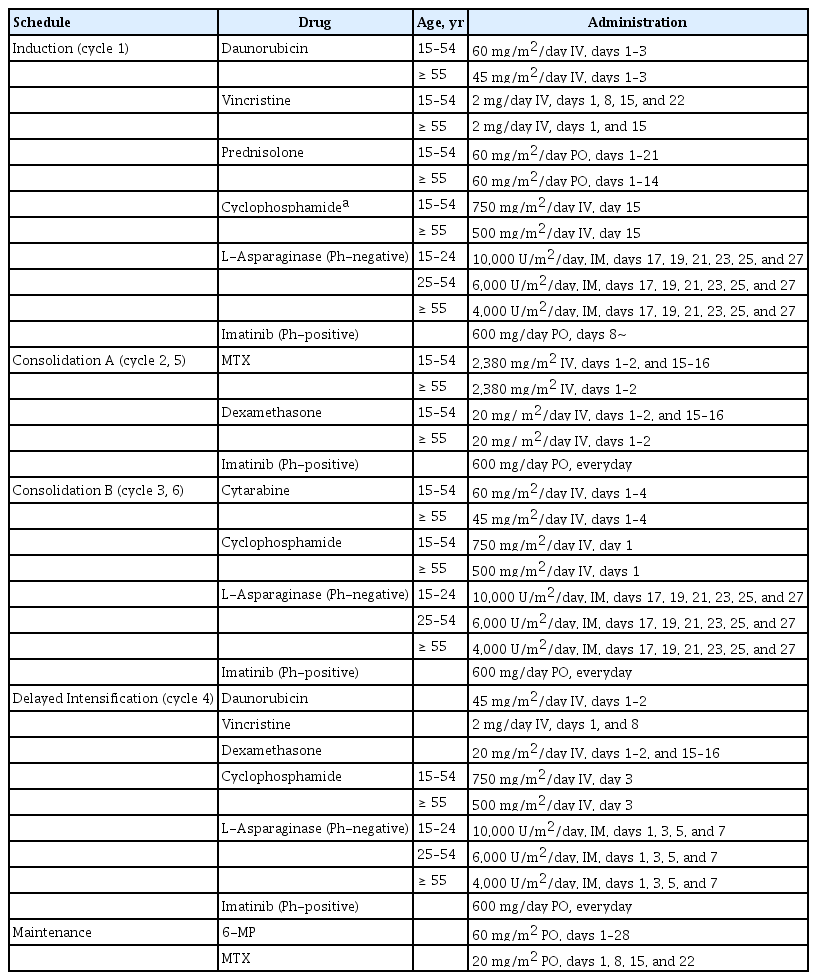
Pediatric-inspired regimen with late intensification and increased dose of L-asparaginase for adult acute lymphoblastic leukemia: the KALLA 1406/1407 study
Article information
Abstract
Background/Aims
The objective of this study was to evaluate the efficacy and feasibility of the pediatric-inspired regimen of the adult acute lymphoblastic leukemia (ALL) Working Party, the Korean Society of Hematology.
Methods
Data of 99 patients with newly diagnosed ALL, who were treated with the KALLA 1406/1407 protocol, were retrospectively analyzed. All patients equally received age-adjusted daunorubicin, vincristine, and prednisolone. L-asparaginase was additionally administered to Philadelphia (Ph)-negative patients according to age, whereas Ph-positive patients received 600 mg/day of imatinib.
Results
A total of 99 patients were enrolled in this study, of whom 62 (62.6%) were diagnosed with Ph-negative ALL and 37 (37.3%) were diagnosed with Ph-positive ALL. The median age of patients in the Ph-negative ALL group was 46 years, and that of patients in the Ph-positive ALL group was 49 years. In patients with Ph-negative ALL, 57 (92%) patients achieved complete remission (CR) and CR with incomplete hematologic recovery (CRi). Disease-free survival (DFS) and overall survival (OS) rates at 2 years were estimated to be 42% and 63%, respectively. In patients with Ph-positive ALL, 32 (86%) patients achieved CR/CRi, and 2-year DFS and OS were 31.2% and 49.1%, respectively. Patients who were able to proceed to the allogeneic hematopoietic cell transplantation and younger patients showed significantly superior survival in both Ph-negative ALL and Ph-positive ALL. Neutropenic fever and bacterial infection were the most common and severe adverse events.
Conclusions
The KALLA 1406/1407 protocol showed tolerable toxicities in adult ALL patients. Especially, younger patients had more survival benefits with KALLA 1406/1407 protocol.
INTRODUCTION
Survival outcomes in patients with acute lymphoblastic leukemia (ALL) have demonstrated improvement in the past few decades [1]. Since the introduction of the Berlin-Frankfurt-Münster regimen for pediatric patients, the 5-year event-free survival and overall survival (OS) rates were up to 85% and 90%, respectively, in children with ALL [2,3]. However, in cases of adult ALL, there are still disappointing outcomes [4]. Although > 80% of adults with ALL achieve complete remission (CR) after induction chemotherapy, a significant proportion of them experience a relapse that results in to poor survival outcomes [5–7].
In recent years, the standard treatment for adult ALL has been typically based on pediatric ALL regimens that include vincristine, anthracycline, corticosteroid, and/or L-asparaginase with or without cyclophosphamide [8–10]. For improving the long-term survival in adult patients with ALL, several clinical trials have explored the effectiveness of various regimens that modified the dosage or schedules of pediatric regimens [11,12]. The Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology (KAALLWP) conducted the phase II clinical study to investigate the effect of higher doses of daunorubicin during the induction cycle in adult patients with Philadelphia (Ph)-negative ALL. The CR rate (88.5%) was found to be comparable to that of previous reports, but the long-term outcomes were unsatisfactory with a 3-year OS rate of 46.1% and a 3-year relapse-free survival rate of 43.1%, thus suggesting the need for stronger postremission strategies [13].
Meanwhile, higher doses of glucocorticoids and L-asparaginase have also been reported to play key roles in the contemporary regimens of pediatric patients [14–16]. Moreover, addition of late intensification and repeated courses of methotrexate after remission were reported as critical components in the treatment of pediatric remissions [17–19]. The Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) 2003 study demonstrated that increased doses of prednisone and L-asparaginase yielded significantly better results in adults with Ph-negative ALL; thus, suggesting that the pediatric therapy-based intensified protocol was promising in adults with ALL having relatively tolerable toxicities [20]. Although delayed intensification has not been validated in many studies for adult patients, it might also contribute to enhancing the positive outcomes in adult patients with ALL [21,22].
Therefore, the KAALLWP has proposed a new treatment protocol termed KALLA 1406 for Ph-negative and KALLA 1407 for Ph-positive patients with ALL, which added the concept of delayed intensification along with increased doses of L-asparaginase to reduce disease relapse. The present study retrospectively analyzed the efficacy and tolerability of KALLA 1406/1407 regimens on adults with newly diagnosed ALL.
METHODS
Patients
The KALLA 1406/1407 regimens have been implemented from April 2014 to April 2019 at six centers in Korea. ALL was diagnosed and classified based on the World Health Organization’s classification through bone marrow examination and cytogenetic studies [23]. Inclusion criteria were patients aged ≥ 15 years, eligible for intensive chemotherapy with an Eastern Cooperative Oncology Group performance status of ≤ 2, and with adequate organ functions. Patients were required to have adequate cardiac function (ejection fraction > 45%) as evaluated by and echocardiogram. Adequate kidney function (creatinine level < 1.5 mg/dL) and liver function (bilirubin level < 1.5 mg/dL; transaminases levels less than three times the upper normal limit) were also required, unless attributable to leukemia. The exclusion criterion was patients with an uncontrolled infection or uncontrolled illnesses and having been treated with other investigational agents. This study was approved by the Institutional Review Board of Kyungpook National University Hospital (KNUH 2019-10-003) and each other participating center. Informed consent by the patients was waived due to the retrospective nature of our study. All procedures in this study that involved human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Cytogenetic abnormalities were categorized based on previous studies as follows [24,25]: (1) good-risk group: hyperdiploid (> 50 chromosomes), trisomy of chromosome 4, 10, or 17, t(12;21)(p13;q22): ETV6-RUNX1, t(1;19)(q23;p13): TCF3-PBX1; (2) poor-risk group: hypodiploidy (< 44 chromosomes), t(v;11q23), t(11;19): KMT2A rearranged, t(9;22)(q34;q11.2): BCR-ABL1, complex karyotype (five or more chromosomal abnormalities), Ph-like ALL; intrachromosomal amplification of chromosome 21; and (3) intermediate-risk group: other patients with normal karyotypes. Significant prognostic factors based on past medical history were also considered according to early prospective multicenter studies. Patients aged ≥ 35 years with poor cytogenetic abnormalities, higher initial white blood cell (WBC) count (30.0 × 103/μL for B-cell lineage, > 100.0 × 103/μL for T-cell lineage), and central nervous system (CNS) involvement were defined as the high risk group [5,26].
Treatment
The treatment schedule is shown in Table 1. Briefly, the KALLA 1406/1407 regimens consisted of induction and two cycles of consolidation followed by delayed intensification plus two additional consolidation cycles. During the induction chemotherapy, patients aged < 55 years received 60 mg/m2/day of daunorubicin on days 1–3; 2 mg of vincristine on days 1, 8, 15, and 22; and 60 mg/m2/day of prednisolone on days 1–21. Patients aged > 55 years received 45 mg/m2/day of daunorubicin on days 1–3; 2 mg of vincristine on days 1 and 15; and 60 mg/m2/day of prednisolone on days 1–14. Patients with Ph-positive ALL received 600 mg/day of imatinib from day 8, whereas those with Ph-negative ALL received intramuscular or subcutaneous L-asparaginase on days 17, 19, 21, 23, 25, and 27 according to their respective age (age 15 to 24 years, 10,000 U/m2/day; age 25 to 54 years, 6,000 U/m2/day; and age ≥ 55 years, 4,000 U/m2/day). Bone marrow aspiration and biopsy were performed on days 14 and 28 of induction chemotherapy to evaluate the response of treatment. CNS prophylaxis was performed using 15 mg of intrathecal methotrexate (IT-MTX) with 50 mg of hydrocortisone during each chemotherapy course considering the patients’ conditions.
Postremission therapies were administered no sooner than every 28 days or 1 week after marrow recovery (absolute neutrophil count [ANC] ≥ 1.5 × 103/μL and platelet (PLT) count ≥ 100 × 103/μL in the peripheral blood), and they could be delayed if patients had a severe infection or any other significant condition. Patients received consolidation and delayed intensification chemotherapy according to their age and treatment schedule. Intravenous leucovorin rescue was conducted immediately after MTX administration. Patients with Ph-positive ALL were maintained on 600 mg/day of imatinib, whereas those with Ph-negative ALL received L-asparaginase according to their age and schedule. For patients with Ph-positive ALL, tyrosine kinase inhibitor (TKI) could be changed to dasatinib if they had an adverse event associated with imatinib. Patients with hematologic relapse received salvage therapy followed by allogeneic hematopoietic cell transplantation (allo-HCT) if they achieved CR. For patients with Ph-positive ALL who showed molecular relapse, TKI was changed to dasatinib or ponatinib based on the mutational status, and allo-HCT was considered after they achieved secondary CR. Patients who discontinued the study because of an adverse event or other reasons received treatment at the discretion of the investigator.
Allo-HCT or maintenance therapy
During the consolidation chemotherapy, patients who had a human leukocyte antigen-matched sibling or unrelated donors were considered to proceed for allo-HCT. Donor selection, conditioning regimen, and graft-versus-host disease prophylaxis were decided according to the investigator’s choice. Patients who completed the consolidation chemotherapy and did not undergo allo-HCT received maintenance chemotherapy consisting of 60 mg/m2 of 6-mercaptopurine per oral (PO) on days 1 to 28; 20 mg/m2 of MTX PO on days 1, 8, 15, and 22 for a maximum of 2 years for Ph-negative ALL; and TKI for at least 2 years for Ph-positive ALL.
Response assessment
CR was defined as fulfilling all the following response criteria for at least 4 weeks: (1) < 5% blasts in the bone marrow and disappearance of bone marrow chromosomal abnormality; (2) ≥ 20% cellularity in the bone marrow biopsy with normal maturation of all cell lines; (3) absence of leukemia blasts in the peripheral blood; (4) ANC ≥ 1,000/μL and PLT count ≥ 100,000/μL with independent transfusion; and (5) no evidence of extramedullary involvement of CNS or soft tissue. CR with incomplete hematologic recovery (CRi) was defined as fulfilling all the following response criteria for at least 4 weeks: (1) < 5% blasts in the bone marrow and disappearance of bone marrow chromosomal abnormality; (2) ≥ 20% cellularity in the bone marrow biopsy with normal maturation of all cell lines; (3) absence of leukemia blasts in the peripheral blood; (4) independent transfusion; and (5) no evidence of extramedullary involvement of CNS or soft tissue. Relapse was defined as the recurrence of disease after achieving CR with ≥ 5% blasts in the bone marrow or blasts in the peripheral blood, or the evidence of newly appeared extramedullary involvement. Patients who never achieved CR or CRi were considered as refractory. Adverse events of chemotherapy were graded according to the Common Terminology Criteria for Adverse Events version 5.0 (NIH, Bethesda, MD, USA).
Statistical analysis
Categorical variables were summarized by counts with proportion, whereas continuous variables were described by their median with range. Categorical variables were analyzed using the chi-square and Fisher’s exact tests. Continuous variables were compared using the Wilcoxon rank-sum test. OS was defined as the time from diagnosis to death from any cause or the last follow-up. Disease-free survival (DFS) was estimated from the time of treatment to disease progression or death. The probabilities of OS and DFS were calculated using the Kaplan-Meier method and compared using the log-rank test. The Mantel-Byar test and Simon and Makuch method were used to evaluate the time-dependent covariate approach for allo-HCT. Cox regression model was used for identifying the factors for long-term survival. Factors with a p value of ≤ 0.1 in the univariate analysis were included in the multivariate analysis. The hazard ratio (HR) and 95% confidence interval (CI) were estimated for each factor. A p value of < 0.05 was considered to be statistically significant. Statistical analyses were conducted using the R statistical software 3.6.2 (the R foundation for Statistical Computing, Vienna, Austria, available at http://www.r-project.org).
RESULTS
Patient characteristics
A total of 99 patients were enrolled in this study, of whom 62 (62.6%) were diagnosed with Ph-negative ALL and 37 (37.3%) were diagnosed with Ph-positive ALL. The median age of patients in the Ph-negative ALL group was 46 years, and that of patients in the Ph-positive ALL group was 49 years. Those with Ph-positive ALL tended to be older than Ph-negative ALL (p = 0.051). All patients with Ph-positive ALL were classified into the poor cytogenetic risk group because of the presence of t(9:22) or BCR-ABL1. In the Ph-negative ALL group, only 13 (21%) patients had poor-risk cytogenetics. The median WBC count at diagnosis was 11.9 × 103/μL (range, 0.27 to 442.8), and 31 (31%) patients had a WBC count of > 30.0 × 103/μL. The proportion of elevated WBC count was higher in patients with Ph-positive ALL than in patients with Ph-negative ALL. Lymph node involvement and CNS invasion were identified in 18 (18%) and six (6%) patients, respectively. All patients with Ph-positive ALL and 49 (79.0%) patients with Ph-negative ALL were classified into the high-risk group in this study. Table 2 summarizes the detailed baseline characteristics of the study patients.
Response to induction therapy
All patients equally received age-adjusted daunorubicin, vincristine, and prednisolone. l-asparaginase was additionally administered to 62 patients with Ph-negative ALL according to their age, whereas 37 patients with Ph-positive ALL received 600 mg/day of imatinib. Among patients with Ph-negative ALL, 38 (61%) achieved CR and 19 (31%) achieved CRi, whereas one (2%) patient had primary refractory disease after induction chemotherapy. Mortality was observed in three (5%) patients, including two due to infection and one due to liver failure. The overall response rate (ORR) was 92%, and age < 50 years (p < 0.001), male (p < 0.001), WBC count < 30.0 × 103/μL (p < 0.001), normal lactate dehydrogenase level (p = 0.004), and absence of extramedullary disease (p < 0.001) were identified as the significant factors for achieving CR/CRi.
Among patients with Ph-positive ALL, 23 (62%) achieved CR and nine (24%) achieved CRi after induction chemotherapy. One (3%) patient had primary refractory disease, and death occurred in three (8%) patients, including two due to infection and one due to uncontrolled disease burden. The ORR of patients with age < 40 years (p = 0.028), male (p = 0.002), WBC count < 30.0 × 103/μL (p < 0.001), and without extramedullary disease (p < 0.001) was higher than that of patients without these factors. No statistically significant differences were observed in ORR, as well as the incidence of induction failure and death, between the Ph-negative and Ph-positive ALL groups (Fig. 1).
Long-term outcomes of Ph-negative ALL
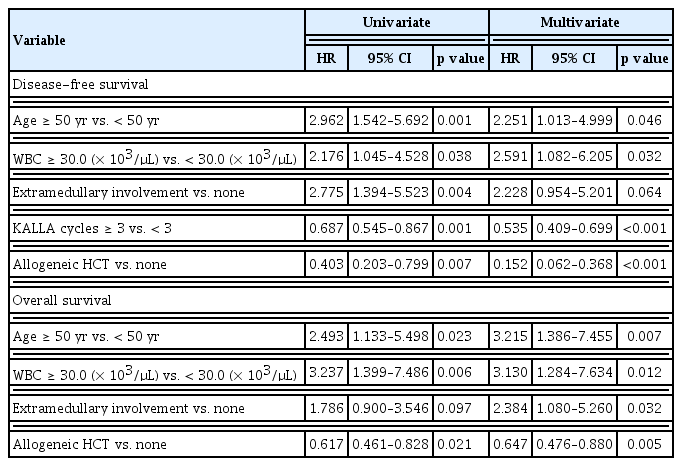
Overall, 62 patients with Ph-negative ALL received a median of three cycles of the KALLA 1406 regimen, and 25 (40.3%) patients underwent frontline allo-HCT. Supplementary Fig. 1 shows the flowchart of all patients. With a median follow-up of 24.5 months, 39 (63%) patients experienced disease relapse and 25 (40%) patients died during the treatment. The rates of DFS and OS at 2 years in patients with Ph-negative ALL were 42% (95% CI, 29.3% to 54.4%) and 63% (95% CI, 49.2% to 74.3%), respectively (Supplementary Fig. 2). In the multivariate analysis, age < 50 years (HR, 2.251; p = 0.046), WBC count < 30.0 (× 103/μL) (HR, 2.591; p = 0.032), KALLA regimen of ≥ 3 cycles (HR, 0.535; p < 0.001), and allo-HCT (HR, 0.152; p < 0.007) were identified as the favorable factors for DFS. Age < 50 years (HR, 3.215; p = 0.007), WBC count < 30.0 (× 103/μL) (HR, 3.130; p = 0.012), and allo-HCT (HR, 0.647; p = 0.005) were identified as the significant factors for better OS, whereas extramedullary involvement was identified as the unfavorable factor for survival (HR, 2.384; p = 0.032) (Table 3).
Long-term outcomes of Ph-positive ALL
A median of three cycles of the KALLA 1407 regimen were administered to 37 patients with Ph-positive ALL. Allo-HCT was administered to 14 patients after they achieved remission. With a median follow-up duration of 26 months, the cumulative incidence rates of relapse and death were 65% and 43%, respectively. The estimated 2-year DFS and OS rates were 31.2% (95% CI, 16.2% to 47.6%) and 49.1% (95% CI, 30.2% to 65.5%), respectively (Supplementary Fig. 3). However, no statistically significant differences were observed between the Ph-negative and Ph-positive ALL groups with regard to long-term survival rates (Fig. 2). In the multivariate analysis, age < 40 years (HR, 5.616; p = 0.015), KALLA regimen of ≥ 3 cycles (HR, 0.384; p < 0.001), and allo-HCT (HR, 0.061; p < 0.001) were identified as the favorable factors for DFS, whereas KALLA regimen of ≥ 3 cycles (HR, 0.616; p = 0.03) and allo-HCT (HR, 0.277; p = 0.006) were identified as the significant factors for better OS (Table 4).
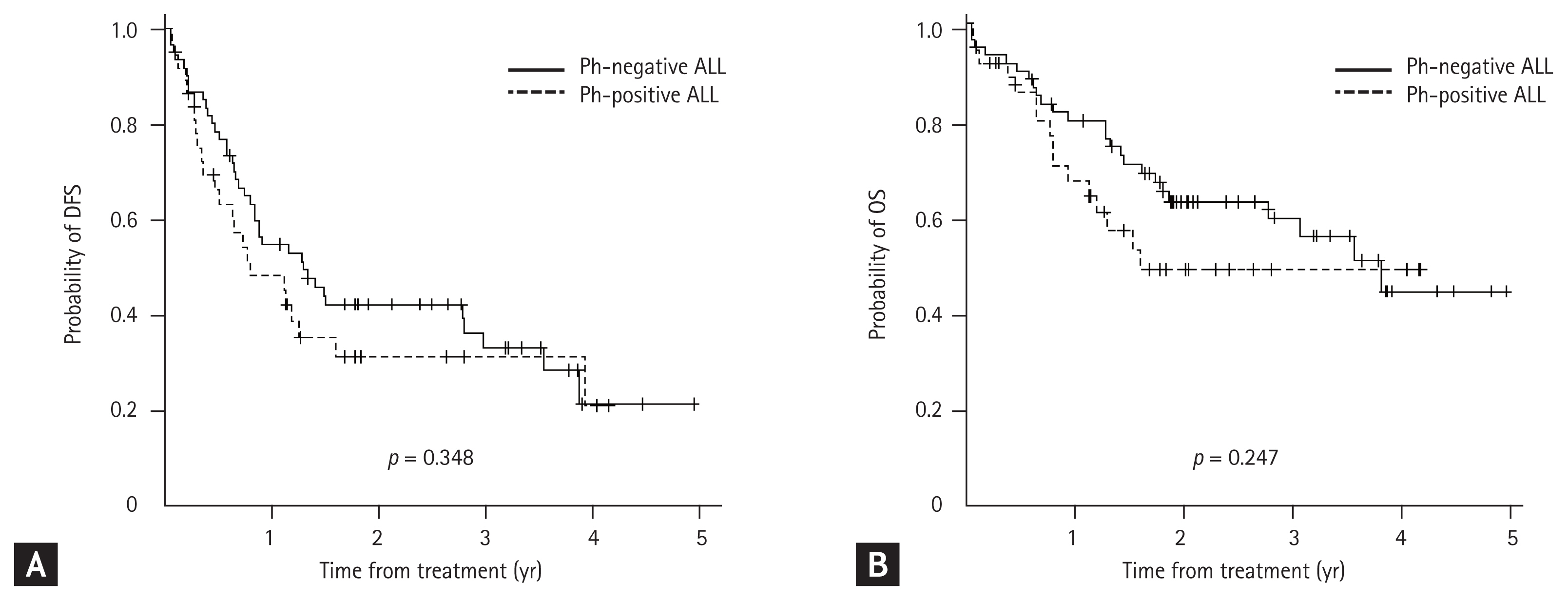
Kaplan–Meier curves according to the diagnosis. There were no statistically significant differences in long-term survival outcomes in terms of (A) disease-free survival (DFS) and (B) overall survival (OS) in patients who received the KALLA 1406/1407 regimens. Ph, Philadelphia; ALL, acute lymphoblastic leukemia.
Adverse events
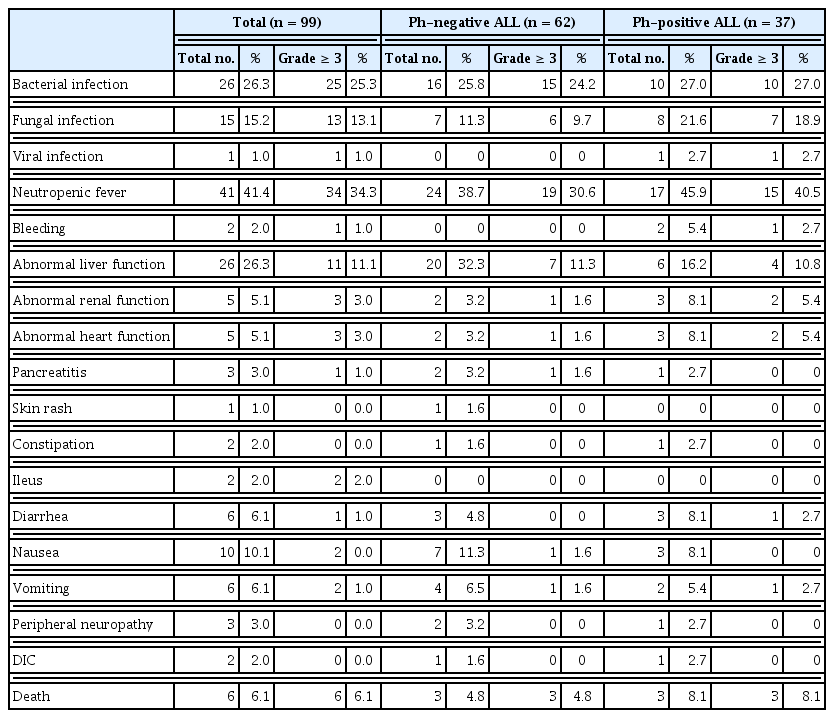
Cytopenia was the most common hematologic adverse event that occurred during induction chemotherapy. All patients received red blood cell and PLT transfusion. At least one dose of granulocyte-colony stimulating factor was also administered to the majority of patients after induction therapy. Regarding nonhematologic adverse events, neutropenic fever (41.4%) and bacterial infection (26.3%) were the most common and severe (grades ≥ 3) (Table 5). The frequency of adverse events in the Ph-negative ALL group was in the order of neutropenic fever, abnormal liver function, and bacterial infection, and that in the Ph-positive ALL group was in the order of neutropenic fever, bacterial infection, and fungal infection. Nausea, vomiting, diarrhea, abnormal renal function, and cardiac toxicity were the minor events. Six patients (four with Ph-negative ALL and two with Ph-positive ALL) discontinued the KALLA regimen after achieving CR/CRi by induction therapy due to toxicities. The frequency of adverse events tended to be higher in patients aged > 40 years. The incidence rates of severe events and death were also higher in patients with advanced age. The adverse events that occurred during consolidation therapies were manageable, and severe events were rare.
Impact of allo-HCT
A total of 25 (40%) patients with Ph-negative ALL underwent frontline allo-HCT, and 14 (38%) patients with Ph-positive ALL received allo-HCT. Patients who could proceed to allo-HCT exhibited significantly superior survival in terms of DFS and OS in both the Ph-negative and Ph-positive ALL groups (Fig. 3). Among those with Ph-negative ALL, 12 (48.0%) had disease relapse, and the median duration from stem cell transplantation to relapse was 10.3 months. Of the 14 patients with Ph-positive ALL, five (35.7%) experienced relapse with 11.1 months of median remission duration.

Kaplan–Meier curves according to the allogeneic hematopoietic cell transplantation (Allo-HCT). Allo-HCT was significantly associated with (A) disease-free survival (DFS) and (B) overall survival (OS) in Philadelphia (Ph)-negative acute lymphoblastic leukemia (ALL) and (C) DFS and (D) OS in Ph-positive ALL.
Outcomes according to the age
In the Ph-negative ALL group, the most significant cut-off age for DFS (p < 0.001) and OS (p = 0.019) was 50 years. The 2-year DFS and OS rates in younger patients (age < 50 years) were 52.8% and 71.4%, respectively, whereas those in older patients (age ≥ 50 years) were 25.4% and 50.4%, respectively. In the Ph-positive ALL group, patients aged < 40 years showed a tendency toward better DFS (p = 0.053) and OS (p = 0.065). The 2-year rates of DFS and OS in younger patients (age < 40 years) were 64.9% and 77.9%, respectively, wheres those in older patients (age ≥ 50 years) were 19.8% and 39.3%, respectively (Fig. 4).
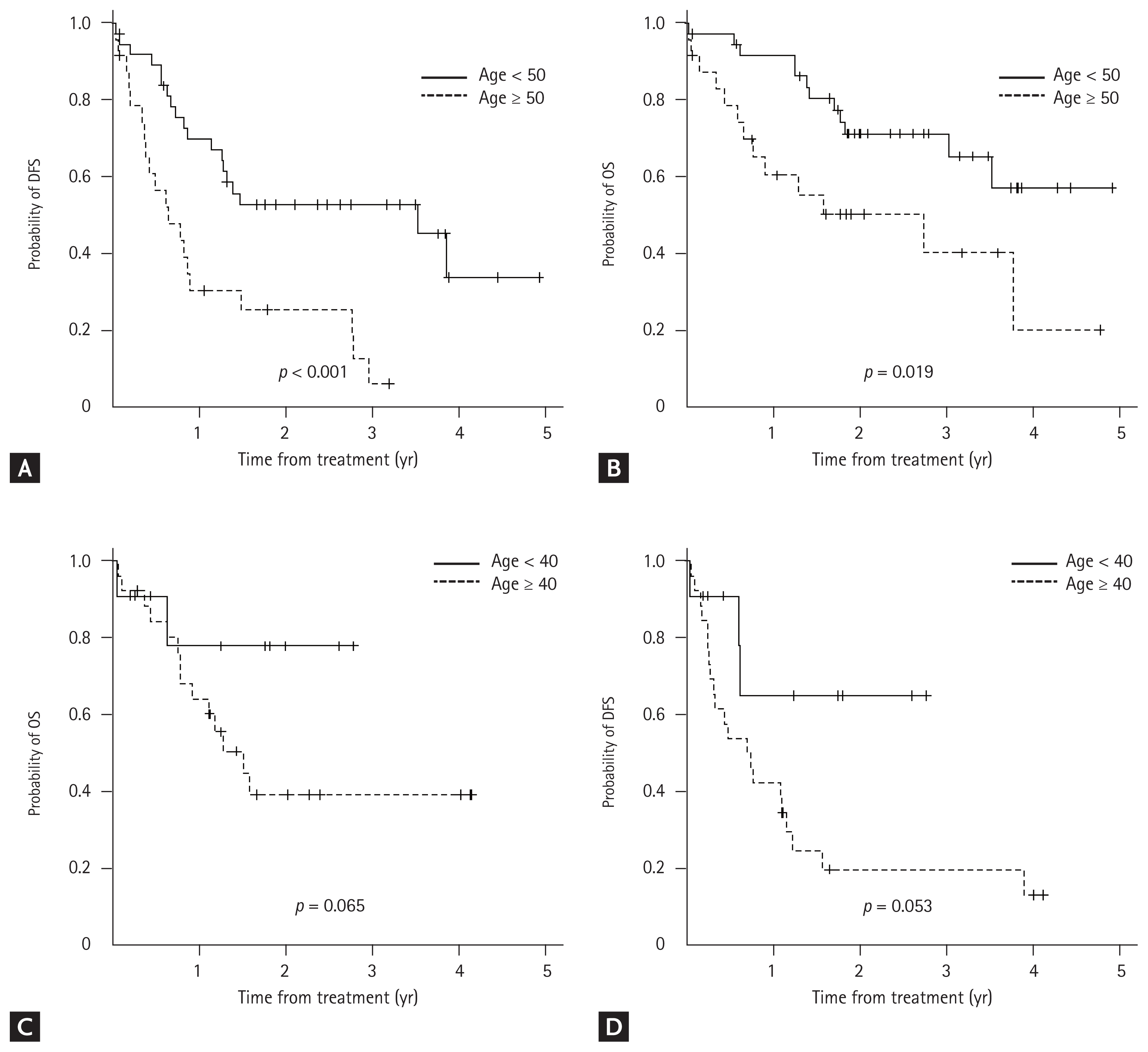
Kaplan–Meier curves according to the cut-off age. Patients with Philadelphia (Ph)-negative acute lymphoblastic leukemia (ALL) aged < 50 years showed a superior (A) disease-free survival (DFS) and (B) overall survival (OS) as compared to those aged ≥ 50 years. Patients with Ph-positive aged < 40 years showed a tendency of better (C) DFS and (D) OS than those aged ≥ 40 years.
The incidence of adverse events was higher in the older age subgroup, especially that of neutropenic fever. Mortality occurred in five patients in the older age group, including three due to infection, one due to uncontrolled leukemia, and one due to treatment toxicity, whereas only one patient died in the younger age group due to infection during induction therapy. In total, 26 patients (26.3%) received reduced doses of KALLA therapy because of toxicities and infections. Although patients aged > 55 years received age-adjusted chemotherapy, they did not tolerate the planned treatment, which resulted in 32.5% of patients (13 of 40 patients) receiving reduced doses of chemotherapy.
DISCUSSION
The KALLA 1406/1407 regimens were a newly developed protocol by the KAALLWP for treating adult patients with ALL, which included the concept of delayed intensification with daunorubicin, vincristine, dexamethasone, and cyclophosphamide, along with an increased dose of l-asparaginase of up to 10,000 U/m2/day during induction, consolidation, and delayed intensification for patients with Ph-negative ALL. In this study, 62 adult patients with Ph-negative ALL and 37 with Ph-positive ALL received the KALLA 1406/1407 regimens, respectively. Overall, the CR rates of Ph-negative ALL and Ph-positive ALL groups were 92% and 86%, respectively. The rates of DFS and OS at 2 years were 42% and 63% in the Ph-negative ALL group and 31.2% and 49.1% in the Ph-positive ALL group, respectively. However, the long-term outcomes between both groups showed no statistical differences.
Previous studies have reported the results of multiagent chemotherapy for adult patients with ALL (Table 6). In the majority of studies, > 90% of patients achieved CR, whereas long-term survival outcomes were still unsatisfactory compared to those in pediatric patients despite the introduction of TKI and monoclonal antibodies [27–33]. A prospective study on 282 patients with Ph-negative ALL reported the clinical impact of modified hyper-CVAD regimen plus rituximab according to CD20 expression. The CR rate was 95%, and the 3-year CR duration and OS were 60% and 50%, respectively. In particular, the long-term outcomes in younger patients (age < 60 years) were superior than those in older patients [28]. Although the TKI-based multiagent chemotherapy demonstrated promising results in patients with Ph-positive ALL, additional approaches are required to improve the long-term survival rates [30–33]. Compared with previous data, the results of KALLA 1406/1407 treatment were not much different. However, in the present study, younger patients with Ph-negative ALL (age < 50 years) and those with Ph-positive ALL (age < 40 years) had 2-year OS rates of 71.4% and 77.9%, respectively, which suggested that younger patients have better survival benefits with the KALLA protocol.
Disease relapse is a major cause of poor prognosis in adult patients, despite achieving CR during intensive chemotherapy [4]. Several previous studies have emphasized the need for further progress in the treatment of adult ALL, which may maintain long-term remission without severe toxicities [21,22]. Till date, allo-HCT has been the standard therapy for ALL in adults, and our results support the need for allo-HCT to maintain long-term remission and survival. Our results showed that patients who received allo-HCT after achieving CR had significantly better survival outcomes than patients who received the KALLA regimen alone. Meanwhile, in 33 (33.3%) patients, an HLA-matched donor could not be found or they were not suitable for transplantation due to old age and weakness. Instead, they proceeded with delayed intensification without severe adverse events. However, 18 (54.5%) patients showed disease relapse after the delayed intensification therapy, which indicated the need for more effective novel approaches to maintain remission for patients who cannot undergo allo-HCT. The antileukemic effect of asparaginase on lymphoblastic leukemia cells is well known; asparaginase interferes with the synthesis of l-asparagine (Asn) and catalyzes the conversion of Asn into aspartic acid and ammonia, depleting materials to synthesize DNA, RNA, and proteins and ultimately leading to cell death [34–36]. One of the key factors for the successful treatment of pediatric ALL was the intensive use of asparaginase [37]. Previous studies have demonstrated that the effect of asparaginase on clinical outcomes was dependent on the frequency or intensity of its use. Although l-asparaginase is known to cause more toxicities in older age patients, the potential for improved survival has been strongly suggested by recent studies on ALL in adults [20,38]. Results from several studies have suggested an asparaginase activity level of 0.1 IU/mL as the target necessary to ensure adequate Asn depletion [39,40]. However, the optimal dose and schedule of asparaginase for adult ALL are not yet known due to limited data. In the phase II study for an escalated dose of daunorubicin during induction therapy for Ph-negative ALL by conducted by the KAALLWP (KALLA 0501), 4,000 U/m2/day of l-asparaginase was administered during induction and first consolidation [13]. As a study to redeem the KALLA 0501 protocol, the KAALLWP increased the dosage of l-asparaginase according to age (age 15 to 24 years, 10,000 U/m2/day; age 25 to 54 years, 6,000 U/m2/day; and age ≥ 55 years, 4,000 U/m2/day) due to the concern of toxicities. In the present study, one of four patients had elevated liver enzymes that were also induced by other agents, whereas the major adverse effects of asparaginase such as pancreatitis and hyperglycemia were rare and manageable even in older aged patients. Therefore, it is worth to consider intensifying the use of asparaginase in patients with Ph-negative ALL based on their condition as well as age for long-term remission and survival of older adults.
In conclusion, the pediatric-inspired regimen with late intensification and increased dosage of l-asparaginase demonstrated tolerable toxicities in adult patients. Although the KALLA 1406/1407 protocol was not significantly superior to previous data, they were effective in younger patients. However, older adults still required more effective strategies to maintain long-term remission. Frontline allo-HCT must be recommended for patients who are eligible for intensive conditioning therapy after achieving CR. Although several studies are ongoing, the outcome of older age patients remains extremely poor. Therefore, there is a need for large-scale clinical trials for combination therapy with novel agents targeting adult patients with ALL. Moreover, minimal residual disease-based effective maintenance strategies should be established.
KEY MESSAGE
1. The KALLA 1406/1407 protocal was effective for adult acute lymphoblastic leukemia, especially for younger patients.
2. An increased dosage of L-asparaginase resulted in tolerable toxicities in adults.
3. Frontline allogeneic stem cell transplantation should be recommended after achieving complete remission.
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.