Risk of colorectal cancer in patients with positive results of fecal immunochemical test performed within 5 years since the last colonoscopy
Article information
Abstract
Background/Aims
Annual fecal immunochemical tests (FITs) are often repeated within the recommended colonoscopy surveillance intervals. However, it remains unclear whether interval FITs are useful. To answer this question, we assessed the risk of colorectal cancer (CRC) according to the interval from the last colonoscopy to an FIT.
Methods
Using the Korean National Cancer Screening Program database, we collected data on patients who underwent FITs in 2011. Patients with positive FIT results were classified into three groups according to their previous colonoscopy interval: 0.5 to 5 years (group 1), 5 to 10 years (group 2), and ≥ 10 years or no colonoscopy (group 3). CRC incidence was defined as CRC diagnosed within 1 year after an FIT.
Results
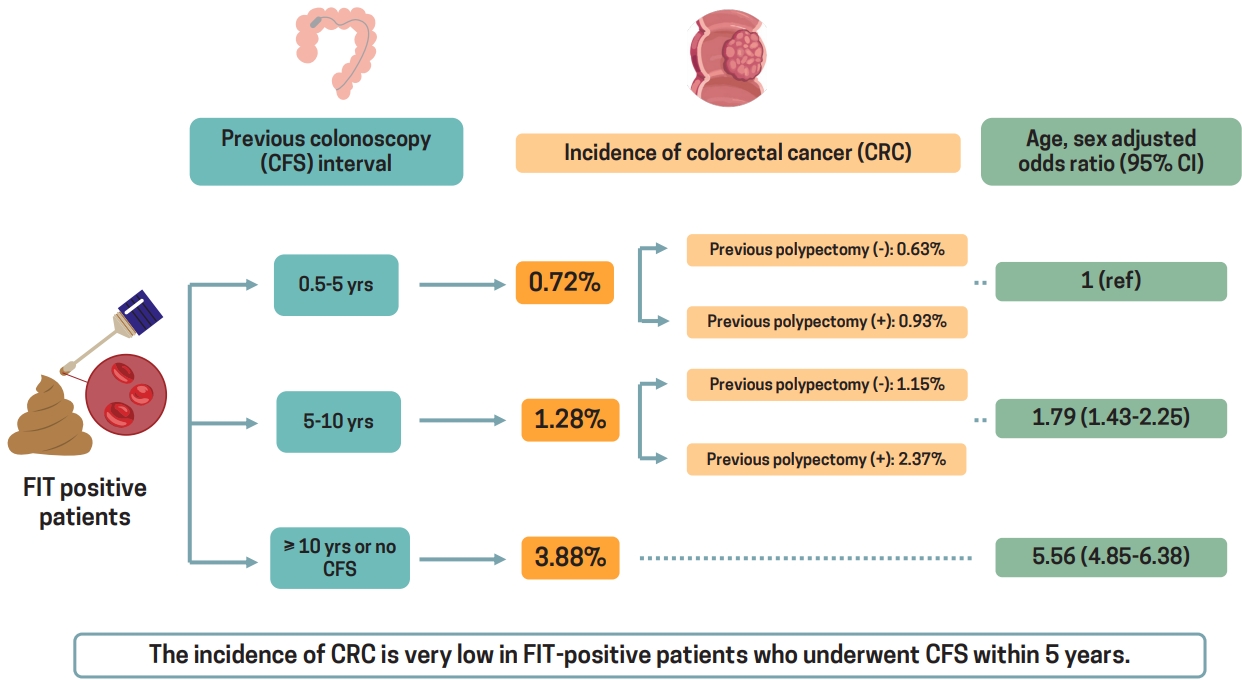
Among 177,660 patients with positive FIT results, the incidence of CRC in groups 1, 2, and 3 was 0.72% (n = 214/29,575), 1.28% (n = 116/9,083), and 3.88% (n = 5,387/139,002), respectively. The age- and sex-adjusted risk for CRC was higher in groups 2 (odds ratio [OR], 1.79; 95% confidence interval [CI], 1.43 to 2.25) and 3 (OR, 5.56; 95% CI, 4.85 to 6.38) than in group 1. Among patients who did and did not undergo a polypectomy during the previous colonoscopy, those in group 2 had a higher rate of CRC than those in group 1 (without polypectomy: 1.15% vs. 0.63%; OR, 1.79; 95% CI, 1.37 to 2.34) (with polypectomy: 2.37% vs. 0.93 %; OR, 2.30; 95% CI, 1.44 to 3.69).
Conclusion
In patients with positive FIT results who had undergone a colonoscopy within the past 5 years, the risk of CRC is very low, regardless of whether a polypectomy was performed, suggesting that interval FITs are not useful.
INTRODUCTION
In randomized controlled trials, colorectal cancer (CRC) screening using the fecal occult blood test (FOBT) has been shown to be effective in reducing the incidence and mortality of CRC [1–4]. The conventional guaiac FOBT (gFOBT) indirectly detects blood in the stool [3]. This test is based on colorimetric detection of peroxidase activity, rather than direct examination of stool for human hemoglobin [3]. Because several foods contain nonhemoglobin peroxidase activity, the gFOBT can be affected by such foods [5]. Recently, important advances in fecal screening have been made with the development of the fecal immunochemical test (FIT). FITs that can directly measure human hemoglobin in the stool using monoclonal or polyclonal antibodies directed against the globin moiety of human hemoglobin have been developed [6,7]. Several studies have reported that an FIT is superior to the gFOBT for the detection of CRC [3,8,9].
Previous guidelines and the Centers for Disease Control and Prevention recommend suspending the gFOBT for at least 5 to 10 years after a normal colonoscopy because repeating the gFOBT can result in subsequent unnecessary colonoscopies and increased health care costs [10–12]. However, these recommendations are based on expert opinions, and there is insufficient evidence to support them. Moreover, in real clinical practice, re-screening with a fecal test is often performed, even if the patient has recently undergone a colonoscopy. This often occurs in countries such as South Korea, which have adopted a program to perform FITs annually as a method of population-based CRC screening.
Recently, the U.S. Multi-Society Task Force on Colorectal Cancer recommended that a colonoscopy should be repeated when the FIT result is positive after a recent colonoscopy because the FIT is superior to the gFOBT [3]. The European Society of Gastrointestinal Endoscopy also suggested considering a repeat colonoscopy based on clinical judgment in patients with unexpectedly positive FIT test results [13]. However, this is a weak recommendation based on low-quality evidence. Whether to perform an interval FIT between colonoscopies remains unclear. To resolve this issue, it is necessary to identify the risk of CRC according to the interval since the last colonoscopy in patients with positive FIT results. Very few studies have investigated this topic. The number of patients studied was too small to evaluate the risk of CRC; thus, these studies had inconsistent results [14,15]. Therefore, we used a large-scale, population-based database to examine the risk of CRC in relation to the time that had elapsed since the last colonoscopy in patients who had undergone an FIT.
METHODS
Study population
The Korean government supports CRC screening via the National Cancer Screening Program (NCSP). The NCSP provides a single annual FIT for all Koreans aged 50 years and older as an initial CRC screening and a colonoscopy as a second test for those with positive FIT findings. Data were extracted from the National Health Information Database (NHID) of the National Health Insurance Service (NHIS), which runs the NCSP. The study population comprised patients who underwent FITs through the NCSP between January 1, 2011, and December 31, 2011. We only collected the initial FIT results for those who had undergone two or more FITs during the study period.
Of the 3,062,459 patients selected, 205,883 were excluded owing to previous diagnoses of cancer (including CRC) (n = 205,883) or inflammatory bowel disease (n = 13,675), and another 7,448 were excluded owing to missing data on screening date, age, and sex. Of the remaining 2,835,453 patients, 180,038 had positive FIT results and 2,655,415 had negative results. We excluded patients who had undergone a colonoscopy within 180 days (0.5 years) from the time of the FIT because the FIT and colonoscopy were considered to have been performed at approximately the same time. Finally, this study included a total of 177,660 patients with positive FIT results and 2,623,765 patients with negative FIT results (Fig. 1).
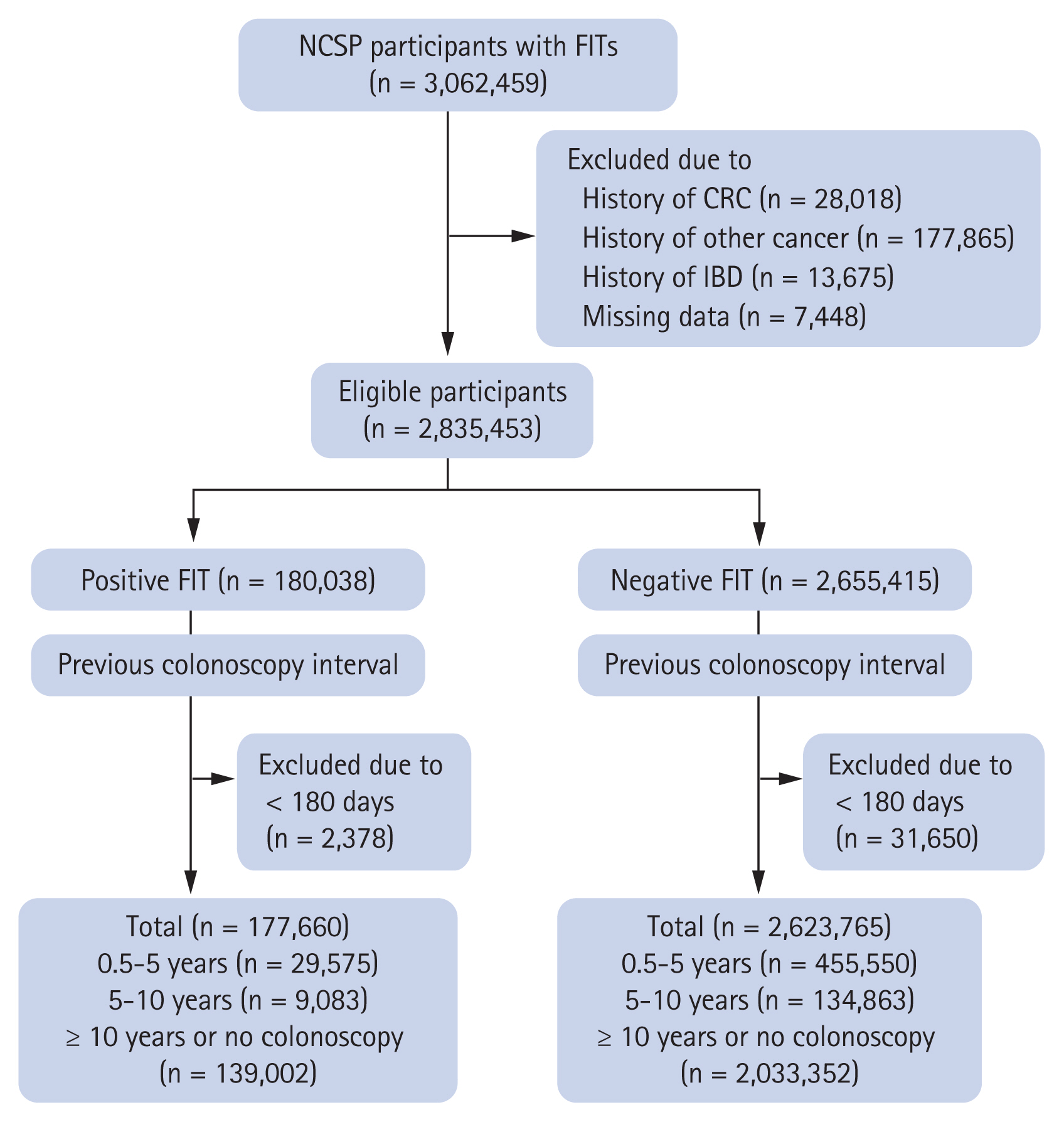
Flow chart of study patients. NCSP, National Cancer Screening Program; FIT, fecal immunochemical test; CRC, colorectal cancer; IBD, inflammatory bowel disease.
The NHIS-NHID is encrypted and does not contain personal identifiers. This study protocol was approved by the Institutional Review Board (IRB) of Ewha Womans University Mokdong Hospital (IRB No. 2018-12-023). Written informed consent by the patients was waived due to a retrospective nature of our study.
Definition of variables and confirmation of CRC
The NHIS-NHID contains information on age, sex, screening date, FIT results (negative, positive), and comorbidities (e.g., cancer) based on the International Classification of Disease 10th revision (ICD-10) codes for all patients. Information regarding CRC development for patients was obtained from the NHIS-NHID. Patients were considered to have CRC if they had both the cancer registration code and appropriate diagnostic code (ICD-10: C18–C21, D01.0–D01.3). CRC incidence was defined as CRC diagnosed within 1 year after an FIT.
Prescription codes for colonoscopies were used to determine whether a colonoscopy was performed prior to an FIT. A previous colonoscopy was defined as having codes for colonoscopy, colonoscopic polypectomy, colonoscopic mucosal resection, or colonoscopic submucosal resection. We identified these colonoscopy-related codes from January 1, 2002, to December 31, 2011, for all patients. The previous colonoscopy interval was defined as the interval between the time of an FIT and the most recent colonoscopy. Patients with positive and negative FIT results were classified into three groups according to previous colonoscopy intervals: 0.5–5, 5–10, and ≥ 10 years or no colonoscopy.
Statistical analysis
Categorical variables were compared among the groups using the chi-square test and continuous variables using one-way analysis of variance. We performed logistic regression analysis to estimate the odds ratios (ORs) with 95% confidence intervals (CIs) of the risk of developing CRC within 1 year after an FIT among the groups. The results were presented as age- and sex-adjusted and crude ORs.
A p < 0.05 was considered statistically significant, and all p values were two-tailed. All data analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline characteristics of the study population
The baseline characteristics of the study population are presented in Table 1. Among 177,660 patients with positive FIT results, the number of patients who had undergone a colonoscopy 0.5–5, 5–10, and ≥ 10 years prior or had not undergone any colonoscopy was 29,575 (16.65%), 9,083 (5.11%), and 139,002 (78.24%), respectively. In patients with positive FIT results, the mean age was 61.93 ± 8.64 years, and the proportion of men was 51.95%. The mean age was the highest among patients who had undergone a colonoscopy ≥ 10 years prior or had not undergone any colonoscopy, and the proportion of men was the highest among those who had undergone a colonoscopy 0.5 to 5 years prior.

Baseline characteristics of the study population grouped according to previous colonoscopy intervals
Among the 2,623,765 patients with negative FIT results, the number of patients who had undergone a colonoscopy 0.5–5, 5–10, and ≥ 10 years prior or not undergone any colonoscopy was 455,550 (17.36%), 134,863 (5.14%), and 2,033,352 (77.50%), respectively. In patients with negative FIT results, the mean age was 60.99 ± 8.21 years, and it was the highest among those who had undergone a colonoscopy 5 to 10 years prior. The proportion of men was 43.81%, and it was the highest in those who had undergone a colonoscopy 0.5 to 5 years prior.
Incidence of CRC according to FIT results
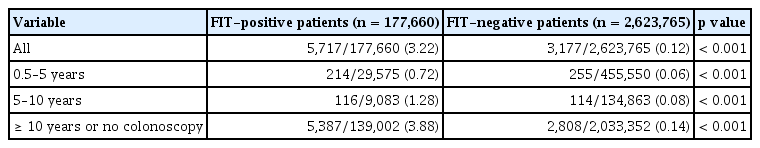
The incidence of CRC based on the interval from the last colonoscopy to an FIT among patients with positive and negative FIT results is shown in Table 2. The incidence of CRC in patients with positive FIT results who had undergone a colonoscopy 0.5–5, 5–10, and ≥ 10 years prior or had not undergone any colonoscopy was 0.72% (n = 214/29,575), 1.28% (n = 116/9,083), and 3.88% (n = 5,387/139,002), respectively, and the corresponding rates in patients with negative FIT results were 0.06%, 0.08%, and 0.14%, respectively. The incidence of CRC was significantly higher in the FIT-positive group than in the FIT-negative group (p < 0.001). In addition, the incidence of CRC was significantly higher in the FIT-positive group than in the FIT-negative group in all three groups based on the previous colonoscopy intervals (all p < 0.001).
Risk of CRC according to the interval from the last colonoscopy to the FIT
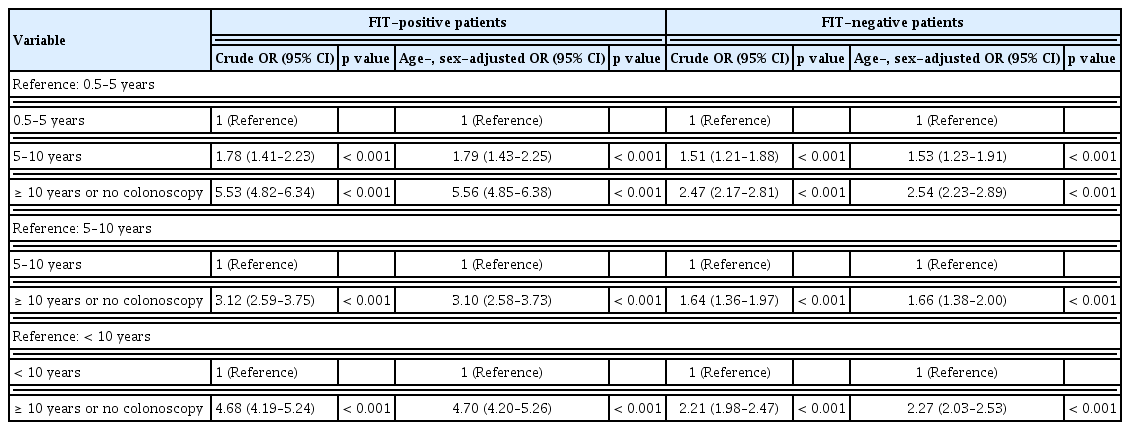
Among patients with positive FIT results, the age- and sex-adjusted risks for CRC were significantly higher in those who had undergone a colonoscopy 5–10 and ≥ 10 years prior or had not undergone any colonoscopy than in those who had undergone a colonoscopy 0.5 to 5 years prior (OR, 1.79; 95% CI, 1.43 to 2.25) (OR, 5.56; 95% CI, 4.85 to 6.38, respectively) (Table 3). The risk was also higher in patients who had undergone a colonoscopy ≥ 10 years prior or had not undergone any colonoscopy than in those who had undergone a colonoscopy 5 to 10 years prior (age- and sex-adjusted OR, 3.10; 95% CI, 2.58 to 3.73).
The results in patients with negative FIT results were similar to those in patients with positive FIT results (Table 3). Patients with negative FIT results who had undergone a colonoscopy 5–10 and ≥ 10 years prior or had not undergone any colonoscopy had a significantly higher risk of CRC compared to those who had undergone a colonoscopy 0.5 to 5 years prior (adjusted OR, 1.53; 95% CI, 1.23 to 1.91) (adjusted OR, 2.54; 95% CI, 2.23 to 2.89, respectively).
We performed a subgroup analysis based on whether a polypectomy was performed during the most recent colonoscopy among patients with positive FIT results (Table 4). We defined the code for colonoscopic polypectomy, colonoscopic mucosal resection, or colonoscopic submucosal resection as “with polypectomy,” while we defined the code for colonoscopy as “without polypectomy.” The incidence of CRC in patients with positive FIT results who had undergone a colonoscopy without a polypectomy 0.5–5 and 5–10 years prior was 0.63% (n = 131/20,671) and 1.15% (n = 93/8,113), respectively, and the corresponding rates in those who had undergone a colonoscopy with a polypectomy were 0.93% (n = 83/8,904) and 2.37% (n = 23/970), respectively. Patients with positive FIT results who had undergone a colonoscopy 5 to 10 years prior were at a greater risk of CRC than those who had undergone a colonoscopy 0.5 to 5 years prior both among those without and with a polypectomy (age- and sex-adjusted OR, 1.79; 95% CI, 1.37 to 2.34) (age- and sex-adjusted OR, 2.30; 95% CI, 1.44 to 3.69, respectively).
DISCUSSION
In this Korean population-based study, we found that patients with positive FIT results who had undergone a colonoscopy within the past 5 years were very unlikely to have CRC. CRC was detected in only 0.72% of those who had undergone a colonoscopy 0.5 to 5 years prior. In addition, the risk of CRC in patients who had undergone a colonoscopy 0.5 to 5 years prior was significantly lower than that in patients who had undergone a colonoscopy 5–10 and ≥ 10 years prior or had not undergone any colonoscopy. These findings suggest that a repeat colonoscopy is not necessary in patients with positive FIT results who have undergone a colonoscopy within the past 5 years because the risk of CRC is very low in these patients. Conversely, patients who have already undergone a colonoscopy may not need to be re-screened with an FIT for at least 5 years.
Very few studies have examined the results of a repeat colonoscopy in patients with positive FIT results and who had undergone a recent colonoscopy. To date, only three studies have assessed the risk of CRC in patients with positive FIT results according to the time elapsed since the last colonoscopy [14–16]. Two studies reported results consistent with those of our study [14,16]. A United States study including 1,119 patients aged ≥ 50 years who were FOBT-positive, asymptomatic, and average-risk reported that CRC was detected in 11.3% (n = 49/434) of patients who had never undergone any colonoscopy, in 8.2% (n = 23/281) of those who had undergone a colonoscopy within > 10 years prior, in 4.5% (n = 10/221) of those who had undergone a colonoscopy within 5 to 10 years prior, and in 0% (n = 0/183) of those who had undergone a colonoscopy within < 5 years prior [16]. In other words, this study showed that none of the 183 patients who had undergone a colonoscopy < 5 years prior were diagnosed with CRC. Recently, a Japanese study including 2,204 patients also revealed that the risk of CRC in patients who had undergone a colonoscopy 0.5 to 5 years prior was very low [14]. In this study, the detection rates of invasive cancer in patients with positive FIT results who had never undergone a colonoscopy, those who had undergone a colonoscopy > 5 years prior, and those who had undergone a colonoscopy 0.5 to 5 years prior were 5.7% (n = 92/1,626), 1.2% (n = 3/248), and 0.3% (n = 1/330), respectively [14]. Similar to our results, the risk of invasive cancer was significantly lower in the 0.5–5- and > 5-year colonoscopy groups than in the no colonoscopy group (adjusted OR, 0.04; 95% CI, 0.01 to 0.37) (adjusted OR, 0.19; 95% CI, 0.06 to 0.61, respectively) [14]. Both aforementioned studies examined the risk of advanced colorectal neoplasia (ACRN, advanced adenoma and/or cancer) and CRC and reported that the prevalence of ACRN in patients with positive FIT results who had undergone a colonoscopy < 5 years prior was also low (1.1% [n = 20/183] in the United States study and 3.9% [n = 13/330] in the Japanese study). Because the United States study excluded patients with a history of adenoma detection during a colonoscopy within the past 10 years and the Japanese study excluded those with high-risk neoplasia (≥ 3 adenomas and/or ACRN), these two studies support the suspension of the FOBT or FIT for at least 5 years “after a negative colonoscopy” and “after colonoscopy without high-risk neoplasia,” respectively.
We could not analyze the results of the previous colonoscopy because the administrative data do not include this information. Instead, we analyzed each of the cases without and with a polypectomy during the previous colonoscopy using the colonoscopy-related procedure code. The results revealed that among patients with positive FIT results who had undergone a colonoscopy within the previous 5 years, the incidence of CRC was very low in cases with and without a polypectomy during the previous colonoscopy (0.63% and 0.93%, respectively). In addition, patients with positive FIT results who had undergone a colonoscopy 0.5 to 5 years prior with and without a polypectomy had a lower risk of CRC than those who had undergone a colonoscopy 5 to 10 years prior. These results suggest that an interval FIT, conducted within the recommended colonoscopy surveillance intervals, is not useful for patients with or without a previous history of colorectal polyps. Our findings support a recently published UK post-polypectomy guideline that does not recommend the performance of an FIT for surveillance after the resection of premalignant colorectal polyps [17].
Contrary to the two aforementioned studies and our study, a Korean study including 2,228 patients with positive FIT results suggested that a repeat colonoscopy should be offered to patients with positive FIT results who have undergone a recent colonoscopy because the risk of ACRN and CRC in patients with positive FIT results who had undergone a colonoscopy < 3 years prior was not low (10.9% [n = 56/514] and 2.1% [n = 11/514], respectively) and the risk was similar to that in patients who had undergone a colonoscopy 3 to 10 years prior [15].
As the aforementioned Japanese and Korean studies were conducted at a single center, and the United States study involved data obtained from the VA New York Harbor Healthcare System, there may have been a selection bias. Additionally, the sample sizes (n = 1,119 to 2,228) were too small to analyze the incidence of CRC. Therefore, it is difficult to draw a reliable and clear conclusion from the three aforementioned hospital-based studies. The strength of the present study is that it is a population-based study with a very large sample size, which enables the analysis of CRC as the main outcome. Our study, which involved 177,660 patients with positive FIT results, provides more reliable information on the risk of CRC according to previous colonoscopy intervals. This is the first large-scale population-based study on this topic. Another strength of this study is that patients with negative FIT results were also evaluated. We observed that the incidence rate of CRC in patients with negative FIT results who had undergone a colonoscopy 0.5 to 5 years prior was extremely low (0.06%), and similar to the findings among patients with positive FIT results, the rate was lower than that in patients who had undergone a colonoscopy 5–10 and ≥ 10 years prior or had not undergone any colonoscopy.
Our study has several limitations. First, the prior colonoscopy examination quality (e.g., endoscopist’s adenoma detection rate, bowel preparation quality, withdrawal time, and cecal intubation), prior colonoscopy results (e.g., low-risk adenomas or high-risk adenomas), and clinical context (e.g., symptoms, worrisome signs, and laboratory values) were not considered because the administrative data did not include such information. However, to overcome these limitations, we analyzed both patients who did and did not undergo a polypectomy during the previous colonoscopy, separately. Additionally, as our study was based on a CRC screening program, most of the study subjects were expected to be asymptomatic. Second, we were unable to investigate the risk of advanced adenomas according to previous colonoscopy intervals because we could not distinguish advanced adenomas by diagnostic codes. However, we included carcinoma in situ of the colon and rectum in the definition of CRC. Additionally, CRC is a more clinically important outcome than advanced adenomas. Third, both qualitative and quantitative FITs were used, but these methods were not analyzed separately. Fourth, the NHIS-NHID does not contain information on colonoscopies that were not covered by medical insurance. Accordingly, when analyzing whether colonoscopies were performed prior to an FIT, the colonoscopies performed through comprehensive health examinations, regardless of medical insurance, would not have been included. This may be why the CRC incidence in our study was lower than that in previous studies. Fifth, in the subgroup analysis based on whether polypectomies were performed, polyp removal using biopsy forceps was not considered. However, when biopsy forceps are used, it is often for the purpose of simple biopsies for lesions other than polyps, such as inflammation, in addition to polyp removal. Therefore, in this study, the use of biopsy forceps was not considered a polypectomy. Lastly, risk factors for CRC, such as a family history of CRC, smoking status, and obesity [18], were not analyzed. Future studies should confirm whether an interval FIT is helpful for patients with these risk factors.
In conclusion, the incidence of CRC was very low (< 1%) in patients with positive FIT results who had undergone a colonoscopy within the past 5 years. Additionally, the risk of CRC in these patients was significantly lower than that in patients who had undergone a colonoscopy 5 to 10 years prior and in those who had undergone a colonoscopy ≥ 10 years prior or had not undergone any colonoscopy. These results were the same for both patients without and with polypectomies during the previous colonoscopy. Even if FIT results are positive, the diagnostic yield of CRC by a repeat colonoscopy may be low for patients who have undergone a colonoscopy within the preceding 5 years. Further research is needed to clarify whether an interval FIT performed within the recommended colonoscopy surveillance interval is clinically useful.
KEY MESSAGE
1. This large population-based study shows that among fecal immunochemical test (FIT)-positive patients who underwent a colonoscopy within the past 5 years, the incidence of colorectal cancer is very low both in those who underwent and those who did not undergo polypectomy during the previous colonoscopy (0.63% and 0.93%, respectively).
2. Even if FIT results are positive, repeat colonoscopy may not be necessary for patients who underwent a colonoscopy within the preceding 5 years.
3. Interval FIT, performed within the recommended colonoscopy surveillance interval, may not be useful both for patients who underwent and those who did not undergo polypectomy during the previous colonoscopy.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1A2C1010786 and 2020R1A5A2019210; Chang Mo Moon).