Efficacy and safety of hydroxychloroquine in osteoarthritis: a systematic review and meta-analysis of randomized controlled trials
Article information
Abstract
Background/Aims
Conventional disease-modifying anti-rheumatic drugs have been trialed in osteoarthritis (OA). Hydroxychloroquine (HCQ), which has shown its effectiveness in rheumatoid arthritis, has been trialed for the treatment of OA; however, its efficacy and safety remain unclear. This systematic review and meta-analysis evaluate efficacy and safety of HCQ for the treatment of OA.
Methods
MEDLINE, EMBASE, and Cochrane Central were searched from inception through June 2020. Two reviewers independently screened for randomized controlled trials (RCTs) comparing HCQ with placebo or other active-comparators for the treatment of knee, hand, or hip OA, extracted data, and performed Cochrane risk of bias assessments.
Results
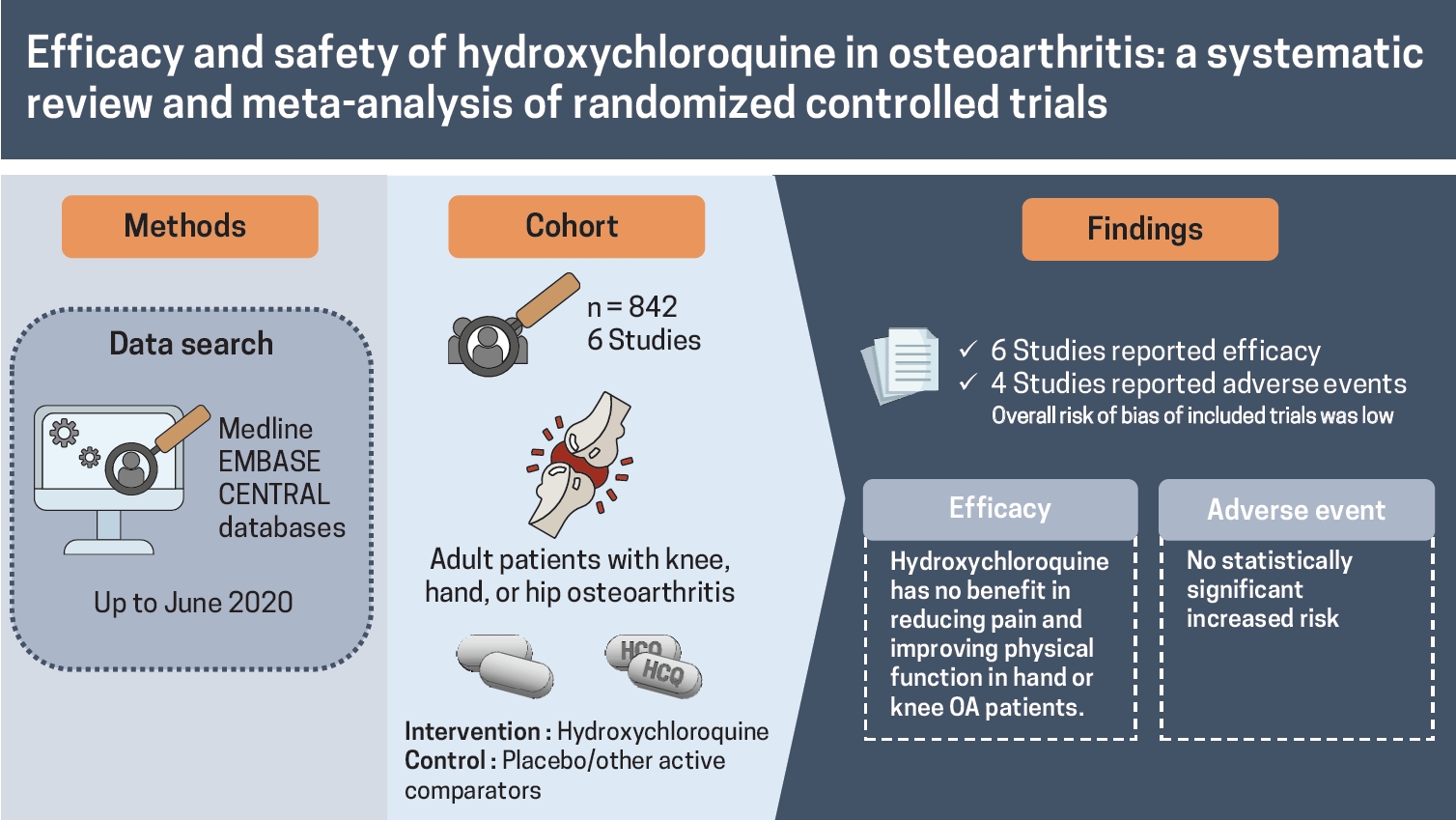
Six RCTs, four in hand OA, two in knee OA, consisting of 842 patients (436 in HCQ arm, 406 in control arm) were included. RCTs were conducted between 2012 and 2020, one each at UK, Netherlands, Germany, Italy, Iran, and Egypt; follow-up period ranged 24 to 52 weeks. High-quality evidence showed no clinically important pain reduction with HCQ compared to placebo/active-control in hand OA (standardized mean difference [SMD], 0.14; 95% confidence interval [CI], –0.20 to 0.48). Effect on pain reduction in knee and hand OA was small and non-significant (SMD, –0.09; 95% CI, –0.44 to 0.25). High-quality evidence showed no improvement in dysfunction with HCQ compared to placebo in hand OA patients (SMD, 0.08; 95% CI, –0.23 to 0.40). Effect on dysfunction improvement in knee and hand OA was modest and statistically non-significant (SMD, –0.20; 95% CI,–0.57 to 0.18). No improvement in quality of life was observed in hand OA.
Conclusions
HCQ has no benefit in reducing pain and improving physical function in hand or knee OA patients.
INTRODUCTION
Osteoarthritis (OA) has become a silent epidemic worldwide in recent years. With the combined effect of a variety of both non-modifiable and modifiable risk factors, including ageing, genetic predisposition, gender, obesity, injury or trauma, this burdensome disease is becoming more prevalent, with the estimated number of people who are suffering from hip or knee OA to be more than 300 million worldwide [1-4]. Accordingly, OA is identified as one of the 10 most common causes of disability in older adults in developed countries, with a higher prevalence in women than men [5]. Due to the absence of a cure, it poses a substantial and increasing health burden with notable implications for individuals and the healthcare systems [6-8].
Pharmacological and non-pharmacological treatments, either individually or in combination, remains as the mainstream intervention for OA [9]. The commonly recommended first-line of treatment included exercise and patient education. Pain medications for OA include paracetamol, topical, and oral non-steroidal anti-inflammatory drugs (NSAIDs) [6,9-11]. In a recent meta-analysis, paracetamol, however, has been shown to have minimal effect compared to placebo [12] and is not recommended in the recent guidelines, including the recent Royal Australian College of General Practitioners (RACGP) and Osteoarthritis Research Society International (OARSI) guidelines for the treatment of OA [13,14]. While both topical and oral use of NSAIDs were shown to be moderately effective for pain relief compared to placebo, oral use carries an increased risk of cardiovascular toxicity [6,15] and is often contra-indicated in OA patients who usually have comorbidities. The efficacy of intra-articular corticosteroids injections remains questionable due to the short-term benefits and the overall low quality of trials [15]. Moreover, a recent randomized controlled trial (RCT) highlights that intra-articular injection reduces the cartilage volume over 2 years than placebo treatment with no difference in knee pain [16]. New therapies are therefore required for the management of OA.
Given the limited efficacy of the current pharmacological treatments and the increasing evidence of the role of inflammation in OA, several conventional disease-modifying anti-rheumatic drugs (DMARDs) have been recently trialed in the hip, knee, and hand OA. One such medication is hydroxychloroquine (HCQ), which has been shown its effectiveness in treating rheumatoid arthritis (RA) with an acceptable safety profile [10,17,18]. However, the exact mechanism of action of HCQ in RA population is poorly understood. As a form of DMARDs, HCQ is suggested to have an inhibitory action on tolllike receptor (TLR) signaling [19]. In OA cartilage lesions, the TLRs have been found to be upregulated, where it stimulates cartilage breakdown via pro-inflammatory pathways [20,21]. Considering the inflammatory response in OA, the HCQ was proposed as a promising option in the treatment of OA.
Due to the moderately acceptable safety profile of HCQ, its popularity has risen in recent years, and several studies have investigated its effect in managing knee and hand OA [22-25]. To date, however, little attention has been made to systematically evaluate its efficacy for the management of OA. While few narrative reviews shed light on the inconsistent effects of HCQ on OA [10,11], the results of these reviews, however, were not based on comprehensive sources and methodical search strategies.
Although a previous systematic review focusing on non-pharmacological, pharmacological, and surgical treatment for hand OA reported a lack of efficacy of HCQ on pain, function, grip strength, and radiographic progression in hand OA [26], it only included three RCTs published as conference abstract with unclear risk of bias. Hence, a carefully constructed systematic review and meta-analysis, with a priori protocol, is essential to assess the effect of HCQ in patients with hip, knee, and hand OA. The present systematic review and meta-analysis, therefore, aimed to evaluate the efficacy and safety of HCQ for the treatment of hip, knee, and hand OA.
METHODS
Search strategy, selection, and data extraction
A systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We followed our protocol published with a priori defined search strategy, study inclusion and exclusion criteria, outcomes, and analyses [27]. Three bibliographic databases—MEDLINE and EMBASE using the Ovid interface and the Cochrane Central Register of Controlled Trials—were searched from inception till June 2020, with English and Chinese language restriction. The search strategy included a combination of Medical Subject Heading (MeSH) terms such as (Osteoarthritis OR “Degenerative Arthritis”) AND (Hydroxychloroquine OR Plaquenil) AND (“Randomized Controlled Trials” OR “Controlled Clinical Trial” OR placebo) (Appendix 1). Hand searching of abstracts from last 2 years conference proceedings of major international associations involved in OA research such as the European League against Rheumatism (EULAR), OARSI, and American College of Rheumatology (ACR) was performed to supplement the literature search. The articles were first screened based on their title and abstracts and then fulltext for their inclusion as per prespecified inclusion criteria by two researchers independently (A.S. and A.K.). Briefly, English or Chinese language articles reporting randomized, quasi-randomized, controlled trials assessing the safety and efficacy of the HCQ in the knee, hand, or hip OA patients above 40 years of age were included. The efficacy outcomes of interest included (1) change in OA associated pain and physical dysfunction assessed using the visual analog scale (VAS), Western Ontario and McMaster Universities Osteoarthritis Index Score (WOMAC), Australian/Canadian Osteoarthritis Index (AUSCAN), numeric rating scale (NRS); (2) quality of life (QoL) assessed using EQ-5D, SF-6D, health assessment questionnaire (HAQ). Safety outcomes included adverse effects (AEs). Serious adverse effect (SAE) reported with HCQ. Other outcomes of interest included radiographic structural damage and biomarker change [27]. In the event of more than one pain measure reported in a study, we used the pain outcomes in the following order: VAS, pain subscale of WOMAC, NRS, AUSCAN, and any other reported pain measures. The relevant data, such as information on study design, population characteristics, intervention/comparator details, and change in efficacy and safety outcomes, were extracted pre-designed excel sheet. Two investigators (A.S. and Z.W.) confirmed all data entries, and any discrepancy at the screening and data extraction stages were resolved by mutual discussion or arbitration by the third reviewer (B.A.).
Quality assessment
The bias risk of the included studies was evaluated according to the Cochrane Handbook 5.0.1 RCT bias risk assessment tool [28]. The quality of the literature was assessed for items such as sequence generation, allocation concealment, blinding of participants, study personnel, outcome assessors, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Two researchers (A.S. and A.K.) evaluated the quality; any difference of opinion was resolved by discussion or arbitration by the third researcher (B.A.).
Statistical analysis
Data pertaining to mean change in continuous outcome was used to estimate the pooled effect size. Efficacy outcome data on change from baseline to follow-up was calculated as the arithmetic difference between baseline and longest reported follow-up. The corresponding reported standard deviations (SD) were used, if not reported, were calculated using reported standard error (SE) or confidence intervals (CI). The change-from-baseline SD was calculated using the methods described in the Cochrane Handbook (Chapter 6; Section 6.5.2.8) [29] and a conservative correlation coefficient value of r = 0.5 [30]. To facilitate the pooling of data, we standardized the results of the studies to a uniform scale using standardized mean difference (SMD), as the studies assessed the outcome measure in a variety of ways using different assessment tools [29]. Review Manager 5 (RevMan 5.3) and STATA version 16.0 (STATA Corp., College Station, TX, USA) were used for data analysis. Statistical heterogeneity was assessed as per Q statistics (p < 0.05 was considered heterogeneous), and I2 statistic (I2 > 50% was deemed to be heterogeneous) [31]. A meta-analysis of the included studies was performed using the generic inverse variance random-effect model.
Reproducible research statements
The study protocol is available online at https://doi.org/10.1101/2020.07.20.20157669.
RESULTS
Literature search
The literature search process is shown in the PRISMA flow diagram (Fig. 1). A thorough literature search retrieved a total of 71 articles from three databases. Overall, 39 articles were sourced from PubMed and EMBASE, 32 were from Cochrane Central, and one article was identified by hand-searching. After duplicate removal, 61 articles were screened, and six articles were included in the final analysis.
Study characteristics
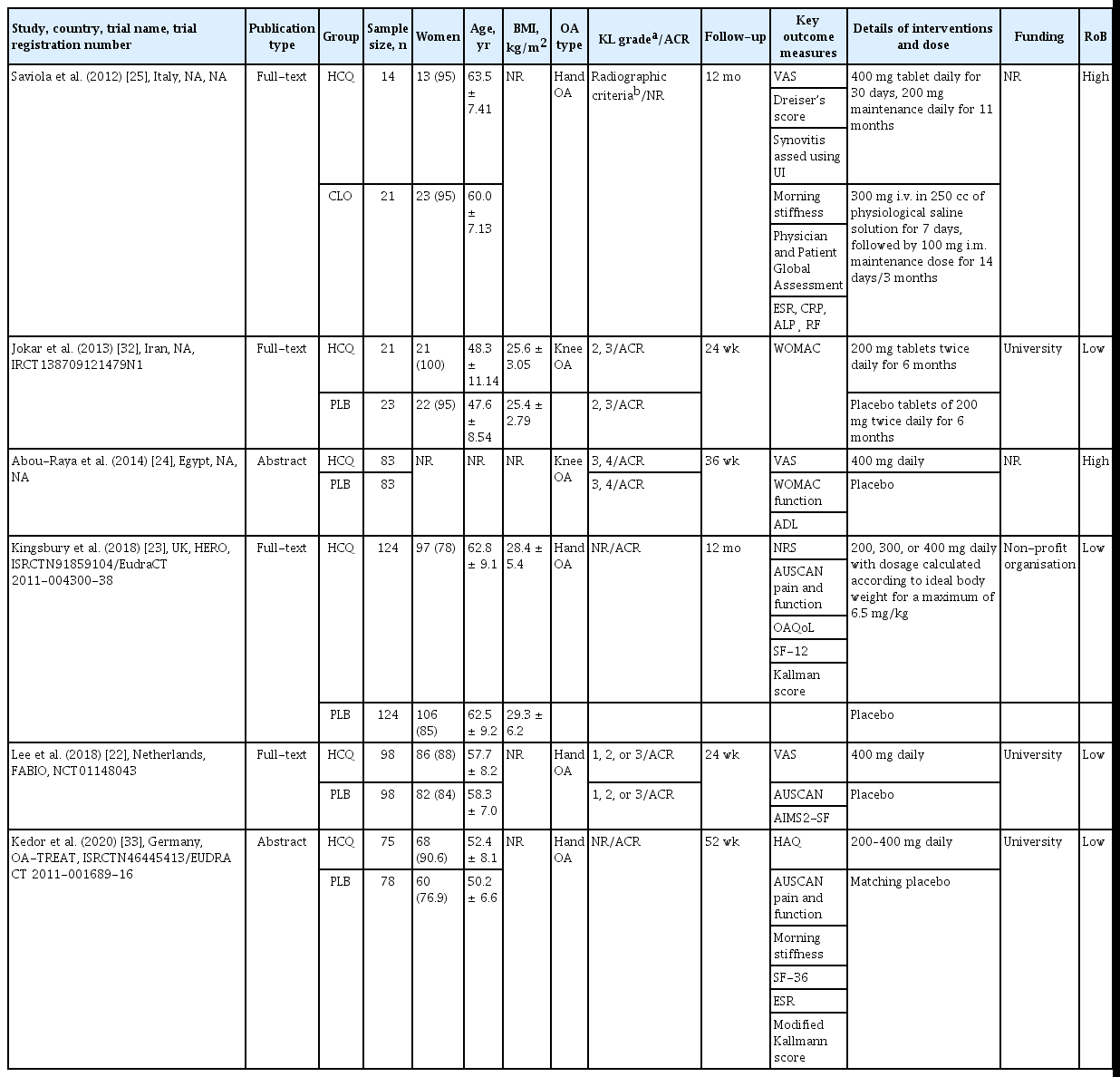
A total of 842 patients were enrolled in six included RCTs, with 436 in the experimental arm and 406 in the control arm [22-25,32,33]. Out of six studies, four were in hand OA [22,23,25,33], two were in knee OA patients [24,32] (overall: 89.6% women; mean age 55.3 years), and none of the studies assessed HCQ in hip OA. The majority of the studies compared HCQ with placebo, while one study in hand OA patients compared HCQ with an active comparator (chlodronate) [25]. The studies were conducted between 2012 and 2020, one each cross UK, Netherlands, Germany, Italy, Iran, and Egypt, and the follow-up period ranging between 24 and 52 weeks. The largest trial, including 248 hand OA patients, was conducted in the UK [23]. Table 1 describes the detailed characteristics of the included studies.
Five studies reported using ACR criteria for the inclusion of the patients [22-24,32,33]. Besides, three studies, two in knee OA and one in hand OA, further employed radiographic OA (Kellgren-Lawrence grade) as the inclusion criteria of the participants [22,24,32]. Three studies, one in knee OA and two in hand OA, assessed pain using VAS [22,24,25]. Three out of four hand OA studies reported pain using the AUSCAN scale [22,23,33], while all studies (n = 2) in knee OA used WOMAC scale to assess pain and function [24,32]. Two studies, reported imaging outcomes, whereas biochemical markers were reported in one study only [23,25,33]. The daily dose of HCQ was 400 mg for knee OA patients, whereas the daily dose in hand OA patients varied from 200 to 400 mg. Four of the included studies were registered with clinical trials registry [22,23,32,33], half of the included studies were investigator-initiated [22,32,33], and 67% were academic/non-profit organization funded [22,23,32,33]. The funding was not disclosed for 33% of the studies [24,25].
Quality assessment
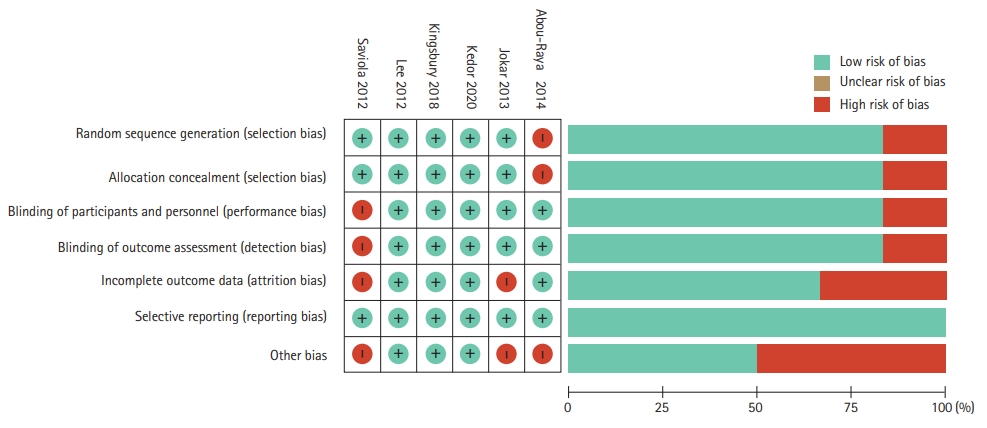
The overall risk of bias of included trials was low, with three trials assessed as having high quality according to the Cochrane ROB tool [22,23,33]. Three of the included studies were assessed as having a high risk for incomplete outcome data either due to loss to follow-up or not providing adequate data for missing information (Fig. 2) [25,32].
Efficacy outcomes
Effect of HCQ on OA related pain
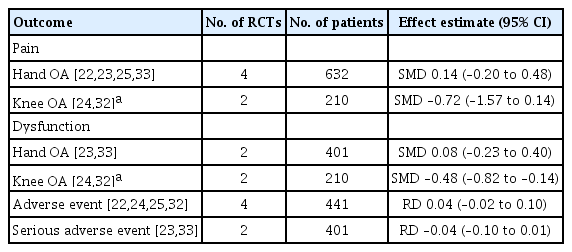
All the six included RCTs assessed pain, four in hand OA, and two in knee OA, using various patients reported outcome (PRO) instruments: VAS, NRS, AUSCAN, and WOMAC. Three RCTs evaluated pain using VAS; two in hand OA one in knee OA [22,24,25]. RCTs in knee OA patients reported pain using WOMAC and VAS each [24,32]. Three studies in hand OA assessed pain using AUSCAN [22,23,33]; whereas, one study reported NRS [23]. Overall, five trials constituting 401 participants in the HCQ groups and 406 participants in the placebo control groups contributed to the analysis of pain in hand or knee OA patients [22-24,32,33]. One trial with 14 participants in the HCQ groups and 21 in the clodronate control groups also contributed to the overall analysis of pain in hand or knee OA patients [25]. We found high-quality evidence that HCQ had no clinically important pain reduction compared to placebo/active control interventions in hand OA patients (SMD, 0.14; 95% CI, –0.20 to 0.48) [22,23,25,33]. Moderate-quality evidence from two studies showed a larger pooled effect size for pain reduction in knee OA; however, it was with a wide CI and statistically non-significant (SMD, –0.72; 95% CI, –1.57 to 0.14) [24,32]. Overall, the pooled effect on pain reduction in OA patients was small and not significant (SMD, –0.09; 95% CI, –0.44 to 0.25). An I2 statistic of 75% and 83% for studies assessing pain in hand OA and knee OA, respectively indicated a high degree of statistical heterogeneity (Fig. 3). Two studies that assessed pain using both AUSCAN and NRS [23], and AUSCAN and VAS [22] found no statistically significant improvement in pain in either of the instruments.
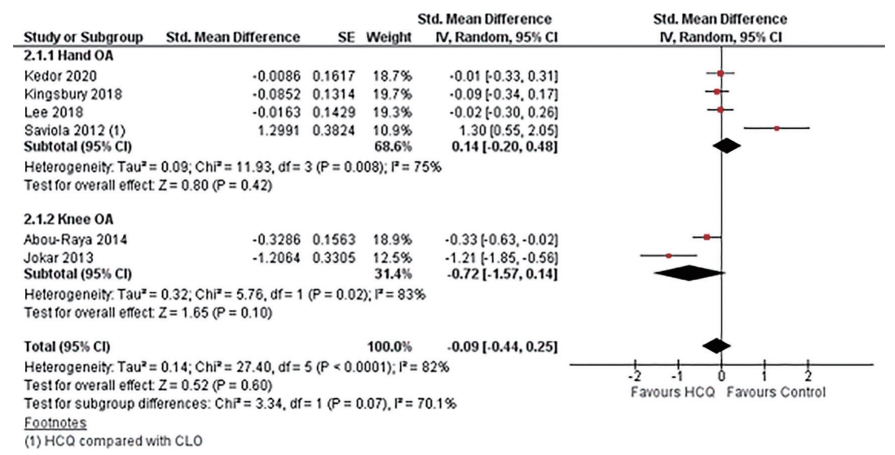
Pooled standardized mean difference (SMD) for change in osteoarthritis (OA) associated pain. Abou-Raya et al. [24] was published as an abstract in 2013 and is still not published as full-text; the author did not make the data available upon e-mail request. SE, standard error; IV, generic inverse-variance random-effect meta-analysis; CI, confidence interval; HCQ, hydroxychloroquine; CLO, clodronate.
Effect of HCQ on OA related dysfunction
Four studies assessed function limitation, two reported AUSCAN function limitation in hand OA [23,33], and two reported WOMAC function limitation in knee OA [24,32]. Overall, four trials constituting 303 participants in the HCQ groups and 308 participants in the placebo control groups contributed to the analysis of dysfunction in hand or knee OA patients [23,24,32,33]. We found high-quality evidence that HCQ had no improvement in dysfunction compared to placebo in hand OA patients (SMD, 0.08; 95% CI, –0.23 to 0.40) [23,33]. However, the moderate-quality evidence suggested a modest, although statistically significant, improvement in knee OA associated dysfunction (SMD, –0.48; 95% CI, –0.82 to –0.14) [24,32]. Overall, the pooled effect on dysfunction improvement in OA patients was modest with a wide CI and statistically non-significant (SMD, –0.20; 95% CI, –0.57 to 0.18). An I2 statistic of 58% for studies assessing dysfunction in hand OA indicated a moderate degree of statistical heterogeneity; however, I2 value of 20% demonstrated low heterogeneity between the studies assessing pain in knee OA (Fig. 4).
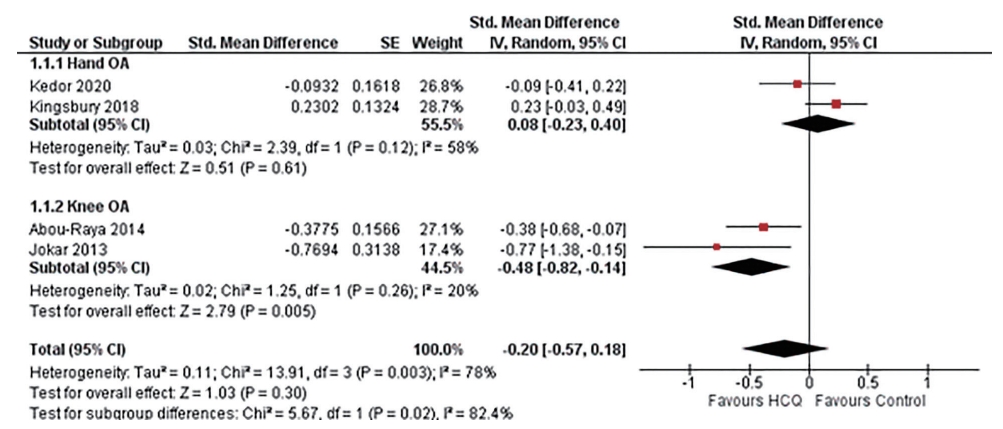
Pooled standardized mean difference (SMD) for change in osteoarthritis (OA) associated dysfunction. Abou-Raya et al. [24] was published as an abstract in 2013 and is still not published as full-text; the author did not make the data available upon e-mail request. SE, standard error; IV, generic inverse-variance random-effect meta-analysis; CI, confidence interval; HCQ, hydroxychloroquine.
Effect of HCQ on OA related quality of life
Three studies assessed the QoL in hand OA patients using PRO instruments: osteoarthritis quality of life scale (OAQoL), 12-item short form survey (SF-12), Arthritis Impact Measurement Scale 2 short form (AIMS2-SF), and 36-item short form survey (SF-36) [22,23,33]. None of the knee OA studies reported QoL outcomes; however, one study in knee OA assessed the impact of knee OA on patient’s activities of daily living (ADL) [24]. Unanimously, high-quality evidence from three studies in hand OA patients reported no systematic treatment differences between HCQ and placebo for the QoL assessed using OAQoL, SF-12, AIMS2-SF, and SF-36 (mental and physical) [22,23,33]. However, low-quality evidence from one study reported a small but statistically significant improvement in ADL (mean difference, 1.1; p < 0.05) in knee OA patients at 36 weeks [24].
Effect of HCQ on OA related imaging markers
Two studies in hand OA patients assessed radiographic progression using Kallman and modified Kallman score [23,33]. Both the studies reported no statistically significant (p > 0.24) improvement in Kallman radiographic scores at 12-month follow-up duration [23,33]. One study in knee OA assessed synovitis using ultrasound imaging and reported a clinically relevant reduction in synovitis in HCQ groups at 52 weeks; however, accompanying data was not reported [25].
Effect of HCQ on OA related biochemical markers
Two studies in hand OA patients assessed biochemical marker outcomes: erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). While one study observed no changes in ESR in both groups [25], others reported a significant difference in ESR (p < 0.01) between both groups at 52 weeks [33]. The CRP levels were reported improved initially in the first 6 months and then decreased at month 12 follow-up [25].
Safety outcomes
Four studies, two in hand and knee OA each, reported AEs [22,24,25,32] comprising 216 patients in the HCQ group and 225 patients in the placebo/active-comparator group, whereas SAEs were reported in two studies comprising 199 hand OA patients in HCQ group and 202 in the placebo group [23,33]. Fewer SAEs were observed in the HCQ group; however, the difference was non-significant (risk difference [RD], –0.04; 95% CI, –0.10 to 0.01) [23,33]. Likewise, no significant difference was observed for AEs (RD, 0.04; 95% CI, –0.02 to 0.10); however, higher AEs were reported in the HCQ arm [22,24,25,32]. Notable SAEs of prolonged QT interval with ventricular arrhythmias, erythema multiforme, and acute generalized erythematous pustulosis were being related to HCQ [23]. Overall, no difference was observed for AE/SAE between HCQ and placebo/active-comparator arm (RD, 0.00; 95% CI, –0.04 to 0.04) (Fig. 5).
DISCUSSION
To the best of our knowledge, this is the first and most comprehensive systematic review and meta-analysis assessing the efficacy and safety of HCQ for the treatment of OA. We found high-quality evidence to support that HCQ is no more effective than placebo/active control in reducing pain in knee or hand OA patients [22- 25,32,33]. Furthermore, high-quality evidence suggested that HCQ demonstrated no improvement in physical dysfunction compared to placebo for patients with hand OA [23,33]. However, moderate-quality evidence showed a modest improvement in knee OA associated dysfunction [24,32]; the benefit was small and may not have been clinically important (Table 2). The overall safety profile HCQ was acceptable with AEs/SAEs comparable to placebo/clodronate. Likewise, no favorable effect of HCQ was observed on QoL in hand and knee OA patients [22,23,33]. Limited evidence was reported assessing the effect of HCQ on radiographic markers and biomarkers in the knee and hand OA patients.
The results were broadly uniform across major studies showing no beneficial effect of HCQ in for the treatment of OA, with an exception in showing improvement in function mainly derived from knee OA studies by Jokar et al. [32] and Abou-Raya et al. [24]. For hand OA associated pain outcome, Saviola et al. [25] study was the sole contributor for heterogeneity; where moderate heterogeneity was observed in hand OA associate function outcome. To be noted, Saviola et al. [25] compare HCQ with clodronate, an active comparator, and was the only open-labeled trial. Furthermore, it is essential to understand the characteristics of the two studies in knee OA [24,32]. The Joker et al. [32] study demonstrated an unusually narrow SD of symptoms and a lack of a placebo effect. RCTs in OA typically show a strong placebo effect, primarily due to alternate flare and remission in symptoms in OA patients [34,35]. The Abou-Raya et al. [24] study, which demonstrated improvement in pain and function with HCQ, was published as an abstract in 2013 and is still not published as full-text after many years; furthermore, the author did not make the data available when requested through email. Additionally, another trial by the same author team exploring the use of methotrexate in knee OA patients was retracted due to data inconsistencies [36]. Hence, any inference should be made considering these facts. Nevertheless, the pooled effect estimate for improvement in OA associated dysfunction was small and was statistically non-significant.
HCQ has a relatively acceptable safety profile and offers modest symptomatic relief in chronic immunity-mediated inflammatory rheumatic diseases [37]. However, a recent systematic review of HCQ in patients with RA demonstrated only a modest improvement in the outcome of interest (ACR20 and ACR50) when used in combination with other conventional synthetic DMARDs [38]. Nevertheless, considering the hypothesis that inflammation has a role in osteoarthritic pathogenesis, researchers have trialled HCQ in OA. Notably, the study by Saviola et al. [25] in erosive hand OA patients was stopped prematurely citing ethical concerns regarding the inefficacy of HCQ. Likewise, the results from HERO trial in nodal hand OA, OA-TREAT trial in inflammatory and erosive hand OA, and FABIO trial in primary hand OA did not support the efficacy of HCQ for pain relief and function improvement [22,23,33]. Thus, while the high-quality evidence from these methodologically rigorous trials, with long follow-up duration (6 to 12 months), put an end to the quest for exploring the efficacy of HCQ in hand OA; the current evidence also indicate that there would unlikely be any promising potential for HCQ for the treatment of knee and hip OA.
The strengths of this review include a registered protocol-oriented approach, extensive literature search, and the use of appropriate statistical techniques to pool the effect estimates. This review had few constraints as well. We restricted our research to English and Chinese language articles and may have missed studies published in other languages. Data were scarce that limited the scope for subgroup analysis and publication bias assessment. The patient population included knee and hand OA patients with varying OA phenotypes. Furthermore, studies comparing HCQ with placebo and active comparator were pooled together for the OA associated pain. However, on removing the study with the active comparator, the results did not change and remained statistically non-significant. Last, due to the inadequate data reporting in some trials, SD values were imputed; however, we used the prescribed methods and assumptions.
In conclusion, this systematic review and meta-analysis found that HCQ has no benefit in reducing pain and improving physical function in hand or knee OA patients. Off-label use of HCQ for patients with OA should be discouraged, considering no additional benefit.
KEY MESSAGE
1. Current evidence shows that, hydroxychloroquine (HCQ) has no benefit in reducing pain or improving physical function in patients with hand and knee osteoarthritis (OA).
2. However, HCQ demonstrated an acceptable safety profile in this population.
3. Off-label use of HCQ for patients with OA should be discouraged.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
Ambrish Singh is supported by International Graduate Research Scholarship, University of Tasmania. Benny Antony is supported by National health and Medical Research Council of Australia Fellowship.