Efficacy of lower dose pirfenidone for idiopathic pulmonary fibrosis in real practice: a retrospective cohort study
Article information
Abstract
Background/Aims
Pirfenidone slows the progression of idiopathic pulmonary fibrosis (IPF). We investigated its efficacy and safety in terms of dose and disease severity in real-world patients with IPF.
Methods
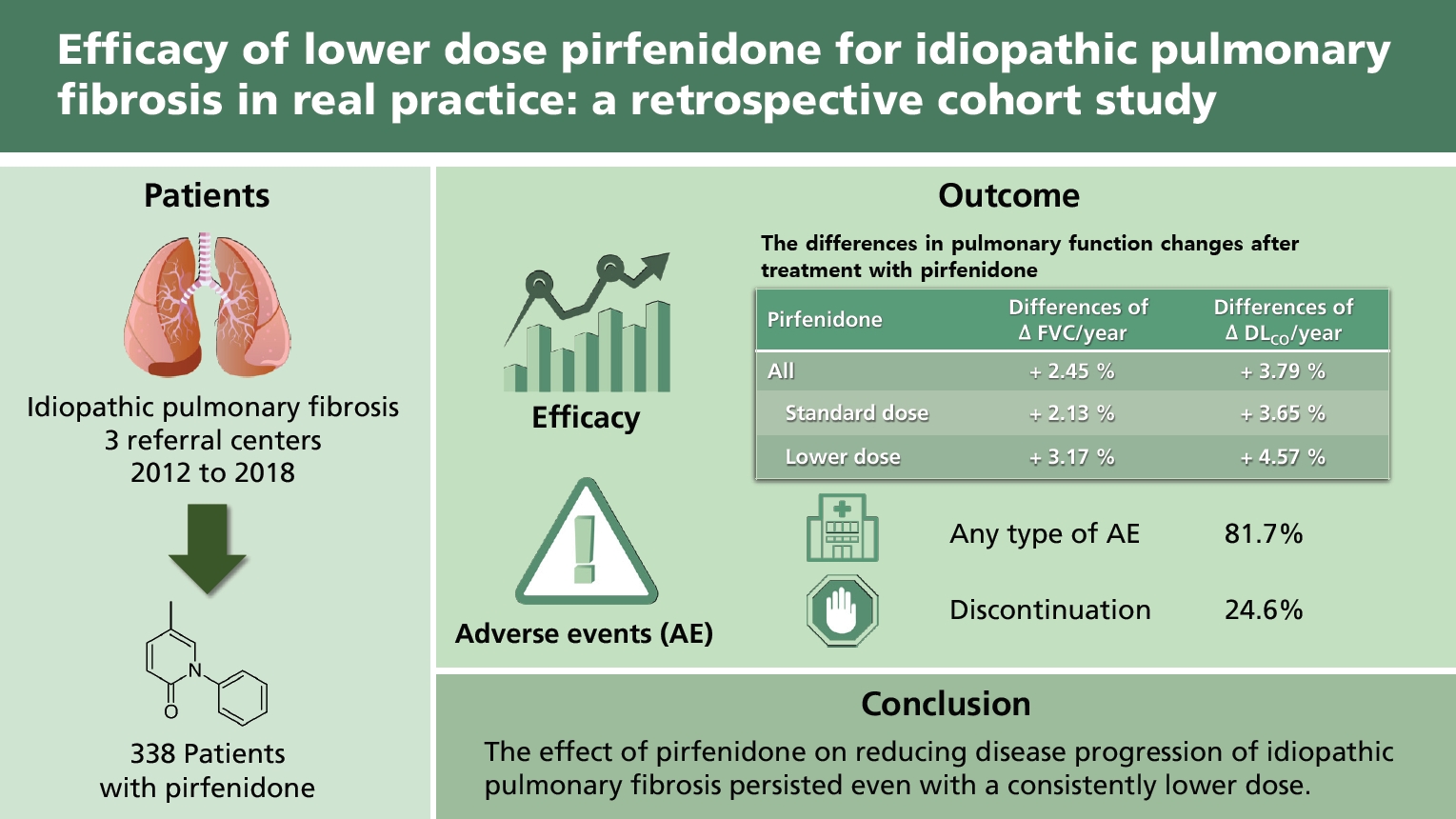
This multicenter retrospective cohort study investigated 338 patients treated with pirfenidone between July 2012 and March 2018. Demographics, pulmonary function, mortality, and pirfenidone-related adverse events were also investigated. Efficacy was analyzed according to pirfenidone dose and disease severity using linear mixed-effects models to assess the annual decline rate of forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO).
Results
The mean %FVCpredicted and %DLCOpredicted values were 72.6% ± 13.1% and 61.4% ± 17.9%, respectively. The mean duration of pirfenidone treatment was 16.1 ± 9.0 months. In the standard dose (1,800 mg/day) group, the mean %FVCpredicted was −6.56% (95% confidence interval [CI], −9.26 to −3.87) per year before, but −4.43% (95% CI, −5.87 to −3.00) per year after treatment with pirfenidone. In the non-standard lower dose group, the mean %FVCpredicted was −4.96% (95% CI, −6.82 to −3.09) per year before, but −1.79% (95% CI, −2.75 to −0.83) per year after treatment with pirfenidone. The FVC decline rate was significantly reduced, regardless of the Gender-Age-Physiology (GAP) stage. Adverse events and mortality were similar across dose groups; however, they were more frequent in GAP stages II–III than in the stage I group.
Conclusions
The effect of pirfenidone on reducing disease progression of IPF persisted even with a consistently lower dose of pirfenidone.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a fibrotic interstitial pneumonia of unknown etiology. It is a chronic, progressive disease with an extremely poor prognosis, and a median survival of approximately 3 years from the time of diagnosis [1]. Pirfenidone, an antifibrotic drug, reduces the rate of decline in forced vital capacity (FVC) and prolongs progression-free survival in large-scale randomized controlled trials. Although it is effective in the treatment of IPF, it is also associated with adverse events [2,3].
As there may be differences between clinical trials and real-world situations, some studies have investigated the efficacy and adverse events of pirfenidone in real clinical settings. In these studies, pirfenidone is effective and well-tolerated. In most of these studies, patients are treated with a standard dose (2,400 mg) of pirfenidone, except in Japan, where the standard dose is 1,800 mg [4–7]. In the Clinical Studies Assessing Pirfenidone in idiopathic pulmonary fibrosis: Research of Efficacy and Safety Outcomes (CAPACITY) 004 study, the pirfenidone-associated attenuation of decline in FVC at 1,197 mg pirfenidone per day was intermediate compared to that with 2,403 mg pirfenidone per day and placebo [2]. However, few studies have investigated the effect of a lower dose of pirfenidone in real-world situations [8].
Additionally, the efficacy of pirfenidone according to disease severity varies in real-world studies [9–11]. The efficacy of pirfenidone is similar in advanced IPF and non-advanced IPF in one study; however, another study shows that it is more beneficial for patients with advanced IPF [10,11].
We speculated whether a lower dose of pirfenidone would also be effective in real-world settings and whether there would be differences in its efficacy and safety according to disease severity. This study investigated the efficacy and safety of pirfenidone according to pirfenidone dose and disease severity in patients with IPF in real-world conditions.
METHODS
This was a multicenter retrospective cohort study of patients with IPF from three referral centers in Korea namely, Seoul National University Hospital, Seoul National University Bundang Hospital, and Seoul Metropolitan Government Seoul National University Boramae Medical Center.
The study included patients who were diagnosed with IPF according to the consensus statement of the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Society (ATS/ERS/JRS/ALAT) [1] and who were treated with pirfenidone between July 2012 and March 2018. To improve the reliability of the results, patients with a minimum of two follow-up pulmonary function tests (PFTs) performed after commencing pirfenidone treatment were included. Patients for whom the initial date of pirfenidone treatment was unclear owing to referral from other hospitals were excluded.
This study was conducted as per the amended Declaration of Helsinki. The study protocol was approved by the Institutional Review Board or ethics committee of each hospital (IRB no.: J-1803-006-924 [Seoul National University Hospital], B-1801/442–104 [Seoul National University Bundang Hospital], 20180129/30-2018-5/023 [Seoul Metropolitan Government Seoul National University Boramae Medical Center]). The need to obtain informed consent was waived owing to the retrospective nature of the study.
Baseline demographic characteristics, information on the diagnosis of IPF, comorbidities, and previous and combined treatments with pirfenidone, pulmonary function, mortality, and pirfenidone-related adverse events were investigated for each patient.
Pirfenidone treatment was initiated with 600 mg, in three divided doses, and gradually increased to 1,800 mg. The attending physician adjusted the dose of pirfenidone, depending on the patient’s adverse event. When a patient was unable to tolerate the adverse events, the pirfenidone dose was reduced or the treatment was discontinued temporarily. When the patient’s condition improved, the attending physician decided whether to resume the treatment. Before the first administration of the drug, the patient’s PFT and 6-minute walk test were administered. PFT was performed every 6 months before and after pirfenidone treatment. Overall death and IPF-related death were recorded as clinical outcomes.
Definition
In this study, the standard dose of pirfenidone was 1,800 mg. The maximum and final doses of pirfenidone were defined as the highest and last doses received during the treatment period, respectively.
The patients were divided into two groups according to the pirfenidone dose. The standard dose group included patients who had received 1,800 mg of pirfenidone per day for more than 6 months. The non-standard dose group included patients who had received less than 1,800 mg of pirfenidone per day for more than 6 months.
Disease severity was classified according to the GAP stage, and patients were divided into GAP stage I and GAP stage II–III groups.
Statistical analysis
For efficacy analysis, PFT data collected from patients were used. A mixed-effects linear regression model was used to analyze repeated-measurement data and to correct missing data. First, FVC and diffusing capacity of the lungs for carbon monoxide (DLCO) data from patients who underwent a minimum of two PFTs after commencing pirfenidone treatment were used to estimate the annual FVC and DLCO decrease rates after treatment with pirfenidone. To evaluate the efficacy of pirfenidone, the annual FVC and DLCO decrease rates before and after treatment were compared using the paired t test. Finally, we examined whether there was a difference in the annual rate of decline in FVC and DLCO in each group before and after treatment, according to dose and disease severity. Differences between the groups were evaluated using an unpaired t test.
For safety analysis, adverse events related to treatment were analyzed, and the rate of discontinuation of treatment owing to adverse events was calculated. The Kaplan-Meier curve was used to plot the probability of survival, and the differences between groups were analyzed using the log-rank test.
Statistical analysis was performed using StataSE version 12 (StataCorp., College Station, TX, USA) and SPSS version 19 (IBM Co., Armonk, NY, USA). A p value < 0.05 was considered to indicate statistical significance.
RESULTS
Characteristics of the study patients
Of the 565 patients who were prescribed pirfenidone in the three participating hospitals, 338 were enrolled in the study. The patients not enrolled in our study included 17 with an unclear date of onset of pirfenidone treatment and 210 without at least two follow-up PFTs. Among the enrolled patients, efficacy analysis was performed in 174 for FVC and 164 for DLCO, including patients who underwent a minimum of two FVC or DLCO measurements before pirfenidone treatment. Subsequently, subgroup analysis was performed according to the pirfenidone dose or the baseline GAP stage. We excluded patients who received 1,800 mg of pirfenidone per day for less than 6 months or whose baseline GAP scores were unavailable (Fig. 1).
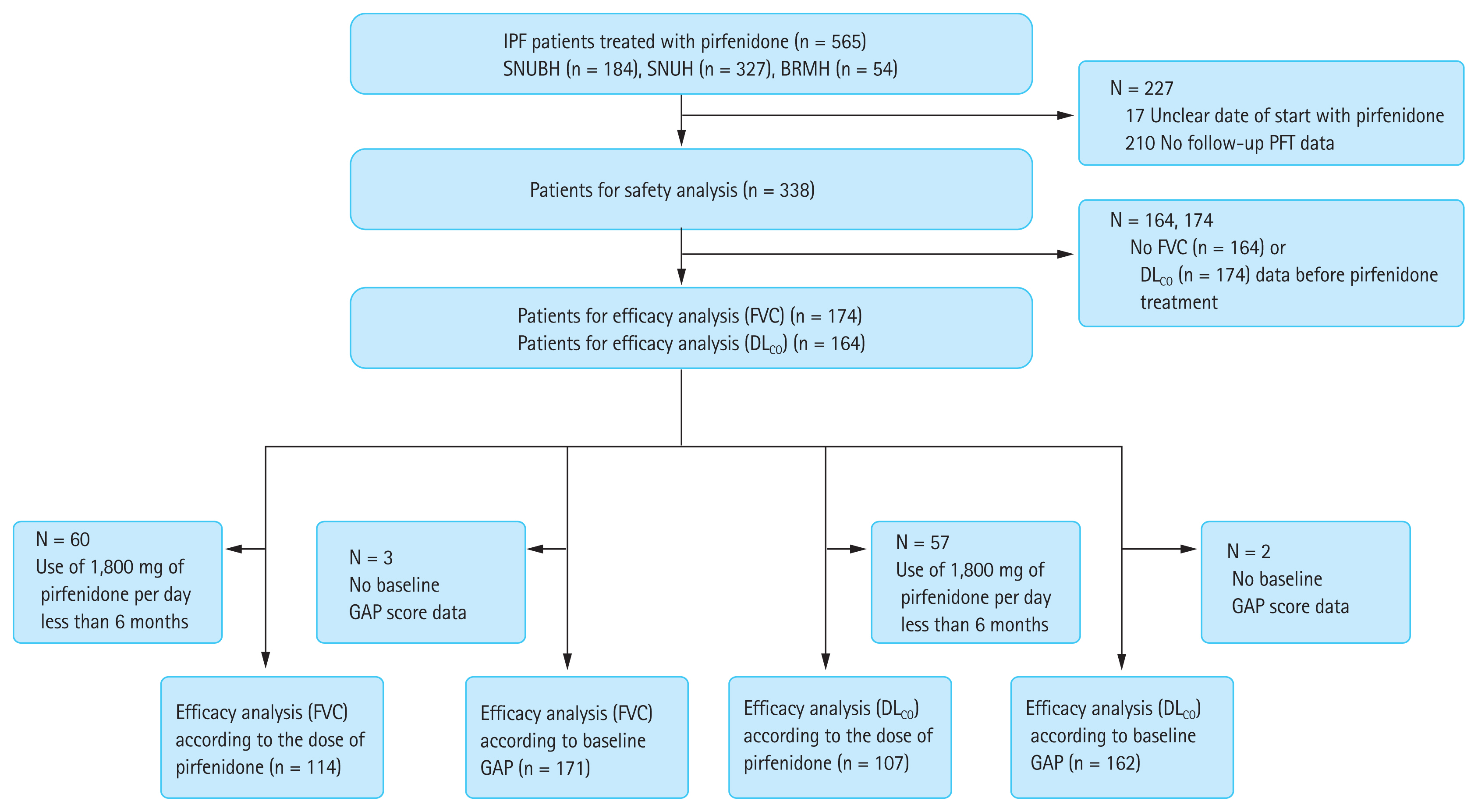
Flow diagram of the study. IPF, idiopathic pulmonary fibrosis; SNUBH, Seoul National University Bundang Hospital; SNUH, Seoul National University Hospital; BRMH, Seoul Metropolitan Government Seoul National University Boramae Medical Center; PFT, pulmonary function test; FVC, forced vital capacity; DLCO, diffusing capacity of the lungs for carbon monoxide; GAP, Gender-Age-Physiology.
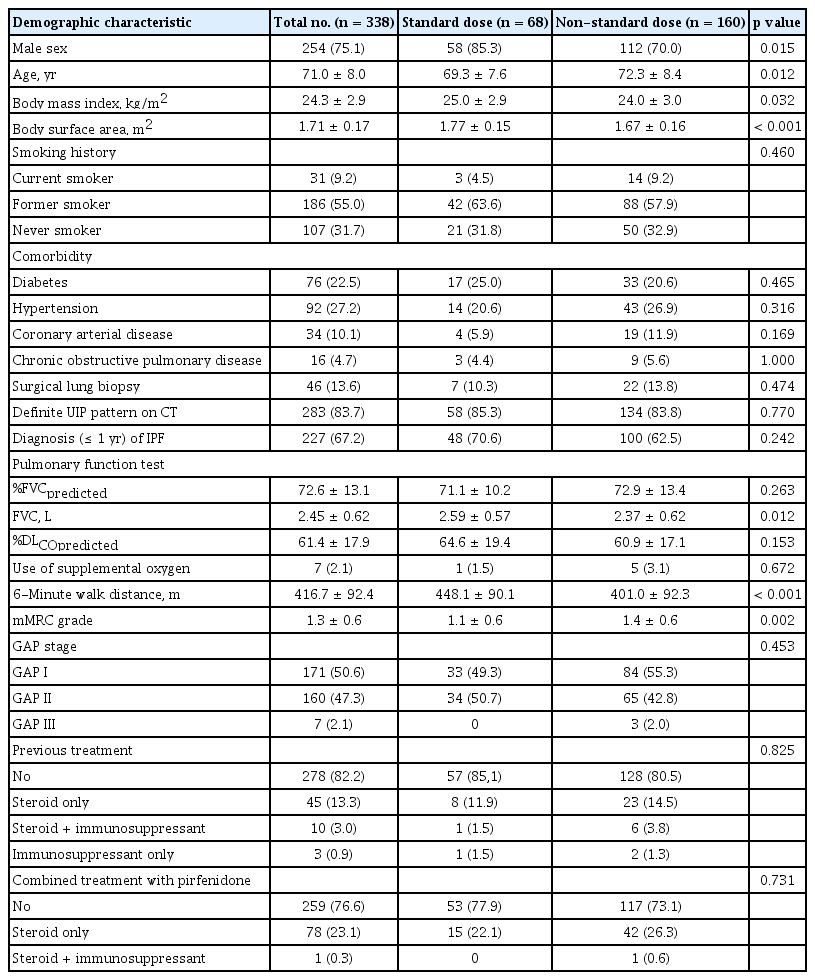
Of the included patients, 75.1% were male and the mean age was 71.0 ± 8.0 years. Most were former smokers (55.0%), and 9.2% were current smokers. Most did not receive other treatments before (82.2%) or during treatment with pirfenidone (76.6%). More than half (67.2%) started pirfenidone treatment within 1 year of IPF diagnoses. Patients in the standard dose group had a higher percentage of males, younger age, and higher body mass index (BMI) and body surface area (BSA) than those in the non-standard dose group. Longer distances in the 6-minute walk test and lower modified Medical Research Council (mMRC) grades were observed in patients treated with a standard dose of pirfenidone (Table 1). The results were similar in the patients included in the efficacy analysis (Supplementary Tables 1 and 2).
In the patient cohort, 46.2% received 1,800 mg (standard dose) of pirfenidone per day as the maximum dose during the entire period of pirfenidone treatment; however, 21.3% of patients were eventually treated with 1,800 mg of pirfenidone. Approximately half of the patients maintained a pirfenidone dose lower than 1,800 mg. The rate of pirfenidone discontinuation for any cause was 26.3%. The overall mean duration of pirfenidone treatment was 16.1 ± 9.0 months, and 68 patients (20.1%) received the standard dose (1,800 mg) of pirfenidone for more than 6 months. In these patients, the overall mean duration of pirfenidone treatment was 18.2 ± 7.8 months (Table 2).
Changes in lung function
Annual FVC and DLCO changes were estimated in patients who underwent a minimum of two PFTs during pirfenidone treatment. The mean percentage predicted FVC (%FVCpredicted) change after pirfenidone treatment was −1.78% (95% confidence interval [CI], −2.37 to −1.20) per year, and the mean percentage change predicted DLCO (%DLCOpredicted) after pirfenidone treatment was −3.11% (95% CI −3.92 to −2.30) per year. The patients were divided into three groups according to the %FVCpredicted or %DLCOpredicted decline rate: Δ FVC or DLCO/year ≤ −10%, −10% < Δ FVC or DLCO/year ≤ −5%, and −5% < Δ FVC or DLCO/year. The changes in %FVCpredicted and %DLCOpredicted are illustrated in Fig. 2.

The decline of (A) forced vital capacity (FVC) and (B) diffusing capacity of the lungs for carbon monoxide (DLCO) during pirfenidone treatment.
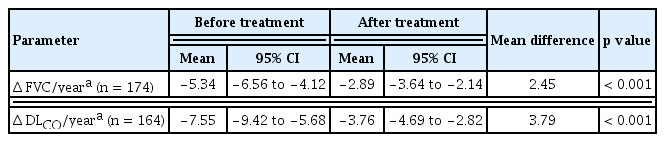
FVC and DLCO changes before and after treatment were investigated in patients with data for at least two PFTs before and after treatment (Table 3, Fig. 3). The %FVCpredicted and %DLCOpredicted decline rates were significantly reduced after pirfenidone treatment (p < 0.001). In addition, lower baseline %FVCpredicted, FVC (L), and %DLCOpredicted and higher mMRC grades were observed in the Δ FVC/year ≤ −10% group than in the other groups. Furthermore, patients with GAP stage II–III and those using steroids at the initiation of pirfenidone treatment were more common in the Δ FVC/year ≤ −10% group (Supplementary Table 3). There were no statistically significant differences in the duration and dose of pirfenidone treatment between the groups (Supplementary Table 4).
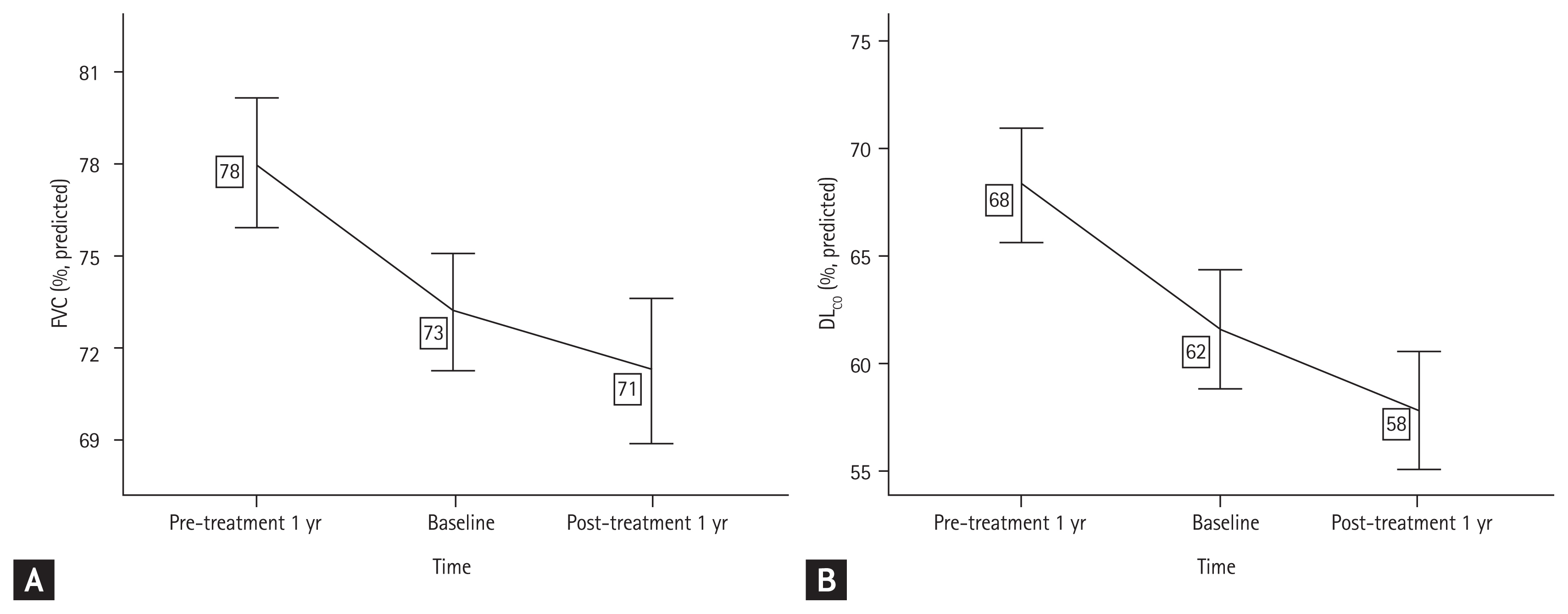
The annual decline of (A) forced vital capacity (FVC) and (B) diffusing capacity of the lungs for carbon monoxide (DLCO) before and after pirfenidone treatment.
The differences in FVC and DLCO changes before and after pirfenidone administration were compared in the standard dose group; the mean %FVCpredicted was −6.56% (95% CI, −9.26 to −3.87) per year before pirfenidone treatment; however, −4.43% (95% CI, −5.87 to −3.00) per year after treatment. In the non-standard, lower dose group, the mean %FVCpredicted was −4.96% (95% CI, −6.82 to −3.09) per year before pirfenidone treatment; however, was −1.79% (95% CI, −2.75 to −0.83) per year after treatment. The rate of decline of %FVCpredicted was significantly attenuated by pirfenidone treatment in both groups (p < 0.05). There was no significant difference in the decline rates between the groups according to dose. Similar findings were obtained for DLCO changes, and there were no significant differences between the groups (Table 4). We performed the additional analysis by dividing the non-standard dose group into a group taking 1,200 to 1,600 mg pirfenidone (1,200 to 1,600 mg dose group) and a group taking < 1,200 mg pirfenidone (< 1,200 mg dose group). The decline rate of %FVCpredicted and %DLCOpredicted was consistently attenuated in the 1,200 to 1.600 mg dose group; however, not in the < 1,200 mg dose group (Supplementary Table 5).

Comparison of FVC and DLCO decline rate before and after treatment according to the dose of pirfenidone
In efficacy analysis according to the baseline GAP stage, the %FVCpredicted decline rates were significantly reduced in both GAP stage I and GAP stage II–III groups (p < 0.001) (Supplementary Table 6). There was no difference in FVC decline between the two groups. However, there was a significant reduction in the decline rate of DLCO in the GAP stage II–III group before and after treatment, but not in the GAP stage I group.
Additionally, efficacy analysis was performed according to the diagnostic method of IPF and smoking status. The decline rate of %FVCpredicted and %DLCOpredicted was more attenuated in patients with IPF diagnosed by surgical lung biopsy than in clinically diagnosed patients (Supplementary Table 7). The ever-smoker patients showed less decrease in %FVCpredicted and %DLCOpredicted than the never smokers (Supplementary Table 8).
Adverse events
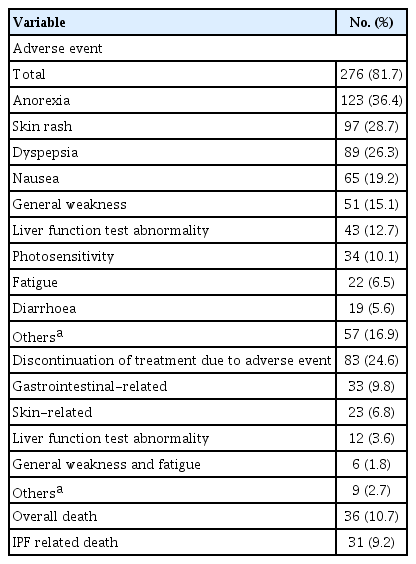
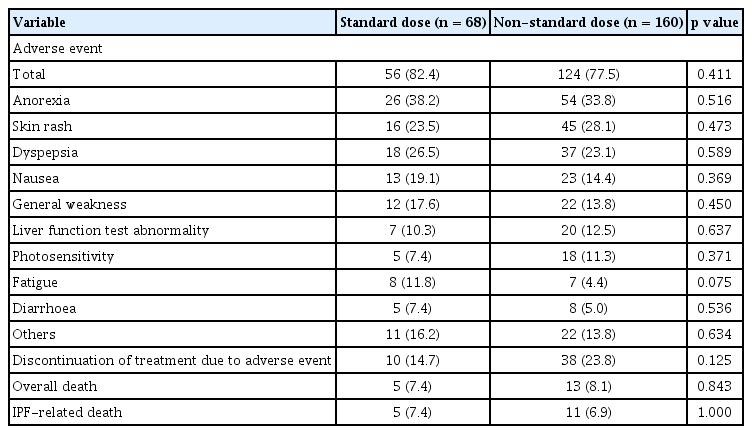
Adverse events occurred in 276 patients (81.7%). Among these, anorexia was the most common, followed by skin rash and dyspepsia. Eighty-three (24.6%) patients discontinued the treatment owing to adverse events. Discontinuation due to gastrointestinal events was the most common (9.8%), followed by skin-related events (6.8%). Overall, death occurred in 36 (10.7%) patients, and IPF-related deaths occurred in 31 (9.2%) (Table 5). There was no significant difference in adverse events between dose groups (Table 6). The overall mean survival time of patients was 48.2 months (95% CI, 41.3 to 55.2) after the initiation of pirfenidone treatment. The mean survival time was not significantly different between the dose groups (mean 41.2% vs. 50.1%, p = 0.847) (Supplementary Fig. 1).
Compared with adverse events and mortality according to disease severity, anorexia, nausea, and general weakness occurred more frequently in the GAP stage II–III group than in the GAP stage I group (Supplementary Table 9).
DISCUSSION
There have been several real-world studies on pirfenidone in IPF [4,10–13]. However, these studies do not focus on pirfenidone dose. Our study comprehensively investigated the effect of pirfenidone on the dose of pirfenidone in real-world practice. Pirfenidone attenuated the rate of decline of FVC and DLCO in both the standard and non-standard lower dose groups. These results suggest that lower doses of pirfenidone may help to prevent disease progression as effectively a full dose.
Our findings are similar to those of a recent post hoc analysis of multinational phase III trials, which revealed that patients receiving pirfenidone at ≤ 90% of the standard dose intensity also showed treatment benefit as compared to placebo [14]. In a study from Japan, changes in %FVC (Δ%FVC) at 12 months were not significantly different between patients taking 1,200 mg and those taking 1,800 mg of pirfenidone. However, when patients were divided into groups based on the BSA-adjusted dose of pirfenidone (876 mg/m2), the Δ%FVC of patients taking higher doses was significantly greater than that of patients receiving lower doses [8]. Although patients in the non-standard dose group received a lower dose of pirfenidone per BSA (719 mg/m2 or less) compared to the standard dose group (1,017 mg/m2), the rate of FVC and DLCO decline was reduced in our study. Furthermore, the decline in FVC and DLCO tended to attenuate more in the non-standard dose group than in the standard dose group, although this was not statistically significant. The differences in baseline characteristics between the groups might have affected the decline rate of FVC and DLCO. Pre-treatment changes in FVC and DLCO tended to decrease less in the non-standard dose group than in the standard dose group. The non-standard dose group may respond well to treatment with pirfenidone. Additionally, the efficacy of pirfenidone in the standard dose group may have been underestimated owing to the small number of patients.
The standard dose of pirfenidone in Asian countries is 1,800 mg according to a clinical trial from Japan, which is lower than the standard dose of 2,400 mg used in Western countries. Despite the use of lower doses, only 20.1% of our patients maintained the standard dose over a period of 6 months. These results are quite different from those in other countries, including Japan, in which most patients received standard doses [6,7,15], and from those of a recent study in which most patients with IPF maintained relatively high doses of pirfenidone [14]. In real-world conditions, pirfenidone may be less tolerable than in a clinical trial setting. Additionally, the high cost of pirfenidone could be one of the reasons for discontinuation. In Korea, the cost of pirfenidone has been covered by the National Health Insurance since October 3, 2015. Some patients could not continue treatment with pirfenidone because of its high cost.
The discontinuation of pirfenidone was more frequent in the non-standard dose group than in the standard dose group. The non-standard dose group had older and more underweight patients than the standard dose group. This is consistent with a recent study showing that the discontinuation rate of pirfenidone is high among older patients, although the rate of adverse events among them is similar to that in younger patients [16].
The FVC decline rate was significantly reduced regardless of the GAP stage in our study. One study showed that the effect of pirfenidone on reducing the rate of FVC decline is greater in patients with advanced IPF [6]. Another concluded that pirfenidone significantly reduces disease progression (≥ 10% decline in FVC or death) at 12 months, regardless of the baseline GAP stage [17]. In terms of attenuating the decline rate of FVC, the present study showed similar results.
Our study further demonstrated that the decline rate in DLCO was significantly reduced in the GAP stage II–III group. A real-world study conducted in Italy showed that pirfenidone does not diminish the rate of decline in DLCO, regardless of the baseline GAP stage [6]. This may be due to differences in the ethnicities of the study populations. A recent study showed that the decline rate of DLCO decreases 6 months after commencing pirfenidone treatment in IPF patients with a mean GAP score of 5 at baseline (stage II) [10]. Thus, pirfenidone should be used in the treatment of patients with severe IPF.
In our study, there was no difference in the frequency of adverse events between the dose groups, contrary to our expectation that the non-standard dose group would have more adverse events. The non-standard dose group included more patients of old age and low BMI than the standard dose group. Although most of the attending physicians tried to escalate the dose of pirfenidone according to protocol, they prescribed pirfenidone conservatively considering the patients’ old age and low BMI without escalating to the full dose of pirfenidone. The degree of adverse events could have been more severe in the non-standard dose group. However, we could not evaluate the severity of the adverse effects due to the retrospective nature of this study.
This study has several limitations. First, as it was a retrospective cohort study, selection bias and missing data were inevitable. A mixed linear regression model was used to calibrate the missing data as much as possible and to adjust for age, sex, and BMI, which may affect the PFT results. Second, the number of patients included in the standard dose group was small. The efficacy of pirfenidone might be underestimated and underpowered in the standard dose group.
Despite several limitations, this study demonstrated that the effect of pirfenidone on reducing disease progression of IPF persisted even with a consistently lower dose of pirfenidone in a real-world clinical setting. These results should be useful to clinicians during daily practice in the treatment of IPF.
KEY MESSAGE
1. The effect of pirfenidone on reducing disease progression of idiopathic pulmonary fibrosis persisted even with a consistently lower dose.
2. Continuing lower dose of pirfenidone would be helpful in patients who can not tolerate standard full dose of pirfenidone.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgments
We thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for the statistical analyses. This study was supported by a grant (No. 06-2019-001) from the SNUBH Research Fund.