for decision-making on adjuvant chemotherapy in older colon cancer patients
Article information
Abstract
Background/Aims
Despite the increasing need for geriatric assessment prior to chemotherapy, the method for this assessment remains inadequate for older cancer patients. We aimed to propose a simple assessment method to predict the performance of adjuvant chemotherapy in older patients after colon cancer surgery.
Methods
This prospective study included patients over 65 years of age who were scheduled for adjuvant chemotherapy after colon cancer surgery. Before initiating chemotherapy, their functional status was assessed on the basis of activities of daily living (ADL)/instrumental activities of daily living (IADL). These parameters were analyzed with clinical characteristics and the patterns of adjuvant chemotherapy. The focus was on the completion rate of adjuvant chemotherapy.
Results
A total of 89 patients with a median age of 72 years were analyzed. Among them, 54 (61%) were non-impaired and 35 (39%) were impaired regarding their ADL/IADL classification. Low body mass index and impairment of ADL/IADLs were significantly associated with chemotherapy interruption. Among toxicities, fatigue and hand-foot syndrome were independent prognostic factors for chemotherapy interruption. Impairments of ADL/IADL were significantly associated with fatigue regardless of age. Based on age and ADL/IADL stratification, younger patients (≤ 72 years) and/or those who were ADL/IADL non-impaired were significantly more likely to complete adjuvant chemotherapy than older patients (> 72 years) and ADL/IADL impaired patients (p = 0.038). This was regardless of the chemotherapy regimen.
Conclusions
Functional assessment using ADL/IADL is a convenient method to predict chemotherapy toxicity and performance. These results suggested that routine screening for ADL/IADLs could guide appropriate patient selection for the completion of adjuvant chemotherapy and predict expected outcomes.
INTRODUCTION
The incidence of colon cancer remains high. It is the second most common cancer in Korea [1] and the fourth worldwide according to GLOBOCAN. With recent increases in the average life expectancy, the age when colorectal cancer is diagnosed has also increased. According to the Health Insurance Review and Assessment Service data in Korea, the incidence of colorectal cancer was over 60% in patients aged 60 and over. This indicated that more than half of patients with colon cancer are older individuals [2]. In addition, stage III colon cancer is the most common (37% of patients), followed by stage II (28%), stage I (21%), and stage IV (14%). The incidence and stage of diagnosis are similar to those in Western countries. They show localized (38%) or regional (35%) stages in the Surveillance, Epidemiology and End Results (SEER) program registries [3]. The current standard treatments for localized colon cancer with high-risk stage II and stage III are surgery and adjuvant chemotherapy. Adjuvant chemotherapy, folinic acid, fluorouracil (5-FU), and oxaliplatin (FOLFOX) and capecitabine and oxaliplatin (CAPOX) are used as standard treatments in these patients. This treatment reduces the relative risk of recurrence by approximately 15% to 20% [4–6]. A recent pooled analysis of patient data from large clinical trials such as National Surgical Adjuvant Breast and Bowel Project C-08 (NSABP C-08), NO16968, Xeloda in adjuvant colon cancer therapy (X-ACT), and Bevacizumab-Avastin Adjuvant (AVANT) trial showed that disease-free survival and overall survival improved with adjuvant CAPOX or FOLFOX over 5-FU/leucovorin in patients 70 years of age or older [4]. However, one study has shown that early mortality increased after adjuvant chemotherapy in older patients [7]. For this reason, older patients with cancer are often under-treated compared to the standard guidelines due to concerns regarding unexpected toxicities. This under-treatment may have a negative impact on survival outcomes [8,9]. Taken together, adjuvant chemotherapy is effective even in older patients. However, it is crucial to select appropriate patients that can tolerate the treatment guidelines and potential toxicities. It is necessary to make treatment decisions in this population carefully. The risk benefit ratio must be considered in addition to the pathologic stage.
Older patients may differ substantially from the general population in their vulnerabilities, inconsistent symptoms associated with treatment, and comorbidities. Thus, it is difficult to design clinical trials to verify the benefit of adjuvant chemotherapy [10,11]. To date, a comprehensive geriatric assessment (CGA) represents a multidisciplinary approach to manage older patients. It includes various geriatric factors such as functional status, social environment, comorbidities, nutritional status, polypharmacy, cognition, and mood. These factors were associated with the prognosis as well as the risk of chemotherapy toxicity [12–17]. Of all the numerous CGAs, the activities of daily living (ADL) and instrumental activities of daily living (IADL) are the most useful. These tools evaluate patients’ functional status and assess the ability of patients to maintain independence at home and in the community. These are more convenient tools than others. They also have the advantage of being able to provide self-evaluations [18,19]. While the Mini-Mental State Exam (MMSE) and IADL have been used to predict factors related to severe toxicities and unexpected hospitalization in metastatic colorectal cancer patients [20], there remains a lack of evidence regarding the role of a functional assessment in the adjuvant setting. Therefore, this prospective study was conducted to evaluate the effectiveness of functional assessment methods using ADL/IADL for predicting the performance of adjuvant chemotherapy in older patients after colon cancer surgery.
METHODS
Patients and treatments
This prospective study included older patients scheduled for adjuvant chemotherapy after complete resection of colon cancer. The patients were enrolled consecutively if they met the following inclusion criteria: provided written informed consent, were aged over 65 years, were diagnosed with adenocarcinoma stage II high-risk (T4, poorly differentiated/undifferentiated tumor, perineural invasion, lymphatic/vascular invasion, less than 12 lymph nodes examined, or localized perforation) or stage III disease, had undergone complete resection, had Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2 (0, fully active; 1, restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; 2, ambulatory and capable of all self-care but unable to carry out any work activities, up and about for more than 50% of waking hours), and received adjuvant chemotherapy.
Three regimens of adjuvant chemotherapy were included: capecitabine monotherapy (1,250 mg/m2 twice daily for 14 days every 3 weeks, 8 cycles), FOLFOX (oxaliplatin at 85 mg/m2 intravenously on day 1, and 5-FU 400 mg/m2 bolus intravenously on day 1 and 1,200 mg/m2 continuous intravenously on days 1 and 2 every 2 weeks, 12 cycles), and CAPOX (oxaliplatin at 130 mg/m2 intravenously on day 1 and capecitabine at 1,000 mg/m2 twice daily for 14 days, 8 cycles). The term “relative dose intensity” is used to define the proportion of actual dose delivered divided by the standard calculated dose during each regimen. It is expressed as a percentage. The completion of chemotherapy was defined as the patient having received the standard cycles for each regimen for 6 months following surgery. Otherwise, it was considered to be an interruption in treatment. The adjuvant chemotherapy was determined by the physician’s preference based on the patient’s condition. Dose reduction was at the discretion of the physician at the initiation of chemotherapy. We performed a stratification for the following factors: sex (male or female), body mass index (BMI; underweight, < 18.5 kg/m2; normal, 18.5–22.9 kg/m2; overweight-obese, ≥ 23 kg/m2), comorbidity (yes or no), hemoglobin level (< 10 or ≥ 10 g/dL), CrCl (estimated glomerular filtration rate < 60 or ≥ 60 mL/min/1.73 m2), ECOG PS (0–1 or 2), and stage (II or III).
Hematologic and non-hematologic toxicities were noted at each cycle and at the end of treatment based on medical records. These data were analyzed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (IRB No. CNUHH-2016-140) and written informed consent was obtained from all patients.
Assessment of the functional status of older patients
Functional status was evaluated by well-trained assistants within 1 week prior to beginning chemotherapy using the ECOG PS, ADL, and IADL. ADLs comprise the following seven items: dressing, washing face and hair, bathing, eating, transferring, toileting, and continence. IADLs comprise the following 10 items: decorating, housework, preparing meals, laundry, going out for short distances, using transportation, shopping, handling money, using the telephone, and taking medicine. The ADL and IADL were based on Katz’s ADL [18] and Lawton’s IADL [19], which reflect the Korean language and culture [21]. We defined the impairment of ADL/IADL as those patients who showed a loss of self-sufficiency in one or more of the domains of ADL or IADL [22].
Statistics
The characteristics of the population were summarized as frequencies and percentages for categorical variables and as medians and ranges for continuous variables. Comparisons between groups were performed using the chi-square or Fisher’s exact tests for categorical variables and the Mann-Whitney or t tests for continuous variables.
Logistic regression analysis was performed to obtain the significant predictors for the interruption of adjuvant chemotherapy. All tests were two-sided, and p < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Windows version 21.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patient characteristics
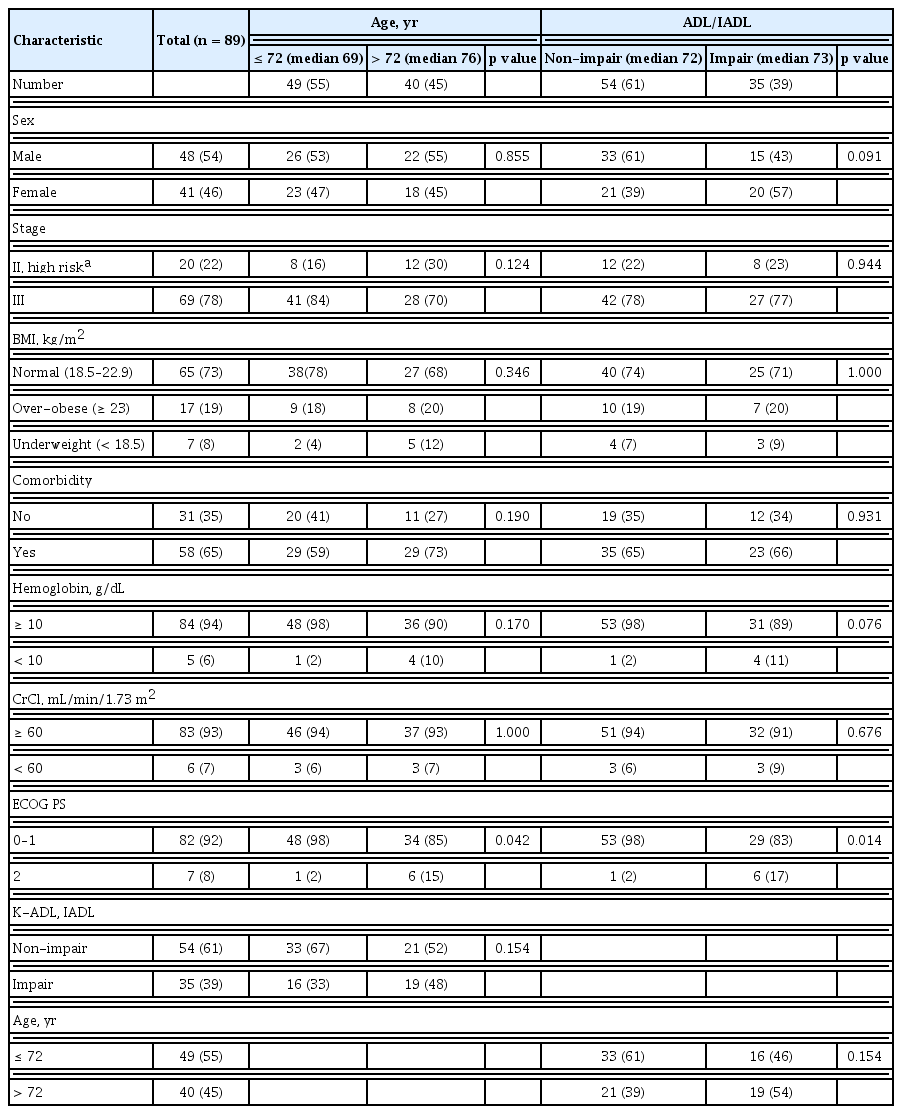
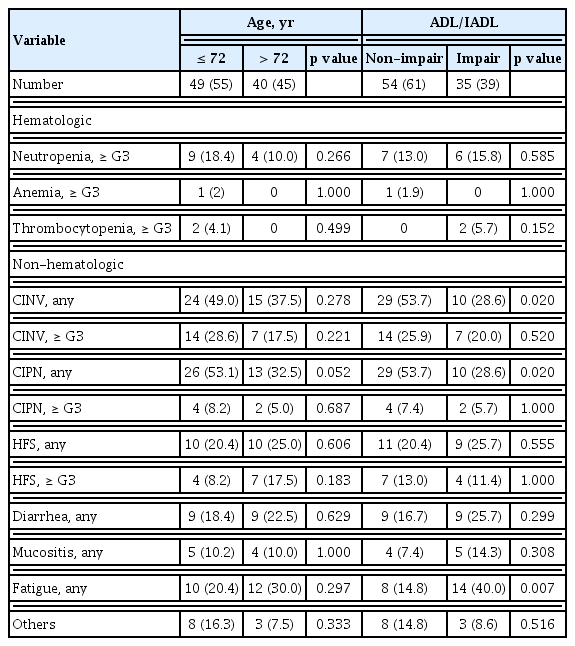
From June 2017 through November 2019, 96 patients were prospectively enrolled. Among them, seven patients were excluded, three for metastatic lesions after surgery, two for rectal cancer requiring radiation therapy, one for refusing chemotherapy, and one diagnosed with double primary cancer. Therefore, 89 patients were analyzed. Their baseline characteristics are described in Table 1. The median age was 72 years (range, 65 to 83). Regarding the age distribution of this study, the age range exists for multiple categories. We analyzed the age range by dividing it into two groups based on median age (72 years). Compared to patients younger than 72 years (n = 49), patients over 72 years (n = 40) showed no difference in characteristics except for ECOG PS.
ADL/IADL analysis classified 54 patients (61%) in the non-impairment of ADL/IADL category and 35 patients (39%) in the impairment of ADL/IADL category. There were no differences in clinical characteristics between the non-impairment and impairment categories except for ECOG PS (p = 0.014).
Physician preferences regarding adjuvant chemotherapy and dosage strength
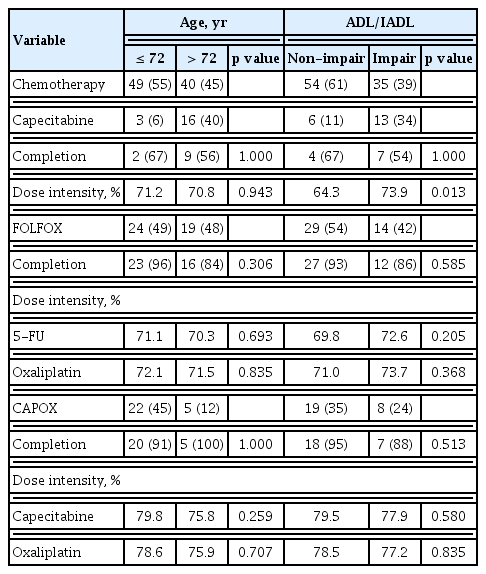
Analysis of the patterns of adjuvant chemotherapy according to the physician’s choice showed that oxaliplatin-based chemotherapy was administered significantly more often to patients who were younger (≤ 72 years) or had non-impairment of ADL/IADL. This was compared to patients who were older > 72 years (p < 0.001; Fig. 1A) or had impairment of ADL/IADL (p = 0.013; Fig. 1B). In total, 19 patients (21%) received capecitabine, 43 patients (48%) received FOLFOX, and 27 patients (30%) received CAPOX. Among them, 75 patients (84%) completed the planned treatment cycle. In detail, 11 patients (58%) were administered capecitabine, 39 patients (91%) were administered FOLFOX, and 25 patients (93%) were administered CAPOX and completed their planned cycles. The dosage was reduced in 81 patients (91%) during the first cycle, and the median dosage strength in the first cycle was 80%. There was no significant difference in the dosage strength for each regimen according to age and the ADL/IADL group, except for capecitabine monotherapy in the ADL/IADL categories. The completion rates of adjuvant chemotherapy did not differ significantly between age or categories by ADL/IADL classification, for each type of adjuvant chemotherapy regimen (Table 2).
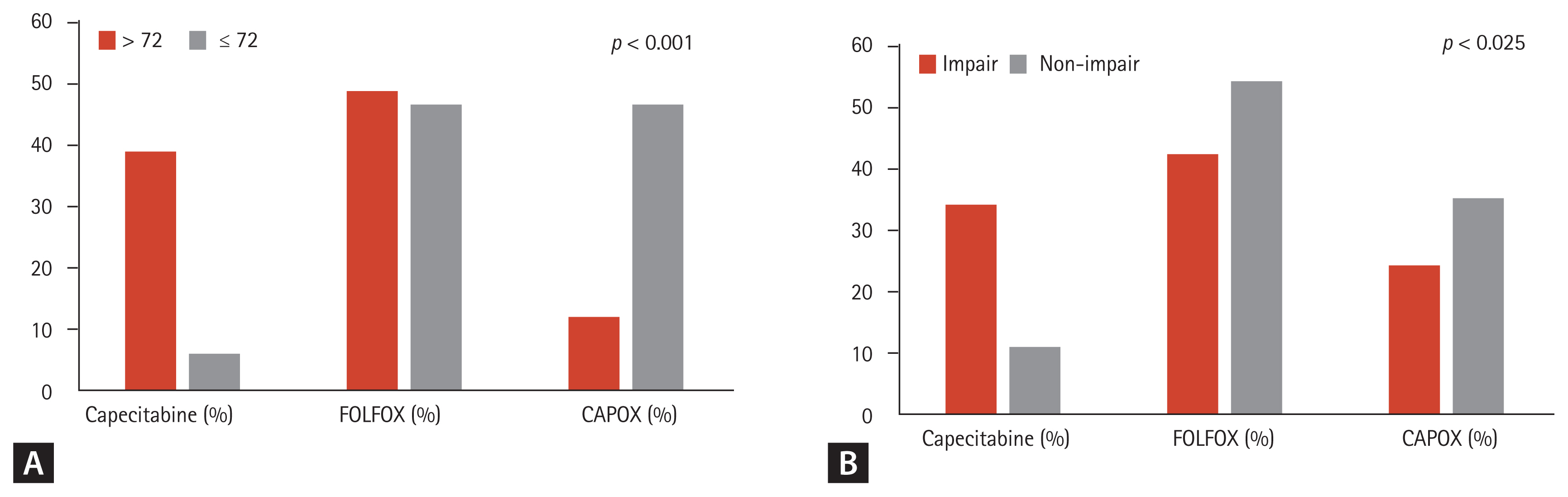
Patterns of adjuvant chemotherapy according to (A) age and (B) activities of daily living/instrumental activities of daily living. FOLFOX, folinic acid, fluorouracil (5-FU), and oxaliplatin; CAPOX, capecitabine and oxaliplatin.
Comparative analysis of the predictive factors associated with the completion of adjuvant chemotherapy
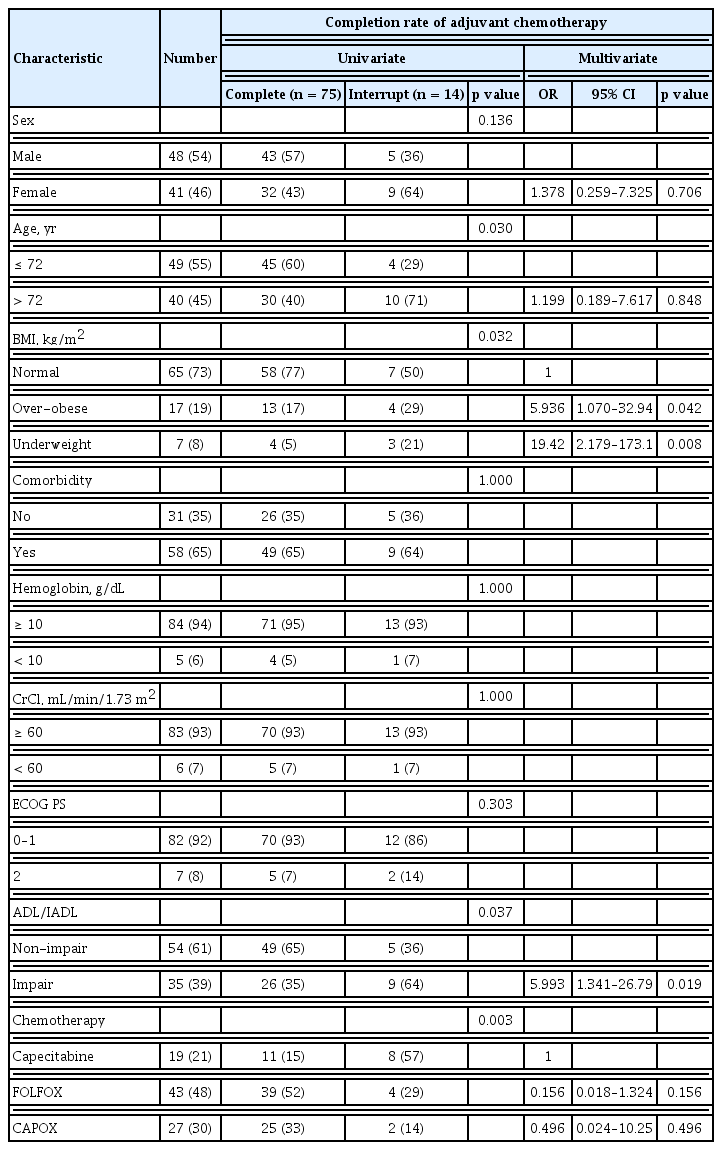
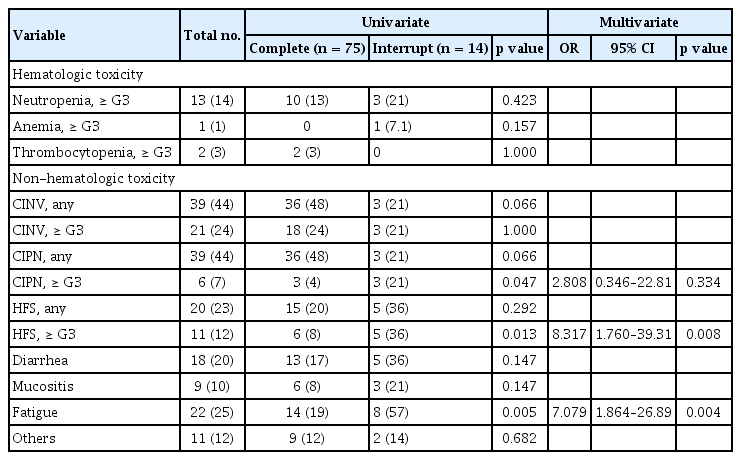
Analysis of the clinical characteristics related to chemotherapy completion showed significant associations with age, the chemotherapy regimen, and BMI in univariate analysis and BMI and ADL/IADL in multivariate analysis (Table 3).
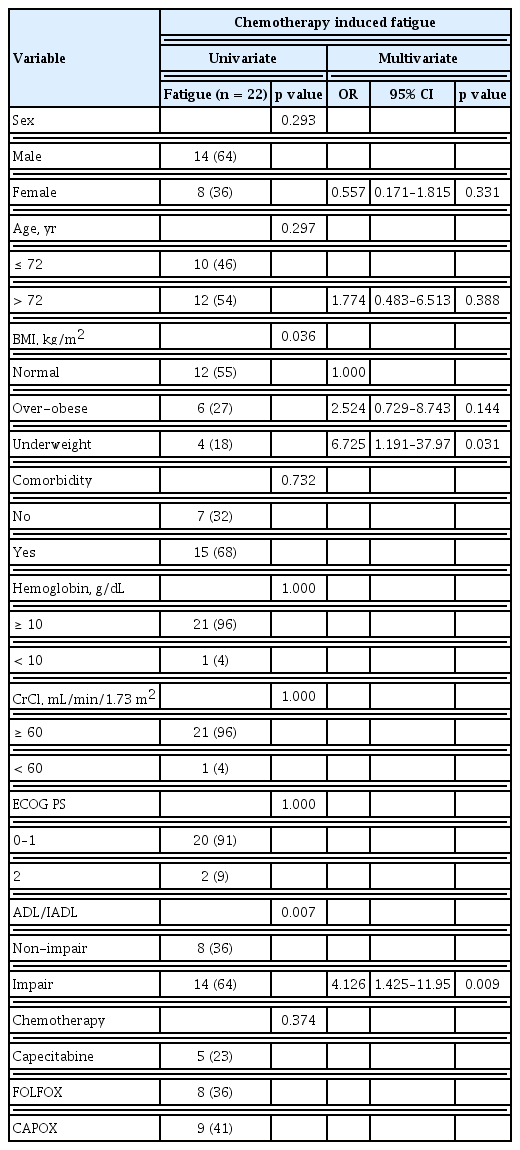
In addition to patient baseline characteristics, toxicity following chemotherapy was also a critical issue related to chemotherapy interruption. Therefore, we analyzed this association with the incidence of toxicity according to age, ADL/IADL, and chemotherapy interruption (Table 4). There was no significant difference in hematologic toxicity. The most common treatment-related hematologic toxicity was neutropenia. One case of febrile neutropenia with severe infection was reported. The patient recovered with antibiotics and in-hospital therapy. However, the patient discontinued chemotherapy because of poor PS. The most common non-hematologic toxicities of any grade were chemotherapy induced nausea and vomiting (CINV), chemotherapy induced peripheral neuropathy (CIPN), hand-foot syndrome (HFS), diarrhea, and fatigue (43%, 43%, 22%, 20%, and 24% respectively). CINV developed more frequently in patients in the non-impairment of ADL/IADL category (53.5%, p = 0.020) and this result would be related with the more use of oxaliplatin based chemotherapy in patients with non-impairment of ADL/IADL than in patients with impairment of ADL/IADL. Likewise, CIPN developed more frequently in patients in the younger-age group (53.1%, p = 0.052) and in the non-impairment of ADL/IADL category (53.7%, p = 0.020). According to age, the cumulative dose of oxaliplatin in younger age group was higher in FOLFOX (younger 756.66 mg/m2 > older 712.21 mg/m2) and CAPOX (younger 808.37 mg/m2 > older 673.40 mg/m2) than in older patients. As with age, the cumulative dose of oxaliplatin in the non-impairment of ADL/IADL category was higher in the FOLFOX (non-impair 743.31 mg/m2 > impair 723.99 mg/m2) and CAPOX (non-impair 818.66 mg/m2 > impair 699.56 mg/m2) than in the impairment category. For these reasons, our results may contribute to an explanation of the risks of developing CIPN due to the cumulative dose of oxaliplatin. Other non-hematologic toxicities were reported in 12 cases, followed by eight cases of oxaliplatin induced hypersensitivity, two cases of infection, one case of hyponatremia, and one case of hepatitis. Interestingly, the incidence of fatigue was significantly higher in patients with impairment of ADL/IADL, regardless of age. Among the toxicities, HFS more than grade G3 and chemotherapy induced fatigue were significantly associated with chemotherapy interruption in multivariate analysis (Table 5). Except for HFS, which is directly related to chemotherapy such as capecitabine, chemotherapy induced fatigue was the only significant factor to predict chemotherapy completion. Among the baseline clinical parameters, low BMI, and impairment of ADL/IADL were associated with chemotherapy induced fatigue in univariate and multivariate analyses (Table 6).
Therefore, we divided patients into four groups based on age (≤ 72 or > 72 years) and functional status by ADL/IADL (non-impairment or impairment) to predict the completion rate of adjuvant chemotherapy according to each regimen. As shown in Fig. 2A, patients with a younger age (≤ 72 years) and non-impairment of ADL/IADL more often received CAPOX, while patients with an older age (> 72 years) and impairment of ADL/IADL more often received capecitabine monotherapy (p < 0.001). In addition, younger patients (≤ 72 years) and/or those with non-impairment of ADL/IADL were significantly more likely to complete adjuvant chemotherapy than were older patients (> 72 years) and those with impairment of ADL/IADL (p = 0.038; Fig. 2B), regardless of the chemotherapy regimen.
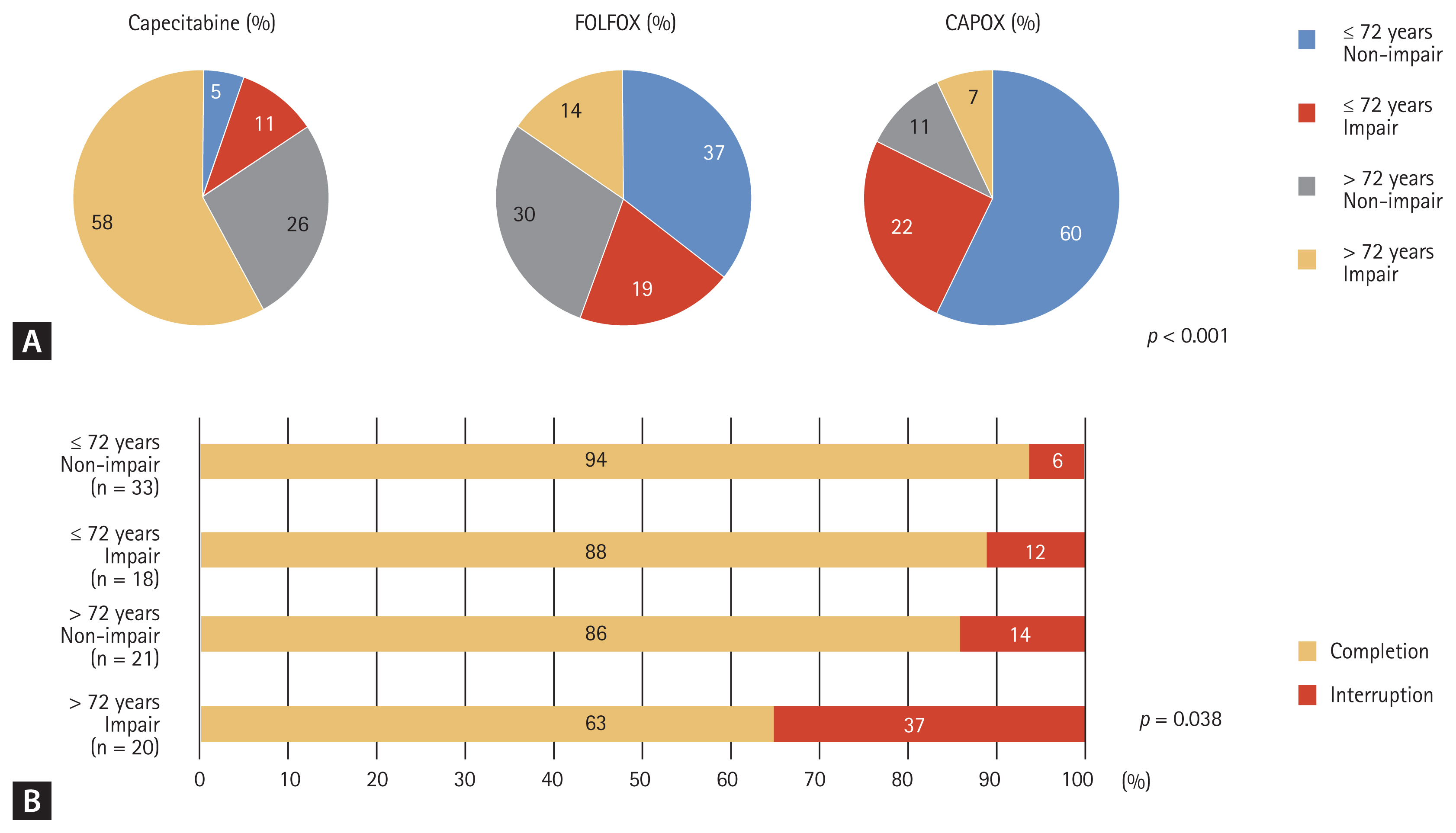
(A) Patterns of adjuvant chemotherapy and (B) completion rates based on age (≤ 72 years vs. > 72 years) and activities of daily living/instrumental activities of daily living (non-impairment vs. impairment). FOLFOX, folinic acid, fluorouracil (5-FU), and oxaliplatin; CAPOX, capecitabine and oxaliplatin.
DISCUSSION
In this study, we reported the predictive role of ADL/IADL assessment for adjuvant chemotherapy completion and its toxicities in older patients after colon cancer surgery. In particular, even though most patients had an ECOG PS 0–1 (92% of patients), the high-risk category could be selected by the impairment of ADL/IADL (41%) for predicting chemotherapy interruption. This is a very meaningful result.
To clarify the vulnerability of older patients, several studies have proposed tools to predict chemotherapy-induced toxicity and mortality through physical or functional assessment. This includes weight loss, BMI, nutritional support, or Karnofsky performance status (KPS) [17,23–25]. One of these studies, the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) study, suggested the use of several models for predicting hematologic toxicity and non-hematologic toxicity in vulnerable older patients [12]. However, the full CGA is too complicated to perform. Previous studies of CGA with cancer patients had variabilities in the patients’ response to treatment. This included tumor heterogeneity, variability in cancer type and stage, and several types of chemotherapy agents. Instead of a complicated full CGA, Hamaker et al. [26], showed that all-cause mortality and chemotherapy tolerance were associated with impairment of ADL or cognitive impairment. This method was relatively less difficult to determine compared to other CGA methods. In addition, the Korean cancer study group geriatric score (KG)-7 was developed by oncologists and geriatricians. It consists of high sensitivity factors from several questionnaires easy to understand and fulfill, such as the ADL, IADL, MMSE, and the mini nutritional assessment. The KG-7 score showed a significant prediction of OS in cancer patients [27]. Our results showed that ADL/IADL impairment or low BMI were significant predictive factors for the completion of chemotherapy. Thus, functional status is a key factor for initiating chemotherapy in older cancer patients.
In this study, chemotherapy induced fatigue was the most significant risk factor for completion of chemotherapy. The European Society for Medical Oncology (ESMO) guidelines recognize the importance of managing older patients with cancer to reduce cancer-related fatigue during and after cancer treatment [28]. Moreover, the National Comprehensive Cancer Network (NCCN) and International Society of Geriatric Oncology (SIOG) guidelines highlight the importance of frailty in older patients with cancer. They recommend the use of CGA to detect frailty [13,29]. Frailty is closely related to fatigue. The incidence of cancer-related fatigue is more frequent in older patients with frailty. However, it is challenging to objectively measure these parameters. Our results showed that a decline in functional status as assessed by ADL/IADL was significantly associated with chemotherapy induced fatigue and chemotherapy completion. Unlike ADL/IADL, ECOG PS has limitations for the prediction of chemotherapy completion or chemotherapy induced fatigue. Therefore, we propose the use of ADL/IADL as an assessment tool for fatigue prior to the determination of a treatment plan.
The limitations of our study include the relatively low dosage strength of chemotherapy agents in this cohort analysis as opposed to those in previous clinical trials. However, in the case of CAPOX, 78% dosage strength in both oxaliplatin and capecitabine was delivered to younger patients (≤ 72 years) and those with non-impairment of ADL/IADL. The median oxaliplatin and capecitabine relative dosage strengths in the reference clinical trials were 80.5% and 78%, respectively in the multicenter international study or oxaliplatin/fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) trial, 84% for oxaliplatin in the NO16968 trial, and 93% for capecitabine in the X-ACT trial [5,6,30]. However, the administered dosage strength cycles of capecitabine were much lower in our study than those for FOLFOX or CAPOX. This may be because capecitabine might be selected as adjuvant chemotherapy for frail patients. Thus, the dosage strength and the completion rate were low. Although the association between the treatment interruption and regimen in older (≥ 72 years) and impaired ADL/IADL categories of patients was not evaluated due to the small sample size, we propose that adjuvant chemotherapy should be carefully considered with active supportive care for these high-risk patients. The omission of adjuvant chemotherapy could be an additional option for these patients, regardless of the regimen.
In conclusion, the results of our prospective study showed that ADL/IADL is a convenient method for the functional assessment of older patients with cancer. This method could be an independent predictor for chemotherapy completion and chemotherapy induced fatigue. Therefore, ADL/IADL could be used as a clinical parameter for older patients receiving adjuvant chemotherapy in clinical practice. Future studies are essential to investigate whether there are benefits for convenience, sensitivity, and specificity of ADL/IADL compared to other geriatric assessments.
KEY MESSAGE
1. Activities of daily living (ADL)/instrumental activities of daily living (IADL) assessment is a useful method for predicting the completion of planned adjuvant chemotherapy for older patients after colon cancer surgery.
2. Older patients with impairments of ADL/IADL are more likely to have chemotherapy induced fatigue and chemotherapy interruption independent of Eastern Cooperative Oncology Group (ECOG) performance status (PS).
3. ADL/IADL assessment could be used as a clinical parameter if older patients are included in practice or clinical trials that use chemotherapy.
Notes
No potential conflict of interest relevant to this article was reported.