Safety of direct oral anticoagulants compared to warfarin in cirrhotic patients with atrial fibrillation
Article information
Abstract
Background/Aims
The safety of direct oral anticoagulants (DOACs) compared with warfarin in patients with both nonvalvular atrial fibrillation (AF) and clinically confirmed liver cirrhosis (LC) has not been well studied. We compared the risk of a major bleeding event between DOAC and warfarin treatments in this patient population.
Methods
A total of 238 cirrhotic patients with AF were retrospectively analyzed. The major bleeding event risk was compared between DOAC- and warfarin-treated groups. The median follow-up duration was 5.6 years.
Results
Among the 238 study patients with LC and AF, 128 (53.8%) received DOACs and 110 (46.2%) received warfarin. The mean patient age was 68.8 years, and 78.2% were men. A major bleeding event occurred in 10 and 20 patients in the DOAC and warfarin groups, respectively, most commonly caused by gastrointestinal bleeding (70.0%). The cumulative risk of major bleeding did not differ between the groups by log-rank test (p = 0.12). This finding did not change when using 60 propensity score-matched pairs. A multivariable Cox regression model indicated that the concomitant use of antiplatelet agents (adjusted hazard ratio [aHR], 2.06; 95% confidence interval [CI], 1.00 to 4.30; p = 0.048) and presence of esophageal or gastric varices confirmed by endoscopic examination (aHR, 2.31; 95% CI, 1.03 to 5.17; p = 0.04) were associated with major bleeding in the entire cohort.
Conclusions
A major bleeding event risk is not increased by DOAC compared with warfarin treatment. Antiplatelet agent use and varices are independently associated with a higher risk of major bleeding during anticoagulation.
INTRODUCTION
Atrial fibrillation (AF) is the most commonly sustained cardiac arrhythmia, occurring in 1% to 2% of the general population. Due to the increased risk of thromboembolic events from AF, current treatment guidelines recommend anticoagulation for stroke prevention in these patients [1–3]. Traditionally, the vitamin K antagonist warfarin has been widely used for this purpose in such cases. Liver cirrhosis (LC) has a unique pathophysiology in terms of hemostasis due to alterations in coagulation factor production, thereby resulting in a disrupted coagulative balance [4–6]. Cirrhotic patients are not only at an increased risk of bleeding but also of venous thromboembolism [7–9]. Due to this unstable balance in their coagulation system, many physicians hesitate to use warfarin to prevent thromboembolic events in patients with LC and AF despite its clinical necessity.
Direct oral anticoagulants (DOACs) have recently begun to replace warfarin in clinical practice owing to their better efficacy and safety, as well as their greater ease of use [10]. However, all of the major clinical trials of DOACs to date have excluded patients with advanced liver diseases, including LC, due to safety concerns [11–15]. In this regard, most DOACs are currently not recommended in patients with advanced cirrhosis, such as cases with a liver function of Child-Pugh class B or C, due to limited evidence. Moreover, there are presently no concrete recommendations for the use of DOACs to prevent thromboembolic events in patients with LC and AF. Several previous studies have evaluated and compared the efficacy and safety of DOACs and warfarin in this complex patient population and have reported that DOACs have a higher level of safety and a comparable efficacy [16–18]. Notably however, these previous studies have only analyzed small numbers of patients with varied treatment indications for DOAC use [17,18]. Other population-based studies have also suggested that DOACs may be considered for use in cirrhotic patients with AF given the lower risk of bleeding with comparable efficacy to warfarin [19–21]. However, these reports lacked detailed information on the liver disease status and ascertained the presence of LC using disease codes rather than clinical diagnoses. Hence, we performed our present study to comprehensively evaluate the safety of DOACs compared to warfarin in a real-life clinical setting among patients with both LC and AF.
METHODS
Study population and clinical information
This study was approved by the Institutional Review Board (IRB) of Asan Medical Center, Seoul, Republic of Korea (IRB number: 2019–1176) and was exempted from requiring patient consent by the IRB due to the retrospective nature of the analyses. We included patients who met all of the following criteria: (1) adult patients over 18 years of age; (2) clinically diagnosed with LC and non-valvular AF; and (3) treated with either warfarin or DOAC. A total of 238 patients were included, of which 128 had received DOACs including apixaban, edoxaban, dabigatran, and rivaroxaban between July 2012 and December 2018. The remaining 110 patients had been treated with warfarin between January 2000 and December 2014 and were included as a historical control group [22]. We excluded patients who met any of the following criteria: (1) a follow-up period of less than 1 month; (2) had undergone a liver transplantation; (3) had an uncontrolled malignancy or metabolic liver disorder; (4) had previously been treated with warfarin before switching to DOAC; (5) lack of clinical evidence of cirrhosis; and (6) lack of relevant clinical information.
LC was defined as the presence of any of the following: a coarse liver echotexture and nodular liver surface on ultrasonography, or clinical features of portal hypertension (e.g., ascites, splenomegaly, or varices) [23]. AF was documented by the absence of the P wave or an irregularity in both the frequency and amplitude of the R-wave on 12-lead electrocardiogram or 24-hour Holter recordings [22]. All information on patient demographics, cirrhosis etiologies, laboratory data, liver-related comorbidities, and the confirmed presence of hepatocellular carcinoma and varices by endoscopic examination were manually reviewed from the electronic medical records database of Asan Medical Center. In addition, known risk factors for ischemic stroke and cardiovascular comorbidities (i.e., hypertension, congestive heart failure [CHF], diabetes mellitus, hyperlipidemia, coronary artery disease, and previous history of ischemic stroke or transient ischemic attack [TIA]) were examined.
The following scores were calculated for all of the included patients: CHADS2 and CHA2DS2VASc to assess the risk of an ischemic event from AF, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile internatinal normalized ratio (INR), Elderly (> 65), Drugs/alcohol used concomitantly (HAS-BLED) to estimate the risk of bleeding events following anticoagulation treatments, and the Child-Pugh score to assess the severity of LC. A detailed description of these scoring systems has been previously described elsewhere [24–26].
Primary and secondary outcomes and assessments
The primary outcome of interest in the present study was the incidence of major bleeding events in patients treated with DOACs compared with those treated with warfarin. A major bleeding event in our current study series was defined using the International Society on Thrombosis and Hemostasis criteria, i.e., (1) fatal bleeding; (2) bleeding in a critical area or organ (intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, or pericardial) or intramuscular bleeding with compartment syndrome; and/or (3) bleeding causing a fall in the hemoglobin level of 2.0 g/dL or more or leading to transfusion of 2 or more units of whole blood or red cells [27]. The secondary outcome of interest was the effectiveness of DOACs in preventing major ischemic events (stroke or TIA) in which a neurologic deficit of sudden onset was confirmed by a physician and by relevant imaging studies in the absence of hemorrhagic events or other possible causes such as infection, trauma, vasculitis, or a tumor. Patients were followed up from their first DOAC prescription until either the development of a major bleeding event or the last follow-up date (i.e., December 2020), whichever came first. The median follow-up duration of the study population was 5.6 years (interquartile range [IQR], 2.5 to 7.4).
Statistical analysis
The Student’s t test was used to compare quantitative variables, and the chi-square test or Fisher’s exact test, as appropriate, was used to compare categorical variables. The cumulative rates of a major bleeding event were estimated using the Kaplan-Meier method and compared using the log-rank test. A Cox proportional hazards model was used to assess independent predictive factors for major bleeding events, with multivariate adjustment for confounding variables that were deemed to be significant in the univariate analysis. Propensity score (PS)-matched analysis was used to minimize potential confounding between patients treated with DOACs and those treated with warfarin. PS values were computed using the following 18 variables: age, sex, diabetes, hypertension, CHF, hyperlipidemia, peripheral vascular disease, coronary disease, chronic kidney disease, serum creatinine, platelet count, serum albumin concentration, total bilirubin, CHADS2 score, CHA2DS2VASc score, HAS-BLED score, and use of anti-platelet agents. Missing values were imputed by linear interpolation using the MICE package. For PS matching, a nearest-neighbor 1:1 matching scheme with the caliper size of 0.1 was employed. None of the standardized differences for any baseline covariates in the matched exceeded 0.2. All statistical analyses were performed using the R program ( http://cran.r-project.org/). All reported p values were two-sided, and p values below 0.05 were considered statistically significant.
RESULTS
Baseline characteristics of the study population
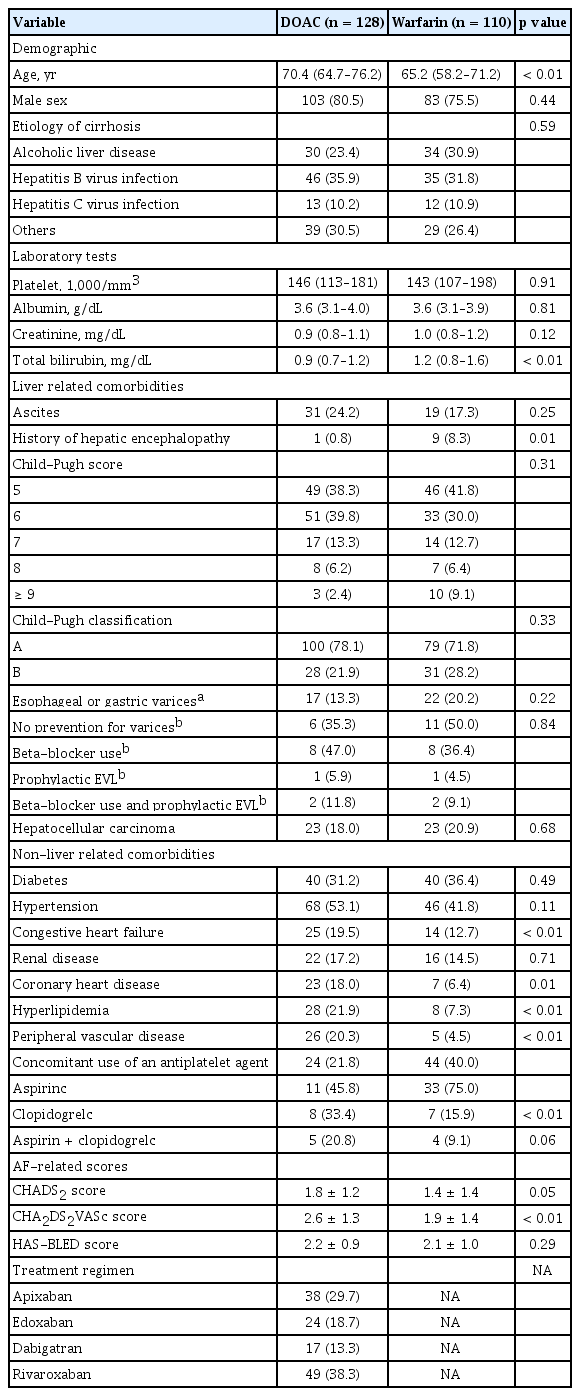
A total of 238 patients undergoing anticoagulation with either DOACs or warfarin were analyzed in this study (Fig. 1). There were 128 DOAC users and 110 warfarin users and the baseline characteristics of these two groups are summarized in Table 1. The median follow-up durations of the DOAC and warfarin groups were 3.3 years (IQR, 2.1 to 4.7) and 7.3 years (IQR, 4.7 to 11.4), respectively, which was a statistically significant difference (p < 0.001). The mean patient age was older in the DOAC group. The major LC etiologies in both groups were chronic hepatitis B and alcoholic liver disease.
No difference was observed between the liver-related comorbidities in the two groups except for the history of hepatic encephalopathy. The numbers of patients with advanced cirrhosis of Child-Pugh class B were 28 (21.9%) and 31 (28.2%) in the DOAC and warfarin groups, respectively. Among the patients with gastroesophageal varices, there was no significant difference between the two groups in the preventive measures for variceal bleeding before commencing the anticoagulation. CHF, coronary heart disease, hyperlipidemia, and peripheral vascular disease were significantly more prevalent in the DOAC group. Neither the proportion of patients treated simultaneously with an antiplatelet agent nor the type of antiplatelet agent used differed significantly between the two groups. There was no difference in the HAS-BLED score between the two groups, whereas the CHADS2 and CHA2DS2VASc scores were significantly higher in the DOAC group. Rivaroxaban was the most frequently prescribed DOAC (49, 38.3%), followed by apixaban (38, 29.7%), edoxaban (24, 18.7%), and dabigatran (17, 13.3%).
Clinical outcomes in the whole study cohort
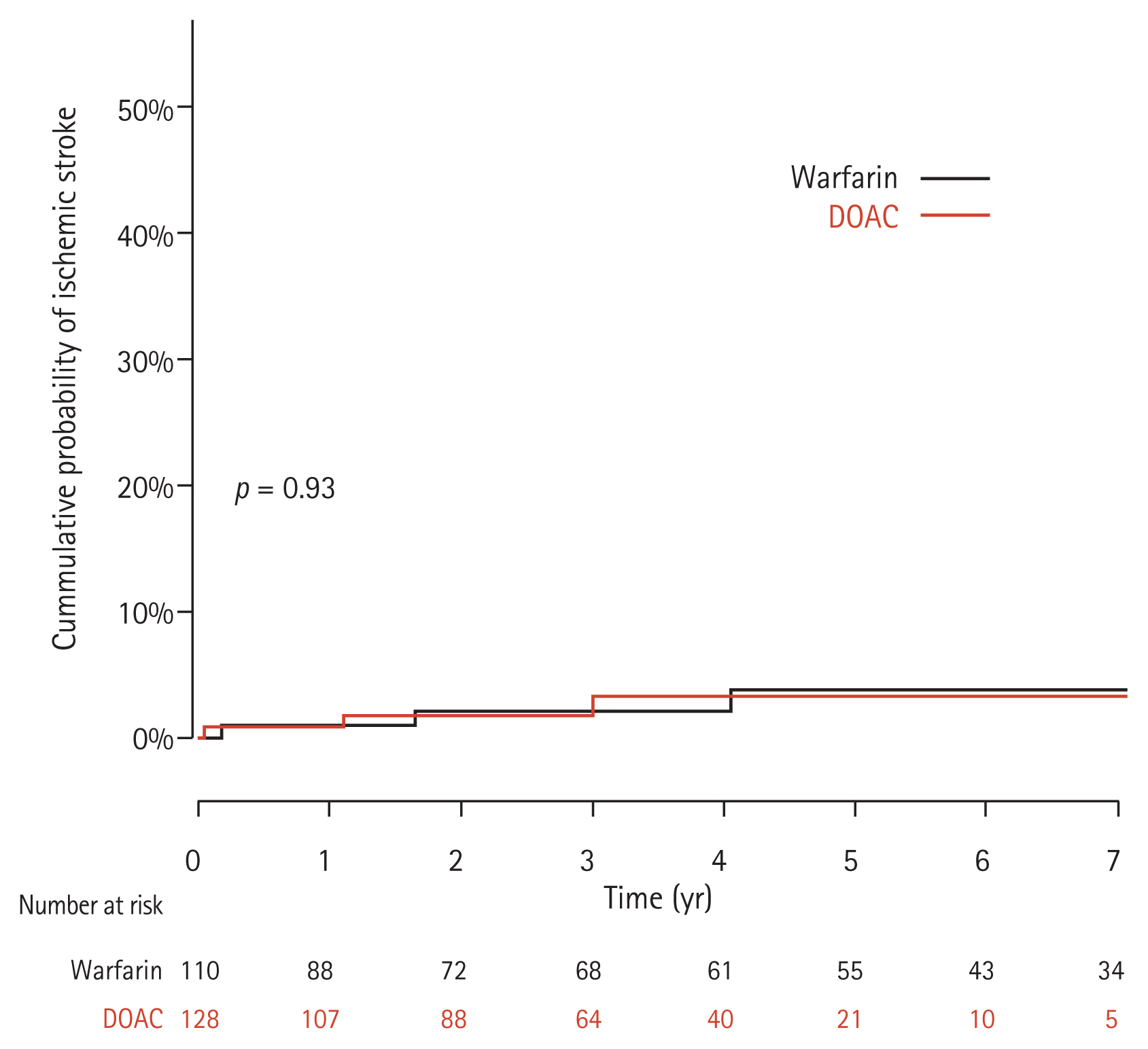
Among our 238 currents study patients, involving 976 person-years (PYs) of observation, 30 cases developed major bleeding events with an annual incidence of 3.1 per 100 PY. The 1-, 2-, 3-, and 5-year cumulative major bleeding incidences determined by Kaplan-Meier analysis were 4.6%, 7.8%, 10.5%, and 13.9%, respectively. In terms of the secondary outcomes, ischemic events occurred in 10 (4.2%) patients with a 1-, 2-, 3-, and 5-year cumulative risk of 0.9%, 1.9%, 1.9%, and 3.6%, respectively.
Comparison of the clinical outcomes between the DOAC and warfarin groups
Major bleeding events occurred in 10 patients in the DOAC group and 20 patients in the warfarin group. Gastrointestinal bleeding (n = 7), hematoma in the musculoskeletal system (n = 2), and intracranial hemorrhage (n = 1) were the major bleeding events in the DOAC group. Gastrointestinal bleeding was also the most common cause of bleeding (n = 14) in the warfarin group, followed by hemoperitoneum (n = 2), hematoma in the musculoskeletal system (n = 2), hemoptysis (n = 1), and intracranial hemorrhage (n = 1). The annual incidences of major bleeding events were 2.6 per 100 PY and 3.4 per 100 PY in the DOAC and warfarin groups, respectively. The cumulative rates of major bleeding events at 1-, 2-, 3-, and 5-years were 3.5%, 3.5%, 8.9%, and 10.7% in the DOAC group and 6.0%, 12.8%, 12.8%, and 17.0% in the warfarin group, respectively. Although the cumulative risk of major bleeding appeared to be lower in the DOAC group, this difference did not reach statistical significance via the log-rank test (p = 0.12) (Fig. 2). During the current study period, there was no significant change in the liver function between the two groups when assessed at 1 year after commencing anticoagulation (p = 0.87).
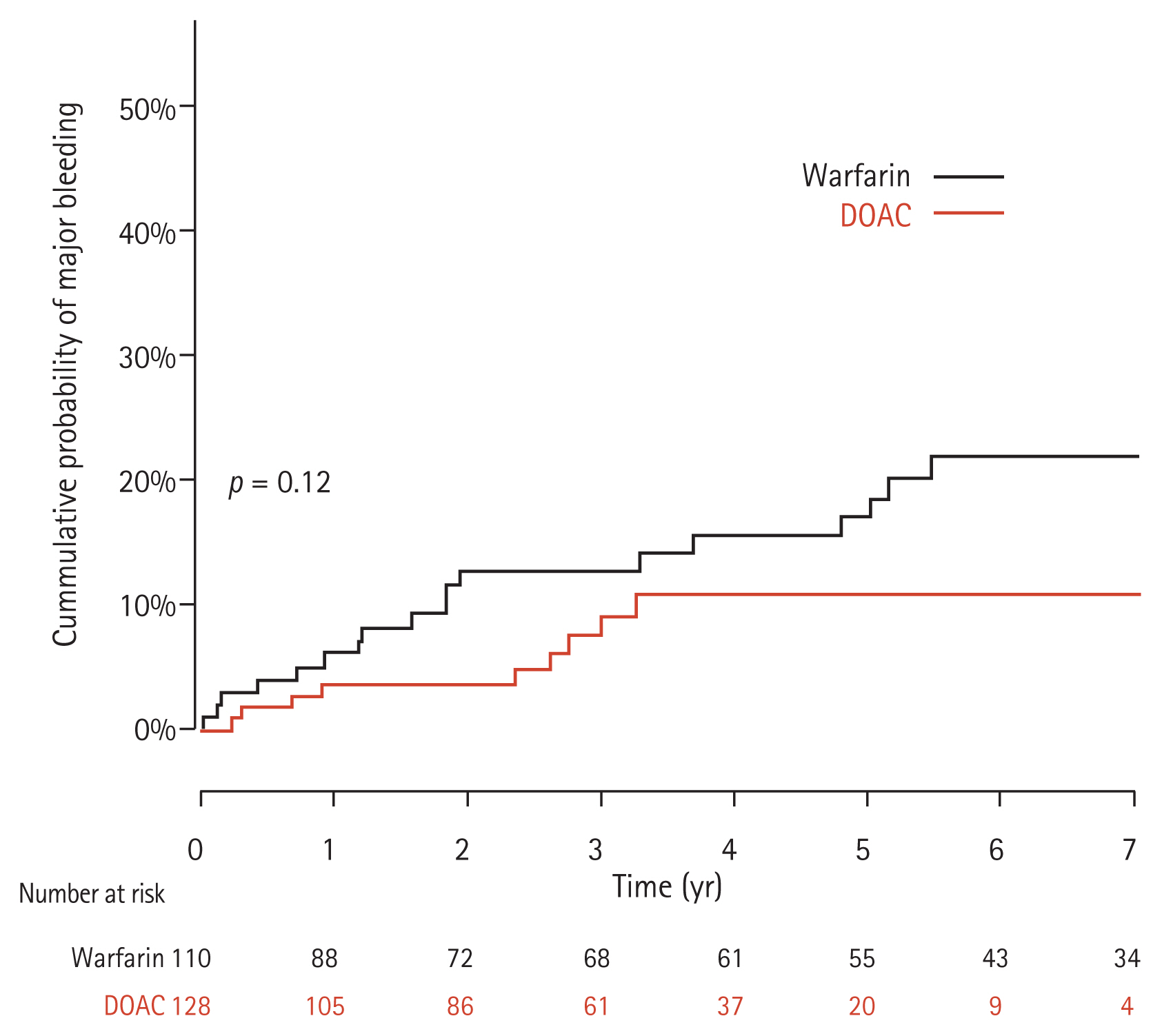
Cumulative incidence of major bleeding events between the full direct oral anticoagulant (DOAC) and warfarin groups.
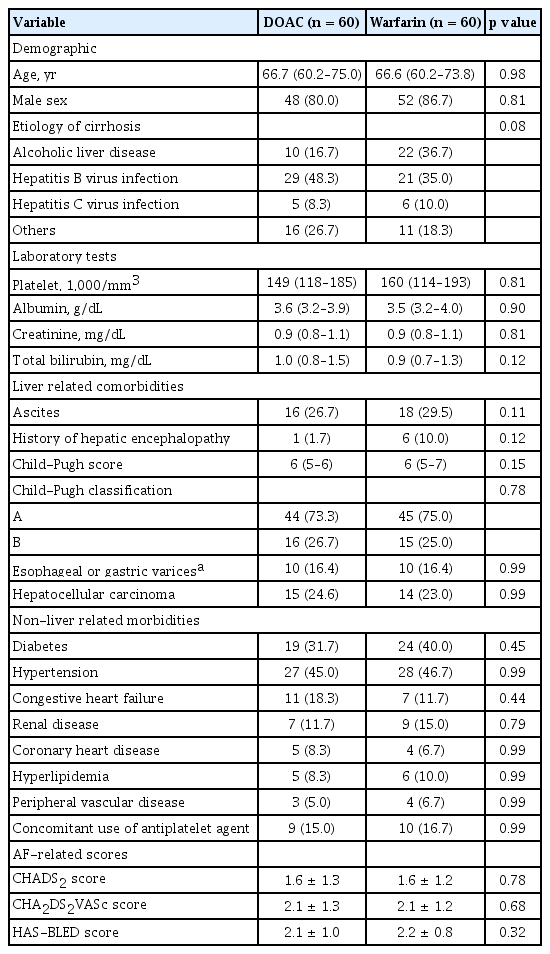
To conduct a more meaningful comparison and to minimize the impact of possible confounders on the measured risk of a major bleeding event in the two groups, 60 PS-matched pairs were generated from the whole study cohort. The baseline characteristics between these two matched groups are presented in Table 2. After PS matching, all baseline covariates were found to be well balanced and did not exceed 0.2 of the standardized mean difference. In these PS-matched pairs, the risk of a major bleeding event did not differ between the DOAC and warfarin treatments (p = 0.34) (Fig. 3).

Cumulative incidence of major bleeding events between the direct oral anticoagulant (DOAC) and warfarin groups after propensity score matching.
With regard to the risk of an ischemic event, three and seven patients developed this condition in the DOAC and warfarin groups, respectively which was not statistically significant (Fig. 4).
Predictive factors for the development of a major bleeding event
Using univariate Cox regression analysis, we found with reference to warfarin treatment that DOAC treatment is not a predictive factor for a major bleeding event (hazard ratio [HR], 0.61; 95% confidence interval [CI], 0.28 to 1.34; p = 0.22). In contrast, concomitant antiplatelet agent use (HR, 2.30; 95% CI, 1.12 to 4.70; p = 0.02) and the presence of esophageal or gastric varices (HR, 2.64; 95% CI, 1.21 to 5.78; p = 0.02) were significant predictive factors in this regard. The latter two variables were also found to be statistically significant predictors by multivariable Cox regression analysis with adjusted hazard ratios (aHRs) of 2.06 (95% CI, 1.00 to 4.30; p = 0.048) for concomitant antiplatelet use and 2.31 (95% CI, 1.03 to 5.17; p = 0.04) for the presence of esophageal or gastric varices (Table 3). Not surprisingly, treatment with DOAC in comparison with warfarin was not a predictive factor for a major bleeding event by multivariable analysis (aHR, 0.78; 95% CI, 0.35 to 1.77; p = 0.55).
DISCUSSION
We found in our present study that DOAC treatment in patients with LC and AF does not increase the risk of a major bleeding event compared with warfarin. Indeed, the risk of major bleeding appeared to be lower in the DOAC group in our current study series although this did not reach statistical significance. The risk of an ischemic event was also comparable between these two anticoagulation treatments.
Previous studies have reported that DOACs significantly reduce the risk of major bleeding and intracranial hemorrhage compared with warfarin [20,21]. Our current findings are in keeping with these prior results although we did not demonstrate statistical significance. However, the aforementioned previous reports were population-based studies which only used disease codes to define LC. This classification of LC is unlikely to be as accurate as the manual review of the medical records we conducted for our present analyses. In addition, we here provided detailed information on liver-related morbidities as well as serum parameters to assess the severity of LC, which was a key strength of our present investigation. Prior retrospective studies have demonstrated that the use of DOACs has a comparable if not marginally lower risk of a major bleeding event compared to warfarin [17,18,28]. Pastori et al. [18] also reported in liver fibrosis patients, as determined by fibrosis-4 (FIB-4) index, the DOAC use was associated with a lower risk of major bleeding compared with use of warfarin, whereas no risk difference was observed between these treatments in patients without liver fibrosis. The evidence to date thus suggests that patients with advanced liver disease receiving warfarin are prone to an increases risk of major bleeding. Patients with advanced liver disease, particularly those with LC, have more sophisticated coagulation systems than healthy subjects and a reduced liver function carries a greater risk of bleeding. Indeed, we have previously reported that warfarin use in patients with advanced LC and AF increases the risk of major bleeding compared with non-use, with a similar thromboembolic risk in both instances [22]. These findings may support the notion that the bleeding risks from anticoagulation treatments in patients with advanced LC and AF outweigh the benefits in preventing a thromboembolism.
DOACs have begun to replace warfarin in patients with AF for preventing thromboembolism over the past decade. The therapeutic range of warfarin is very narrow and can be affected by various factors such as diet, drug-drug interactions, and liver function, thereby requiring frequent monitoring of the serum levels. In contrast, DOACs are safer and are associated with a lower all-cause mortality compared to warfarin, driven primarily by a decrease in the fatal intracranial bleeding risk [29]. In addition, DOACs can generally be used without the need to monitor their levels. Hence, DOACs are more convenient to administer for both clinicians and patients than warfarin. These notions of better safety and convenience with the use of DOACs in patients without LC may be applied to patients with LC given our current results the findings of previous studies. Additional supporting evidence for this comes from recent national data from the United States indicating that DOACs are associated with a lower incidence of bleeding compared to warfarin in secondary analyses with a HR of 0.49 [30]. Notably, the risk of major bleeding appeared to be lower in the DOAC group than warfarin group in our entire current study cohort and in our PS-matched cohort. However, the primary outcome of our present study is a rare clinical event, and the incidence of major bleeding was therefore relatively small. This may well have hampered the statistical power of these analyses.
Our present findings also addressed the incremental risk of a major bleeding event in patients who received concomitant antiplatelet agents with their DOAC or warfarin treatment. This finding was consistent with those of previous studies that suggested that a combined use of anticoagulants and antiplatelet agent increased the incidence of bleeding [31,32]. Another important finding from our present study was that presence of esophageal or gastric varices was significantly associated with a higher risk of major bleeding events. Of note also, the most common cause of major bleeding in the present study was gastrointestinal bleeding. This emphasized the importance of evaluating of the risk of variceal bleeding prior to the commencement of anticoagulation treatment. Hence, preemptive treatments involving the concomitant use of beta-blockers or endoscopic interventions should be considered if necessary.
The strength of our present study was its large sample size and ample follow-up period. As a retrospective study based on manually reviewed clinical records rather than a disease-code based study, our study also analyzed a larger number of cirrhotic patients who had been treated using DOACs for AF than any prior report. Our current analyses also included a homogeneous indication for DOAC use, i.e., non-valvular AF. This helped to simplify the purpose and required duration of anticoagulation, and also the monitoring of clinical outcomes during the treatment period. We also applied PS-matching analysis to minimize the effects of existing confounders and provide a fairer comparison between the DOAC and warfarin groups. However, our current study also had some notable limitations. First, due to the retrospective nature of the analyses, some biases were unavoidable. Despite our use of PS-matched analysis, unmeasured biases cannot be inherently overcome. Second, although a relatively large number of cirrhotic patients receiving DOACs was included in the present study, this resulted in a small number of patients manifesting a primary outcome during our study period. Our analysis may therefore have been statistically underpowered in relation to revealing differences between the two treatment groups. Third, few of our included DOAC patients changed their regimen or dose during study period for various reasons. However, we considered the duration of DOAC administration to be the period from the first prescription until the last prescription of any types of DOAC agent, regardless of any dose changes. Fourth, among the patients in the DOAC group, 61 (47.7%) had a dose modification during the study period or started with a reduced dose from the beginning of treatment. However, the warfarin dose, in general, was determined by the level of INR, which made it difficult to calculate the average maintenance dose of warfarin. This might have caused a bias that affected the risk of major bleeding. Lastly, comparisons of the bleeding risk among different types of DOAC drugs were not evaluated due to the small number of patients analyzed. In addition, the definition of LC in the present study was based on clinical criteria and not on the results of a histologic examination which are more prone to subjectivity.
In conclusion, in a relatively large cohort of patients with LC and AF, a comparable risk of a major bleeding event was observed between cases receiving DOACs and those taking warfarin. The concomitant use of antiplatelet agents and the presence of esophageal or gastric varices at the time of treatment initiation are significant predictive factors for major bleeding events. DOACs appear to be a feasible alternative to warfarin in patients with AF and LC given their comparable safety and greater ease of use. However, this finding needs to be validated in a future prospective study of a large cohort.
KEY MESSAGE
1. A major bleeding event risk is not increased by direct oral anticoagulant compared with warfarin treatment in cirrhotic patients with atrial fibrillation.
2. Concomitant use of antiplatelet agent and varices are independently associated with a higher risk of major bleeding during anticoagulation.
Notes
No potential conflict of interest relevant to this article was reported.