Risk factors for neutropenic fever in non- Hodgkin’s lymphoma patients with primary granulocyte colony-stimulating factor prophylaxis
Article information
Abstract
Background/Aims
Febrile neutropenia (FN) interferes with the proper chemotherapy dose density or intensity in non-Hodgkin’s lymphoma (NHL) patients. Chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) ± rituximab has an intermediate FN risk. Prophylactic granulocyte colony-stimulating factor (G-CSF) support is recommended for patients with other host-related risk factors.
Methods
We evaluated the risk factors for FN-related admission in NHL patients who have received primary G-CSF (lenograstim) prophylaxis.
Results
Data from 148 patients were analyzed. The incidence of neutropenic fever was 96 events (12.2%), and the median period was 3.85 days (range, 0 to 5.9); the median duration of neutropenia was 4.21 days (range, 3.3 to 5.07). Eighty-three FN-related admissions were reported. Advanced age (> 60 years), female sex, a low albumin level, and prednisone use were associated with FN-related admission in multivariable analysis (p = 0.010, p < 0.001, and p = 0.010, respectively). A comparison between diffuse large B-cell lymphoma patients treated with R-CHOP and pegylated G-CSF and those treated with R-CHOP and lenograstim did not reveal significant differences in the FN-related admission rate between the two groups, although the lenograstim-treated group had a higher incidence of severe neutropenia.
Conclusions
Elderly patients, female patients, and patients with low albumin levels need to be actively followed-up for FN even when primary prophylaxis with G-CSF has been used.
INTRODUCTION
Even though a large proportion of non-Hodgkin’s lymphoma (NHL) patients could achieve a complete response, chemotherapy-induced febrile neutropenia (FN) is a major barrier to the completion of the planned chemotherapy. FN is frequently responsible for hospital admission, prolonged hospitalization, and immediate administration of intravenous (IV) antibiotics [1]. It is also associated with increased infection-related mortality, with an overall FN-related mortality of 6.8% to 20% [2,3]. Moreover, it can preclude the delivery of the intended dose of chemotherapeutic agents, interfere with its schedule, or even lead to discontinuation of chemotherapy. Decreasing the dose density or intensity can adversely affect the treatment outcome in NHL [4]. Prevention of chemotherapy-related FN is the most important supportive care for improving treatment outcomes in NHL patients. Therefore, the identification of high-risk patients is of primary relevance in prevention. Although granulocyte colony-stimulating factor (G-CSF) support can help reduce FN-related early deaths and achieve the planned dose and schedules, some patients still experience serious FN-related complications [5,6].
The American Society of Clinical Oncology and the European Organization for Research and Treatment of Cancer recommend primary prophylaxis with G-CSF with a chemotherapy regimen of ≥ 20% FN risk or regimens with intermediate FN risk (10% to 20%), wherein the overall risk takes into consideration the patient-related risks [7,8]. The FN risk for cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP)-21 ± rituximab chemotherapy was reported as 17% to 50% [7,9], which classifies it as intermediate risk. As previously mentioned, patient-related risk factors should be assessed when using intermediate-risk chemotherapy to assure that prophylactic G-CSF is provided to patients with an overall high risk.
Based on the National Comprehensive Cancer Network guideline, advanced age (≥ 65 years), previous exposure to chemotherapy or radiotherapy, persistent neutropenia, bone marrow involvement of the tumor, poor performance status, recent surgery and/or open wounds, renal or liver dysfunction, and human immunodeficiency virus infection are considered high-risk patient-related factors [10,11]. Among the high-risk patients treated with prophylactic G-CSF, identifying vulnerable populations for FN-related complications is clinically useful for FN prevention and management.
In this study, we evaluated the FN-related outcomes, including FN-related admission, in NHL patients with high-risk patient-related factors treated with CHOP-21 ± rituximab and prophylactic G-CSF, to identify the predictive factors for FN-related admission.
METHODS
Patients
We enrolled 158 patients who were diagnosed with NHL between 2012 and 2015. Ten patients withdrew their consent; therefore, data from 148 patients were analyzed. All patients were scheduled to receive frontline CHOP or R-CHOP chemotherapy. All patients were aged over 20 years and had one or more of the following patient-related factors for FN: age ≥ 65 years, Ann Arbor stage ≥ III, previous chemotherapy or radiation therapy, bone marrow involvement, absolute neutrophil count (ANC) < 1.5 × 109/L or hemoglobin level < 12.0 g/dL before starting chemotherapy, active infection, open wounds, Eastern Cooperative Oncology Group performance status ≥ 2 or poor nutritional status, hepatic or renal dysfunction, chronic obstructive pulmonary disease, cardiovascular disease, and diabetes mellitus.
Chemotherapy
All patients were treated with CHOP ± R chemotherapy every 3 weeks. Chemotherapy was composed of cyclophosphamide (750 mg/m2 IV on day 1), doxorubicin (50 mg/m2 IV on day 1), vincristine (1.4 mg/m2 up to 2 mg, IV on day 1), prednisone (100 mg oral on day 1 to 5), and rituximab (375 mg/m2 IV on day 1). The treatment response was assessed based on the revised International Workshop Criteria [12]. The relative dose intensity (RDI) was calculated as the ratio of the actual dose intensity to the planned dose intensity for a fixed time period [6,9,13]: RDI = actual dose intensity / planned dose intensity; actual dose intensity = actual dose received for each agents (mg/m2) / actual duration of chemotherapy for all agents (days); planned dose intensity = planned total dose for each agent (mg/m2) / planned duration of chemotherapy for all agents (days). Any adverse event was assessed by the National Cancer Institute-Common Terminology Criteria for Adverse Events, 2009 (version 4.0).
Study drug
Lenograstim (Neutrogin, JW Pharmaceutical Corp., Seoul, Korea) is a recombinant human G-CSF. For prophylaxis, 5 μg/kg/day of lenograstim was injected subcutaneously from days 4 to day 6 after chemotherapy and was continued until the ANC reached 3.0 × 109/L. In case of outpatients, G-CSF was administered on weekdays only, while it was administered continuously without interruption to hospitalized patients. FN was defined as a single oral temperature ≥ 38.3°C or a temperature ≥ 38.0°C sustained for > 1 hour with an ANC < 0.5 × 109/L or < 1.0 × 109/L predicted to fall below 0.5 × 109/L within 48 hours [14,15].
Statistical analysis
The primary endpoint of this study was admission associated with FN during chemotherapy. The secondary endpoints were the development of severe neutropenia (defined as ANC < 0.5 × 109/L), duration of neutropenia (defined as ANC < 0.5 × 109/L or ANC < 1.0 × 109/L), presence and duration of FN, the incidence and duration of infection, use of IV antibiotics, admission not related to FN, reduction of dose density or intensity, complete response rate, and progression-free survival (PFS) or overall survival (OS). PFS was defined as the time from the study enrollment to the date of disease progression or death from any cause. OS was calculated as the time from the study enrollment to death from any cause or the last follow-up. Treatment outcomes of lenograstim-treated patients were compared with those of pegylated G-CSF treated patients, which were obtained from the historical records. We retrieved the data of patients who completed R-CHOP chemotherapy and received pegylated G-CSF between 2014 and 2019. To adjust for the distribution of baseline characteristics, we matched age, sex, and the international prognostic index. Propensity score matching was performed using a 1:1 matching protocol without replacement. Survival analysis and comparisons were performed using the Kaplan-Meier method and the log-rank test. A logistic regression model was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs). A p value < 0.05 was considered statistically significant for all analyses. All statistical analyses were performed using SPSS for Windows version 24.0 (IBM Corp., Armonk, NY, USA) and R package version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical statement
The study protocol was approved by the Institutional Review Board of each participating institution (Severance Hospital IRB No. 4-2011-0432). Informed consent was obtained from all individual participants included in the study.
RESULTS
Patients’ characteristics
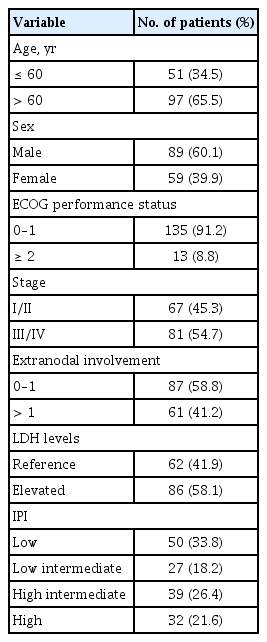
Baseline characteristics are shown in Table 1. Median age was 69 years (range, 24 to 83) with 89 (60.1%) male patients. All patients had at least one risk factor for FN before chemotherapy: four (2.7%), previous chemotherapy; two (1.4%), previous radiation; 14 (9.5%), bone marrow involvement at diagnosis; 4 (2.7%), low ANC (< 1.5 × 109/L); 63 (42.6%), hemoglobin level below 12.0 g/dL, 34 (23.0%), low albumin level (< 3.5 g/dL); four (2.7%), infections; one (0.7%), open wound; eight (5.4%), renal impairment; 14 (9.5%), hepatic disease; one (0.7%), chronic obstructive pulmonary disease; 37 (25.0%), cardiovascular disease; 23 (15.5%), diabetes mellitus; 43 (29.1%), hypertension; 12 (8.1%), hepatitis; and seven (4.7%), history of tuberculosis.
Treatment outcomes
Median follow-up duration was 10.0 months (range, 0 to 38). The OS was 84.0% and PFS was 74.0% at 1-year. Among the 109 patients who completed the planned chemotherapy, 86 (78.9%) achieved complete response, 14 (12.8%) achieved partial response, five (4.6%) had stable disease, and four (3.7%) had progressive disease. The RDIs of the chemotherapeutic agents were as follows: rituximab 0.95 (0.38 to 1.02), cyclophosphamide 0.89 (0.32 to 1.18), doxorubicin 0.86 (0.34 to 1.02), vincristine 0.73 (0.27 to 1.05), and prednisone 0.96 (0.20 to 1.35). Throughout the chemotherapy cycles, in total, 775 G-CSF were administered and the median number of G-CSF used was 5 per cycle (range, 1 to 30).
Safety
The safety profiles of lenograstim were assessed by adverse events. These results were assessed as secondary endpoints. Severe neutropenia (ANC < 0.5 × 109/L) was observed in 322 events among the 786 chemotherapy cycles, and the median duration of neutropenia was 4.21 days (range, 3.3 to 5.07). The incidence of FN was 96 events (12.2%); the median duration of FN was 3.85 days (range, 0 to 5.9). The number of cases of FN-related infection was 103; 95 patients received IV antibiotics, and the median duration of IV antibiotics was 8.5 days (range, 7.2 to 15). In total, 90 admissions were reported, of which 83 were related to FN, while the rest were due to general weakness, and infection without neutropenia, among other causes.
Predictive factors for FN-related admission
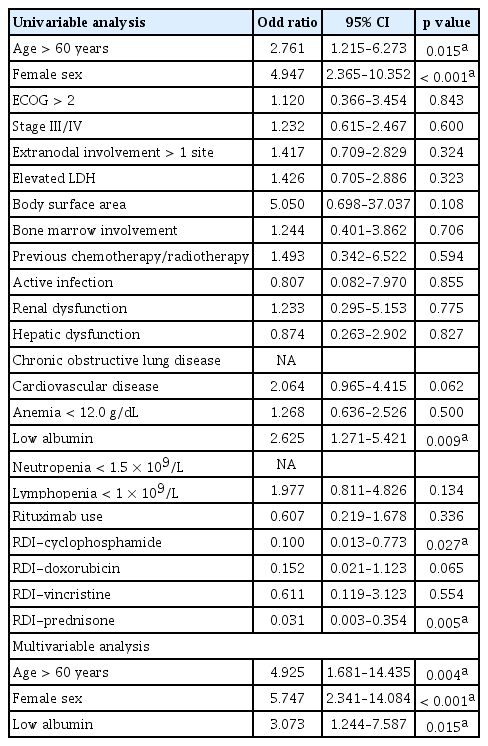
The primary endpoint of this study was FN-associated admission during chemotherapy. The total number of FN-associated admissions was 83 (10.5%) among 786 cycles of chemotherapy. Three factors were significantly associated with FN-related admission on multivariable analysis (Table 2): advanced age (> 60 years) (OR, 2.761; 95% CI, 1.1215 to 6.273; p = 0.015), female sex (OR, 4.947; 95% CI, 2.365 to 10.352; p < 0.001), and low albumin level (OR; 2.625; 95% CI, 1.271 to 5.421; p = 0.009). Other risk factors such as poor performance status (p = 0.843), advanced disease stage (p = 0.600), extranodal involvement at more than one site (p = 0.324), elevated lactic dehydrogenase levels (p = 0.323), lymphopenia < 1 × 109/L (p = 0.134), and the use of rituximab (p = 0.336) were not related with FN-associated admission. Among patients who received R-CHOP chemotherapy, advanced age (> 60 years) (OR, 4.730; 95% CI, 1.468 to 15.241; p = 0.009) and female sex (OR, 3.782; 95% CI, 1.506 to 9.501; p = 0.005) were predictive factors for FN-related admission.
Comparison with pegylated G-CSF-treated patients
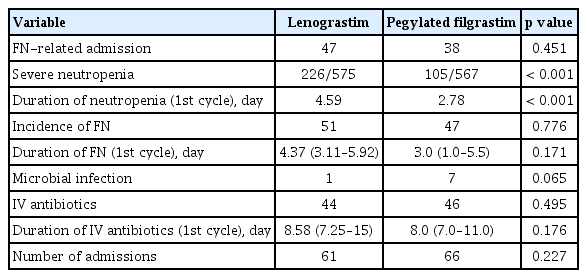
A total of 97 patients were included in each group using propensity score matching (Table 3). The total number of FN-related admissions was not different between the two groups (47 events in the lenograstim group and 37 events in the pegylated G-CSF-treated group; p = 0.451). The incidence of severe neutropenia was 39.3% (226 events of 575 cycles) in the lenograstim-treated group and 18.5% (105 events among 567 cycles) in the pegylated G-CSF-treated group (p < 0.001). The duration of neutropenia during the first cycle was 4.59 days in the lenograstim-treated group and 2.78 days in the pegylated G-CSF-treated group (p < 0.001). Other secondary endpoints were not different (Table 4).
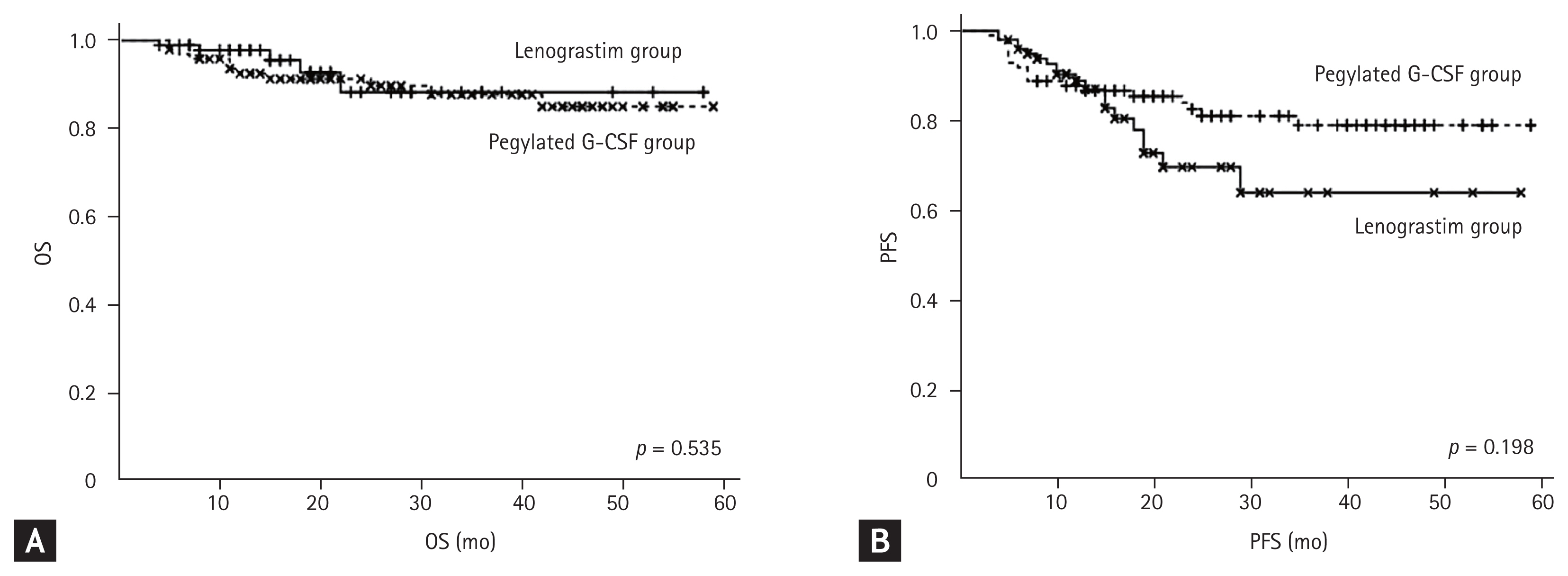
OS rate was 98.0% in the lenograstim-treated group and 94.0% in the control group at 1 year (p = 0.535) (Fig. 1A). PFS rate was 89.0 % in lenograstim-treated group and 87.0% in the pegylated G-CSF-treated group at 1 year (p = 0.198) (Fig. 1B). The RDI of rituximab, cyclophosphamide, doxorubicin, and vincristine were higher in the pegylated G-CSF-treated group (p = 0.001, p = 0.001, p < 0.001, and p < 0.001); the RDI of prednisone was not different (p = 0.422).
DISCUSSION
This multicenter prospective observational study evaluated the FN-related outcomes of primary G-CSF support in patients who received CHOP ± R chemotherapy. The primary endpoint of this study was the FN-related admission rate, which was found to be 10.5%. Advanced age, female sex, and low albumin levels were associated with FN-related admission. Compared with pegylated G-CSF-treated patients as a historical control, primary prophylaxis with lenograstim showed non-inferior FN related admission rate, although the incidence of severe neutropenia was higher in the lenograstim group.
The chemotherapy regimen, patient-related factors, and tumor type and burden should be considered as the risk factors for FN [10]. All enrolled patients had at least one patient-related risk factor for FN, and we evaluated the predictive factors for FN-associated admission among these patients. The incidence of FN was 96 events of 786 cycles (12.2%), and the FN-related admission rate was 10%. This value was lower than that previously reported [7,9]. Among lymphoma patients treated with CHOP-like chemotherapy without primary G-CSF prophylaxis, the FN-related admission rate was reported to be 17% to 50% [9,16]. Prophylactic G-CSF initiated in the first chemotherapy cycle was associated with a 0.84-time lower risk of FN [5]. The most common adverse reaction to G-CSF administration was musculoskeletal pain, which could be managed with analgesics [17]. In this study, toxicity was mainly related to chemotherapy.
Age ≥ 65 years, low albumin level, neutropenia, presence of hepatic disease, planned average RDI > 80%, and no early G-CSF use increased the initial hospitalization rate [9]. We used prophylactic G-CSF support in all high-risk patients; however, advanced age, low albumin level, and female sex still had statistical significance with respect to FN-related admission. The RDI of rituximab, cyclophosphamide, and prednisone were risk factors for FN-related admission on univariable analysis but it failed to show statistical significance on multivariable analysis.
Age is an established risk factor for FN, and advanced age has been related to serious complications, including death [18–20]. The cut-off value for age is usually 65 years, although 60 years has been used in some reports [21]. In this study, 60 years of age was a significant risk factor for FN that requires admission. Albumin levels have been known to be related to FN and reflect the nutritional status of cancer patients [22]. Female sex is also a predictive factor for FN [9,17]. Advanced age, female sex, and low albumin level led to a 4.9-, 5.7-, and 3.0-times increase in the risk for FN-related admission in patients with prophylactic support, respectively. Therefore, more aggressive approaches and supportive care are needed to prevent FN-related admission in patients with these risk factors.
After propensity score matching, there were no differences in FN-related admissions between the lenograstim and pegylated G-CSF groups. Although the incidence of severe neutropenia was higher in the lenograstim group, this could be because blood test was not done regularly in pegylated G-CSF group. Response rate was not different between the groups, although the chemotherapy RDI was higher in the pegylated G-CSF group. Pegylated G-CSF could be superior in maintaining a consistent dose density, and observation that needs further investigation. Finally, long-term follow-up is required to reveal the relation between dose density and treatment outcome. Previous studies revealed that four single doses of filgrastim are equivalent to pegylated filgrastim in terms of maintaining the dose intensity of an R-CHOP regimen administered every 2 weeks [23,24]. According to this study, a daily dose of 5 μg/kg lenograstim until ANC recovery or one dose of 6 mg pegylated G-CSF can work as treatment options for NHL patients with patient-related risk factors.
This study has some limitations. We used daily G-CSF for NHL patients instead of pegylated G-CSF, which is now widely used for primary prophylaxis with CHOP ± R chemotherapy for NHL. At the beginning of the study, prophylactic pegylated G-CSF was not widely used in Korea. Moreover, it was usually used for secondary prophylaxis after neutropenia development. Therefore, we compared the efficacy of daily primary prophylactic use of G-CSF and prophylactic use of pegylated G-CSF using propensity score matching from historical control. Furthermore, the follow-up period was not sufficiently long, and there was no relation between dose intensity and treatment outcome. Long-term follow-up is needed to elucidate this issue. This study was significant in terms of determining the predictive factors for FN-related admission in high-risk patients who receive CHOP-21 ± R chemotherapy and primary prophylactic G-CSF support. Most importantly, the final goal of R-CHOP chemotherapy in diffuse large B-cell lymphoma was not palliative but curative; therefore, reducing the infection-related complications during chemotherapy is very important.
In conclusion, this study showed the FN-related outcomes of primary G-CSF prophylaxis in NHL patients with high-risk patient-related factors. Patients with advanced age, female sex, or low albumin levels before chemotherapy should be managed cautiously to minimize the risk of FN-related admission despite early primary prophylactic G-CSF support.
KEY MESSAGE
1. Febrile neutropenia (FN)-related admission rate was not different between non-Hodgkin’s lymphoma patients who received primary granulocyte colony-stimulating factor (G-CSF) prophylaxis and those who received pegylated G-CSF prophylaxis.
2. Elderly patients, female patients and patients with low albumin levels need to be actively followed-up for FN.
Notes
This study was sponsored by JW Pharmaceutical (Seoul, Korea).