Survey of the management of patients with bronchiectasis: a pilot investigation in Asian populations
Article information
Abstract
Background/Aims
Although international guidelines for bronchiectasis management have been published in Western countries, there is a lack of data about their application in Asian populations including patients with different phenotypes. We aimed to investigate the current status of bronchiectasis management in Asian populations.
Methods
A nationwide questionnaire survey was performed of Asian respiratory specialists from South Korea, Japan, Taiwan, Singapore, Vietnam, and Sri Lanka. Participants were invited by e-mail to answer a questionnaire comprising 25 questions based on international guidelines for the management of bronchiectasis.
Results
A total of 221 physicians participated in the survey. About half of them were Korean (50.2%), with the next most common nationalities being Japanese (23.1%), Taiwanese (13.6%), and Singaporean (7.7%). Only 18 (8.1%) responders had local guidelines for bronchiectasis. While 85 (38.5%) responders checked sputum acid-fast bacillus smear/culture about 1 to 3 times per year, only a small proportion of responders routinely performed a serum immunoglobulin test (36/221, 16.3%) or evaluated for allergic bronchopulmonary aspergillosis (41/221, 18.6%). Less than half (43.4%) of responders performed eradication treatment in patients with drug-sensitive Pseudomonas aeruginosa infection, mainly due to the limited availability of inhaled antibiotics (34.8%). In addition, 58.6% of responders considered physiotherapy such as airway clearance and pulmonary rehabilitation.
Conclusions
Discrepancies might exist between guideline recommendations and practice for bronchiectasis management in Asian populations, partly due to the limited availability of treatment in each country. The development of local guidelines that consider the phenotypes and situation will help to standardize and improve the management of bronchiectasis.
INTRODUCTION
Bronchiectasis is a chronic respiratory disease defined by irreversible dilatation of the bronchi [1]. Patients with bronchiectasis suffer from respiratory symptoms and exacerbations [2,3], which cause a substantial disease burden for both patients and society [4,5]. In addition, recent studies showed that the incidence and prevalence of bronchiectasis have been increasing [6,7]. Accordingly, social interest in bronchiectasis is growing, and several guidelines for bronchiectasis have been published and updated worldwide [8–11]. The guidelines of the European Respiratory Society (ERS) address nine key questions about the management of adult bronchiectasis [10], while the British Thoracic Society (BTS) suggests recommendations at the evidence level [9].
Bronchiectasis has a heterogeneous nature and etiology [12], and therefore displays ethnic and geographic variations. Indeed, Dhar et al. [13] reported that patients with bronchiectasis in India have distinct characteristics from those in other countries and observed poor adherence to guideline-recommended care in real-world practice. In addition, Choi et al. [14] showed that the prevalence of cardiovascular diseases is low in Korean bronchiectasis patients compared with Western patients. A prior history of tuberculosis is one of the main causes of bronchiectasis in Asia [15]. Considering these findings, Asian patients with bronchiectasis have different phenotypes, and thus different practice guidelines are required. However, there is a lack of guidelines for Asian bronchiectasis patients with potentially different phenotypes. Moreover, there are scarce data regarding adherence to and discrepancies among commonly available guidelines in Asian populations.
We therefore planned a survey of Asian physicians to improve understanding of the current status of real-world practice and adherence to guideline-recommended care in this region. This may be the first step to determine the exact situation and thus guide appropriate management. We conducted a survey of Asian respiratory specialists using a questionnaire comprising 25 questions based on bronchiectasis guidelines [9,10]. This survey is an important basis to understand and improve bronchiectasis management in Asian populations.
METHODS
Study participants and methods
A nationwide survey of physicians was conducted using a web-based questionnaire. To investigate adherence to management based on widely used guidelines, 25 questions were proposed based on the guidelines of the ERS and BTS [9,10]. The contents of each question regarding agreement to participate in the survey and the demographics, diagnosis, and treatment of patients with bronchiectasis are presented in Supplementary Table 1. The questionnaire was distributed to potential participants via e-mail from April 2019 to October 2019. Participants provided informed consent. The study protocol was approved by the Institutional Review Board of Asan Medical Center (IRB No. 2019-0198).
RESULTS
Characteristics of responders
A total of 221 questionnaire recipients agreed to participate in the survey. A summary of the demographics of participants from various countries is shown in Supplementary Table 2. Approximately half of all responders (n = 111, 50.2%) were Korean, 51 (23.1%) were Japanese, 30 (13.6%) were Taiwanese, and 17 (7.7%) were Singaporean. Nearly half of responders worked in a general hospital with 500 to 999 beds. A total of 137 (62.0%) and 62 (28.1%) participants reported managing fewer than 50 and 50 to 100 bronchiectasis patients in outpatient clinics per month, respectively. Responders had a variety of clinical experience as respiratory specialists, with more than half (63.8%, n = 139) being professors in academic institutes. Only 8.1% of responders (n = 18) reported having awareness of local guidelines regarding bronchiectasis in their country.
Diagnostic evaluation
At the time of initial diagnosis, more than half (52.0%) of responders checked serum immunoglobulin levels (e.g., immunoglobulin G [IgG], IgA, and IgM) (Fig. 1A), 18.6% routinely tested for allergic bronchopulmonary aspergillosis (ABPA), and 46.2% performed this evaluation only in asthmatic patients (Fig. 1B). For managing stable bronchiectasis, 38.5% of responders checked sputum acid-fast bacillus (AFB) smear/culture about 1 to 3 times per year (Fig. 1C) and 42.5% of responders checked sputum Gram stain/culture about 1 to 3 times per year.
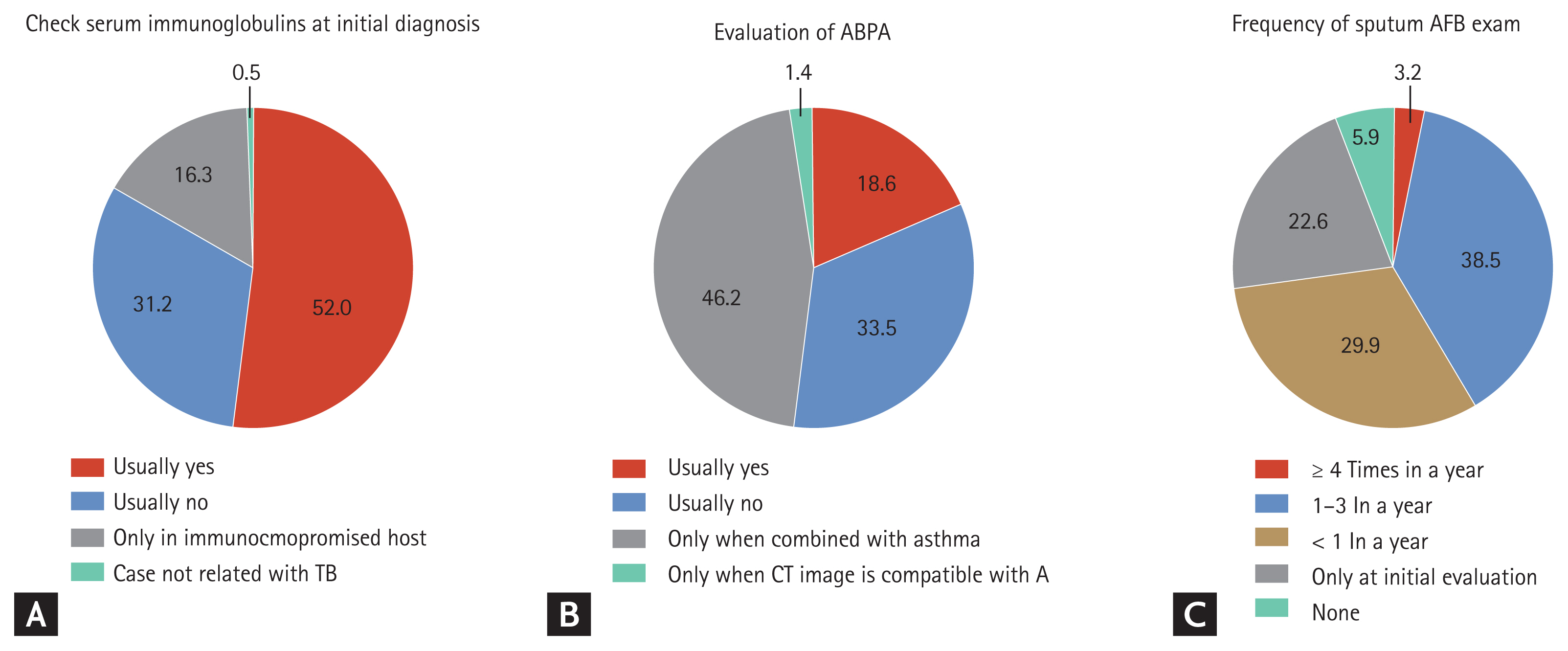
Responses of respiratory specialists regarding diagnostic evaluation. (A) Responses regarding whether a serum immunoglobulin test was performed when diagnosing patients with bronchiectasis. (B) Responses regarding whether a check for allergic bronchopulmonary aspergillosis (ABPA) was performed when diagnosing patients with bronchiectasis. (C) Responses regarding the frequency with which sputum acid-fast bacillus (AFB) smear and culture was checked for patients with stable bronchiectasis. TB, tuberculosis; CT, computed tomography.
Patient management and treatment
Only 77 (34.8%) responders reported the availability of inhaled antibiotics in their institution (Fig. 2A). A total of 143 (65.0%) responders reported that they treated patients with acute exacerbations with oral antibiotics in outpatient clinics (Table 1). Approximately half (51.6%) of responders treated bronchiectasis patients with acute exacerbations for 7 to 10 days. Eradication treatment in patients with drug-sensitive Pseudomonas aeruginosa infection was performed by 43.4% of responders (Fig. 2B); however, only 23.7% of responders performed eradication treatment in patients with drug-resistant P. aeruginosa infection (Table 1).
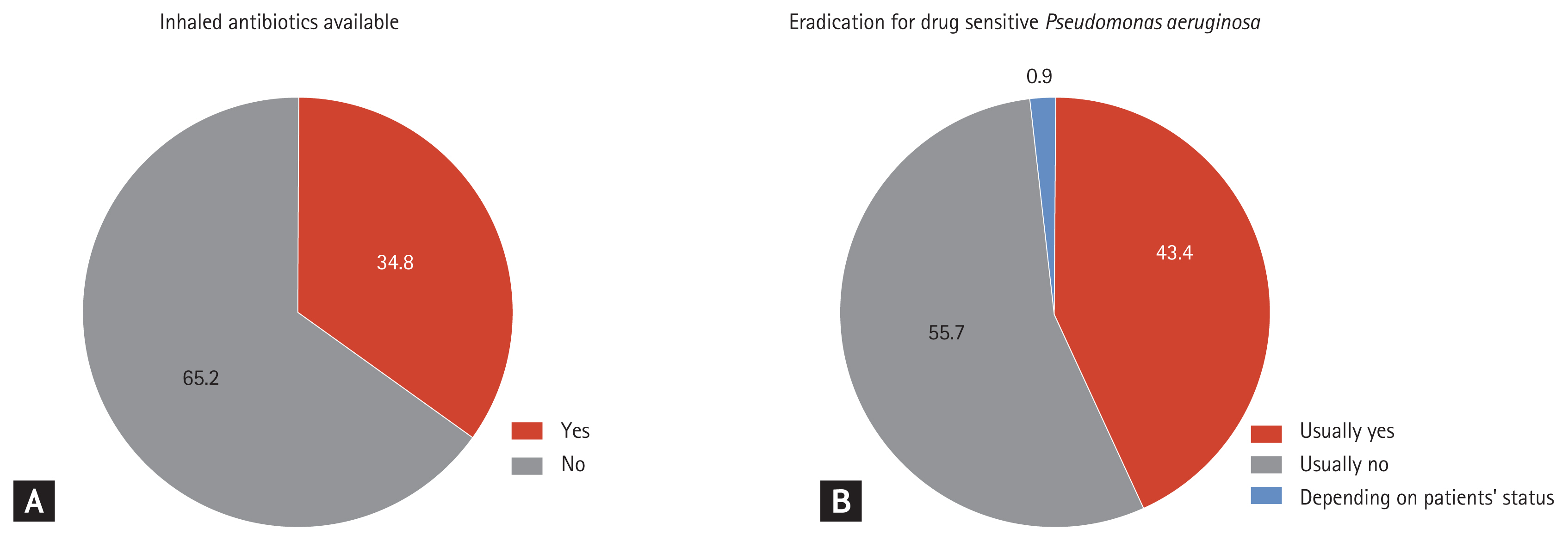
Responses of respiratory specialists regarding patient management. (A) Responses regarding the availability of inhaled antibiotics. (B) Responses regarding whether eradication treatment was performed for patients with drug-sensitive Pseudomonas aeruginosa infection.
Use of anti-inflammatory agents, usually for > 3 months, was reported by 100 (47.4%) responders. Long-term antibiotic treatment (> 3 months) was used by 49.8% of responders for patients with ≥ 3 exacerbations per year (Table 1). In addition, 88.4% of responders used long-term mucoactive treatment (> 3 months) for symptomatic patients. A total of 129 (58.4%) responders considered physiotherapy in their institution. Surgical intervention for patients with localized disease and a high frequency of exacerbations was considered by 40.9% of responders.
Differences between countries
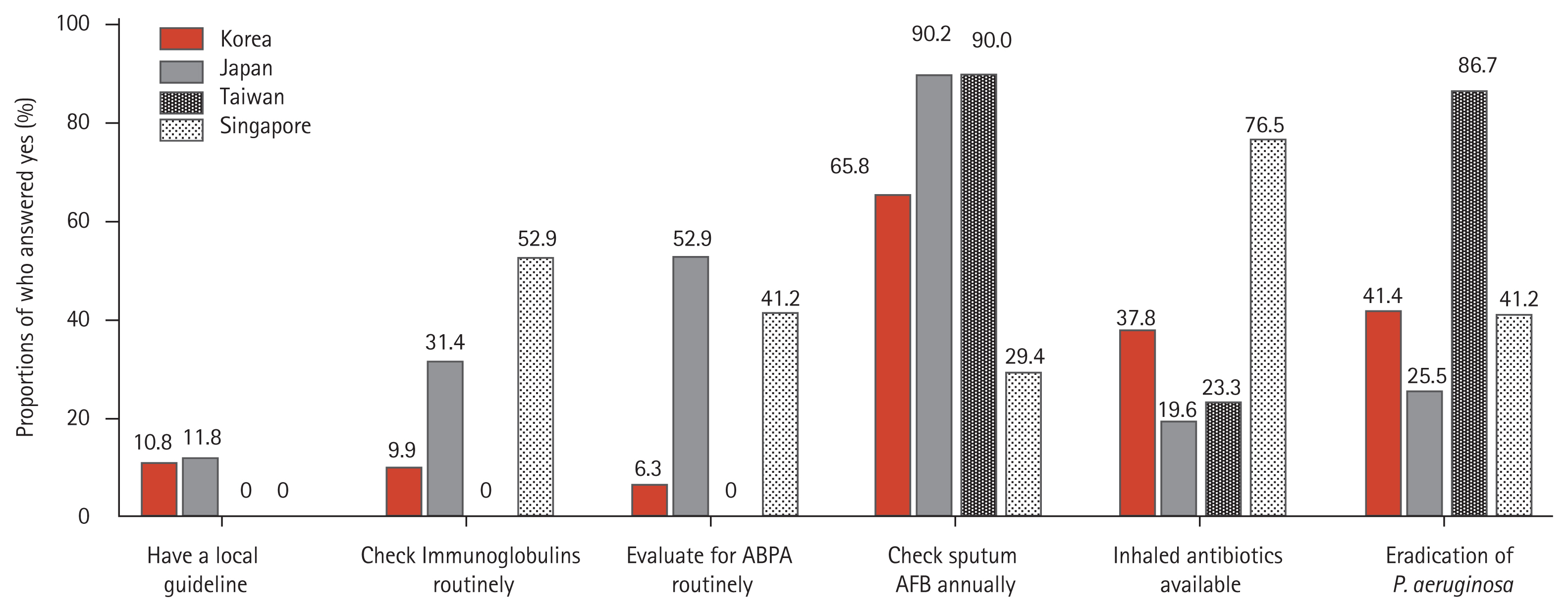
The key differences between countries are summarized in Fig. 3. A serum immunoglobulin test and test for ABPA were most frequently performed in Singapore and Japan, respectively. Nearly 90% of responders in Japan and Taiwan checked sputum AFB smear/culture annually, compared with only 29.4% of responders in Singapore. The proportion of available inhaled antibiotics was highest in Singapore, and eradication treatment of P. aeruginosa was most frequently performed in Taiwan.
DISCUSSION
This study reviewed the current status of real-world practice for patients with bronchiectasis in Asian countries. Although guidelines for management of patients with bronchiectasis have been proposed by Western countries, there is a considerable gap between guideline recommendations and real-life practice in Asian populations. To the best of our knowledge, this is the first survey of bronchiectasis management by Asian respiratory specialists.
There are international guidelines for bronchiectasis, mainly in Western countries [8–10,16,17]. Despite some discrepancies between guidelines, there are informative data concerning causes, related diseases, diagnosis and treatment of bronchiectasis, and patient management. However, there is a lack of guidelines and large-scale data for Asian populations. A recent systematic review revealed that idiopathic bronchiectasis (44.8%) was the most common etiology in 56 previous studies involving 8608 bronchiectasis patients [18]. It also reported that the prevalence of idiopathic bronchiectasis was higher in Asia (59.2%) than in Europe (41.1%), South America (37.3%), Africa (25.0%), and North America (6.6%). Likewise, Huang et al. [15] investigated the etiology and characteristics of 15,729 bronchiectasis patients in Taiwan, and reported that tuberculosis (1,950 patients, 12.4%) was one of the main causes of post-infectious bronchiectasis. Dhar et al. [13] recently reported a marked difference in bronchiectasis between India and Western countries using the Indian bronchiectasis registry including 2,195 patients. Different guidance is needed for different populations and clinical situations; however, there are no international data regarding patients with bronchiectasis from Asian populations.
The etiology of bronchiectasis, including diverse respiratory or systemic diseases, has been previously reported [19,20]. A prior history of tuberculosis was one of the main causes of bronchiectasis in Asian studies [13,15,21,22]. In addition, non-tuberculous mycobacteria (NTM) infection might be a cause and consequence of bronchiectasis [7]. Park et al. [23] evaluated 155 Korean patients with bronchiectasis and reported that 7.1% of patients had a history of NTM infection and that NTM was cultured in samples of 44.5% of patients during a mean follow-up duration of 7.11 years. Lee et al. [24] reported that bronchiectasis was the second most common underlying disease (43.6%) in 33,974 cases of NTM infection over 10 years in South Korea. The prevalence and incidence of NTM infection have increased worldwide, not just in Asian countries [25]. Aksamit et al. [26] recently reported that 63% of 1,826 patients in the bronchiectasis research registry of the USA had a history of NTM disease, suggesting that NTM is also a major etiology in Western populations. Consistently, the current study showed that physicians in Asia usually checked sputum AFB smear and culture regularly (68.4% of responders) or at the initial visit (21.3% of responders).
Our data demonstrated that adherence to European guidelines for diagnostic evaluation of bronchiectasis, with the exception of NTM analysis, was relatively low, consistent with an Indian study [13]. In the current study, only 16.3% and 18.6% of responders checked for immunoglobulins and ABPA, respectively. This might be due to the low prevalence of primary immunodeficiency and ABPA in Asian populations. Rhim et al. [27] reported that the prevalence of common variable immunodeficiency in Korea is only 1.16 per million, which is much lower than in Western countries (about 1:25,000) [28]. In addition, despite a lack of evidence, Denning et al. [29] reported that the disease burden of ABPA is lower in Asian countries than in Western countries. However, considering that immunodeficiency and ABPA are treatable, physicians in Asian countries should be aware of these conditions in patients with bronchiectasis.
The management of bronchiectasis in our survey differed from the guidelines of the ERS [10]. Although there is evidence that antibiotic treatment is of clinical benefit to eradicate P. aeruginosa infection [30,31], only 43.4% of responders performed such treatment in the current study. One reason for this finding was a lack of inhaled antibiotics in their institution. Indeed, only 34.8% of responders had available inhaled antibiotics in their institution. For the same reason, only about half (49.8%) of responders used long-term antibiotic treatment for bronchiectasis patients with ≥ 3 exacerbations per year. Another reason is that the preference of the attending physician in each country might have influenced the results. In another important difference from the recommendations, our study reported that only 58.4% of responders considered physiotherapy, including pulmonary rehabilitation, for patients with bronchiectasis. Pulmonary rehabilitation improves quality of life [32] and may reduce exacerbations [33]. Considering the low risk of pulmonary rehabilitation, Asian physicians should try to establish and deliver this treatment in patients with bronchiectasis.
This study has some limitations. First, our survey was based on physician-reported responses, and there may be some discrepancies between our results and real-world practice. Second, this study focused on respiratory specialists and therefore the majority of responders were professors working in academic institutions, meaning that the data do not reflect the situation in primary clinics. A survey of specialists may be more appropriate to check the limitations of management in each country. Third, our study was conducted in several Asian countries, which may limit the generalizability of our results in all Asian countries. Fourth, the hospital size of responders was diverse, which might have led to differences in diagnostic methods and treatment patterns between countries. Finally, some physicians misunderstood some questions, and this might have affected their responses. For example, although a few responders (12 Korean and six Japanese) answered that they had local guidelines for bronchiectasis, it is unclear whether such guidelines exist in these countries. Despite these limitations, our study partially revealed the current status of bronchiectasis management in Asian countries and suggested which diagnostic work-up and treatment modalities are necessary in these countries. In conclusion, our results suggest that some discrepancies might exist between guideline recommendations and treatment of bronchiectasis in Asian populations. The use of some treatment methods was limited in practice due to their restricted availability, and evidence to support the further introduction of these methods is required. New guidelines that consider different phenotypes and available treatment options in Asia must be developed to improve the management of patients with bronchiectasis.
KEY MESSAGE
1. There are discrepancies between guideline recommendations and real-world practice for bronchiectasis management in Asian populations.
2. Our findings suggest that development of local guidelines that consider specific phenotypes and situations will help to standardize and improve the management of bronchiectasis.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by grants from the Asan Institute for Life Sciences (2020-7043) and the Korea Medical Device Development Fund grant funded by the Korean government (Project Number: 202011C07-00).